Abstract
The molecular mechanisms underlying acute leptin and serotonin 2C receptor induced hypophagia remain unclear. Here we show that neuronal and pro-opiomelanocortin (Pomc)-specific loss of transient receptor potential cation 5 (TrpC5) subunits is sufficient to decrease energy expenditure and increase food intake resulting in elevated body weight. Deficiency of Trpc5 subunits in Pomc neurons was also sufficient to block the anorexigenic effects of leptin and serotonin 2C receptor (Ht2Cr) agonists. The loss of acute anorexigenic effects of these receptors was concomitant with a blunted electrophysiological response to both leptin and Ht2Cr agonists in arcuate Pomc neurons. We also demonstrate that the Ht2Cr agonist lorcaserin-induced improvements in glucose and insulin tolerance were blocked by TrpC5 deficiency in Pomc neurons. Together, our results link TrpC5 subunits in the brain with leptin- and serotonin 2C receptor-dependent changes in neuronal activity as well as energy balance, feeding behavior, and glucose metabolism.
Keywords: melanocortin, obesity, diabetes, transient receptor potential cation channels, thermogenesis, glycemia, patch-clamp, electrophysiology, leptin, serotonin, lorcaserin
Graphical abstract
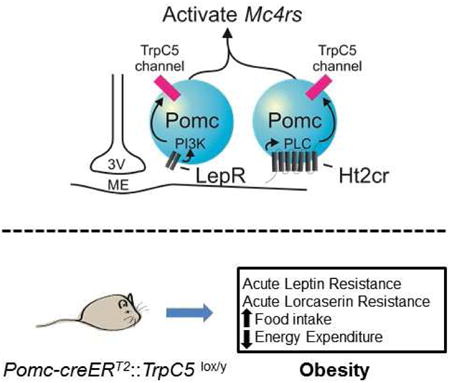
TrpC5 subunits are essential for the negative energy balance associated with Pomc neuronal activation. TrpC5 subunits not only link the acute activities of leptin and serotonin receptors in Pomc neurons, but also modify direct effects on basal metabolism.TrpC5 subunits may provide an endogenous target to manipulate the activity of key neurons involved in the regulation of energy balance and glucose metabolism.
Introduction
Leptin receptors (LepRs) in the brain produce robust anti-obesity and anti-diabetic effects (Bjorbaek and Kahn, 2004; Coll et al., 2007; Elmquist et al., 1999; Flier, 2006; Friedman, 2004; Spiegelman and Flier, 2001). Similarly, the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) contributes to the regulation of feeding behavior, energy expenditure and glucose homeostasis by acting via serotonin 2C receptors (Ht2Crs) within the CNS (Giorgetti and Tecott, 2004; Heisler et al., 2003). The hypothalamic arcuate pro-opiomelanocortin (Pomc) neurons are vital in mediating both leptin's and serotonin's beneficial effects on metabolism (Balthasar et al., 2004; Berglund et al., 2012; Sohn et al., 2011). Very recently, lorcaserin, an Ht2Cr specific agonist became the first FDA-approved weight-loss drug in the last 15 years independent of adverse cardiopulmonary effects. In order to better understand the effectiveness and safety of newly approved anti-obesity drugs it is imperative to delineate cellular mechanisms underlying Ht2Cr and LepR activity in the brain.
There is an emerging role for TrpC channels in the regulation of energy homeostasis by leptin (Qiu et al., 2010). Similarly, activation of Pomc neurons by Ht2Crs is mediated by a phospholipase C (PLC)-dependent activation of putative TrpC channels (Sohn et al., 2011). Single cell RT-PCR of arcuate Pomc neurons revealed that TrpC 1,4, and 5 subunits predominate in murine Pomc neurons (Qiu et al., 2010). However, TrpC1 alone may not be sufficient to form a functional ion channel (Strubing et al., 2001), suggesting that TrpC4 or TrpC5 may be a common target in Pomc neurons of these two potent anorexigenic signals, leptin and serotonin. In the current study, we studied the requirement of TrpC5 in the acute effects of leptin and serotonin to depolarize hypothalamic Pomc neurons. We also examined the requirement of TrpC5 subunits in the acute effects of leptin and serotonin on energy expenditure as well as glucose homeostasis.
Results
Neuronal TrpC5 subunits are required for proper energy balance
Disturbance of acute leptin or Ht2Cr signaling pathways in Pomc neurons is correlated with blunted acute anorexia by leptin and serotonin 2C receptor-agonists as well as deficits in energy and glucose homeostasis. (Hill et al., 2008; Xu et al., 2008; Xu et al., 2010b). We hypothesized that leptin and Ht2Crs may acutely activate TrpC5 subunits within Pomc neurons thus being required in vivo for the feeding effects of these receptors as well as regulating energy balance and glucose homeostasis. To determine the metabolic effects of TrpC5 subunits within the CNS during postnatal development, we generated a conditional mouse model (TrpC5lox/Y) in which we could selectively reduce the expression of TrpC5 subunits in key brain sites. This was accomplished by utilizing TrpC5-targeted ESCs from the European Union Conditional Mouse Mutagenesis (EUCOMM) program in order to generate conditional TrpC5lox/y mice via the insertion of loxP sites surrounding exon 5 of the TrpC5 gene (Figure 1A). Subsequent breeding to ZP3-cre (Figure 1B), CamkIIα-cre or Pomc-creERT2 mice generated mice deficient for TrpC5 in a cell/tissue-type dependent manner.
Figure 1.
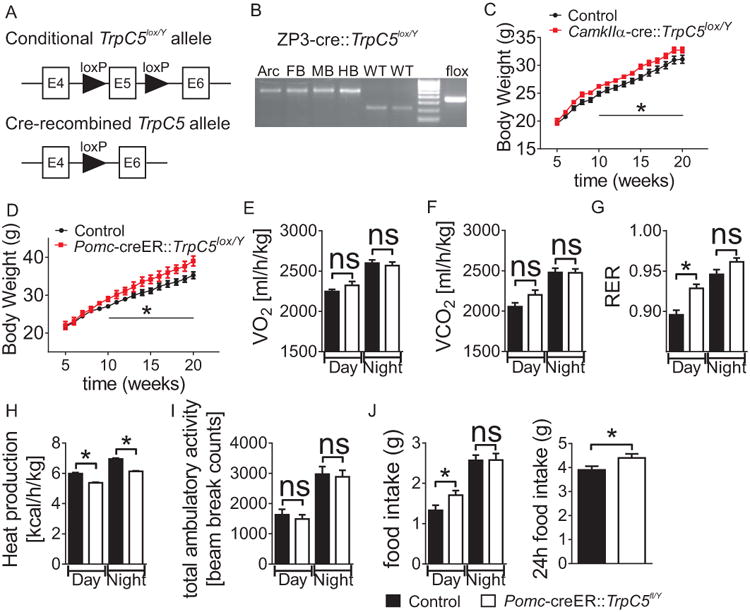
Body weight and metabolic assessment of male WT and Pomc-creERT2∷TrpC5lox/Y mice on chow diet. (A) Schematic of genomic DNA region around exon 5 of TrpC5 in mice carrying a targeted TrpC5 allele (TrpC5lox/Y). After Cre-mediated excision of exon 5, a PCR amplification product identifying the deleted TrpC5 allele (TrpC5lox/Y) becomes detectable. (B) PCR amplification products from genomic DNA using primers detect wild type, floxed, and deleted TrpC5. Tissues were dissected from a ZP3-cre∷TrpC5lox/Y mouse. From left to right; lane 1 Arcuate nucleus (positive for recombination); lane 2 forebrain (positive for recombination); lane 3 midbrain (positive for recombination); lane 4 hindbrain (positive for recombination); lane 5 and 6 wildtype hypothalamus (negative for recombination); lane 6 DNA ladder; and lane 7 hypothalamus from floxed mouse negative for ZP3-cre. Body weight curve of (C) male CamkIIα-cre∷TrpC5lox/Y and (D) male Pomc-cre∷TrpC5lox/Y mice (*p<0.05). (E-I) Depicts (E) unchanged oxygen consumption - VO2, (F) unchanged carbon dioxide production - VCO2, (G) increased respiratory exchange ratio - RER (H) decreased heat production, and (I) unchanged ambulatory activity in male Pomc-cre∷TrpC5lox/Y mice. (J) Male Pomc-cre∷TrpC5lox/Y mice exhibited increased food intake in the light cycle which resulted in hyperphagia over 24h. For (A)–(I), n = 8-15 per group; *p < 0.05. Error bars indicate SEM. Note: mice used in (E-I) were age-matched male littermates (8 weeks of age), and had comparable body weight and lean mass.
When fed a chow diet, neuron- [CamkIIα –cre; in which Cre recombinase is expressed widely in the forebrain and hindbrain (Casanova et al., 2001)] or Pomc- [Pomc-creERT2-cre -allows temporal control of cre recombinase activity in Pomc neurons (Berglund et al., 2013)] specific deficiency of TrpC5 subunits resulted in an age-dependent increase in body weight (Figures 1C and 1D). The increased body weight was more pronounced in mice deficient for TrpC5 subunits in Pomc neurons alone (Figure 1D). Age and weight matched Pomc-creERT2∷TrpC5lox/Y males were hypometabolic, as demonstrated by significant decreases in energy expenditure (Figures 1E-1H) independent of altered activity levels (Figure 1I). Components of total energy expenditure include energy required for physical activities and basal metabolism. In particular, Pomc-creERT2∷TrpC5lox/Y mice exhibited decreased heat production (Figure 1H) suggestive of decreased metabolic rate. Pomc-creERT2∷TrpC5lox/Y mice also showed increased ad libitum food intake (Figure 1J).
Neuron- and Pomc-specific TrpC5 deficiency blunts the acute anorexia by LepR and Ht2Cr activation
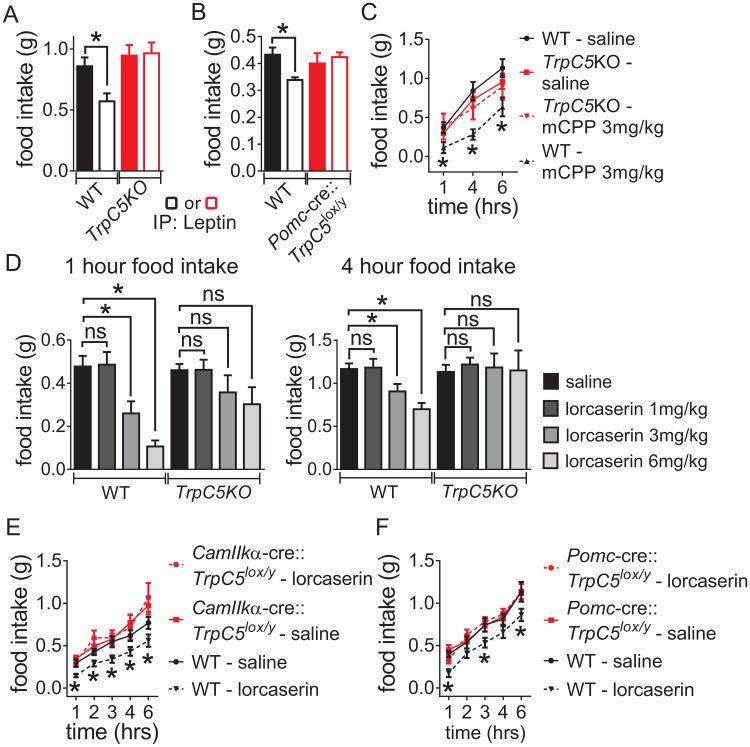
Wildtype, TrpC5KO (Riccio et al., 2009) and Pomc-creERT2∷TrpC5lox/Y mice were fasted overnight and intraperitoneally (i.p.) injected with either saline or leptin (5 mg/kg). Consistent with previous reports (Hill et al., 2008), i.p. injections of leptin reduced food intake in wildtype mice (at 1h) compared to the saline-injected group (Figures 2A and 2B). However, i.p. injections of leptin failed to decrease food intake in TrpC5KO and Pomc-creERT2∷TrpC5lox/Y mice (at 1h) versus the saline-injected group (Figures 2A and 2B).
Figure 2.
Leptin-, mCPP-, and lorcaserin-induced hypophagia is blunted in mice deficient for TrpC5 subunits. (A) and (B) Food consumption was measured 1 hour after leptin administration (5mg/kg, i.p.) and compared with food consumption in each animal following saline administration. (C) and (D) Cumulative food intake measured at 1, 4 and/or 6 hours after administration of Ht2Cr agonists mCPP (3mg/kg) or lorcaserin (1, 3, or 6mg/kg). (E) and (F) Food intake measured in response to lorcaserin (3mg/kg, i.p.). For (A)–(F), n = 9-14 per group; *p < 0.05.
We performed a similar series of experiments with both the non-selective Ht2Cr agonist (meta-Chlorophenylpiperazine – mCPP) (3 mg/kg) and lorcaserin (1, 3, and 6 mg/kg). As reported previously, the Ht2Cr agonists significantly reduced food intake in wildtype mice versus saline-injected groups (at 1h, 4h, and/or 6h Figures 2C-2F). However, i.p. injections of mCPP or lorcaserin failed to suppress food intake (at 1h, 4h, and/or 6h) in TrpC5KO, CamkIIα∷TrpC5lox/Y and/or Pomc-creERT2∷TrpC5lox/Y mice compared to saline injections. Of note 3mg/kg of lorcaserin appears to be a threshold dose for the acute biological activity of food intake. Taken together, TrpC5 subunits are required in Pomc neurons for the acute anorexia induced by leptin receptor and Ht2Cr activation.
TrpC5 subunits are required for the acute activation of Pomc neurons by leptin
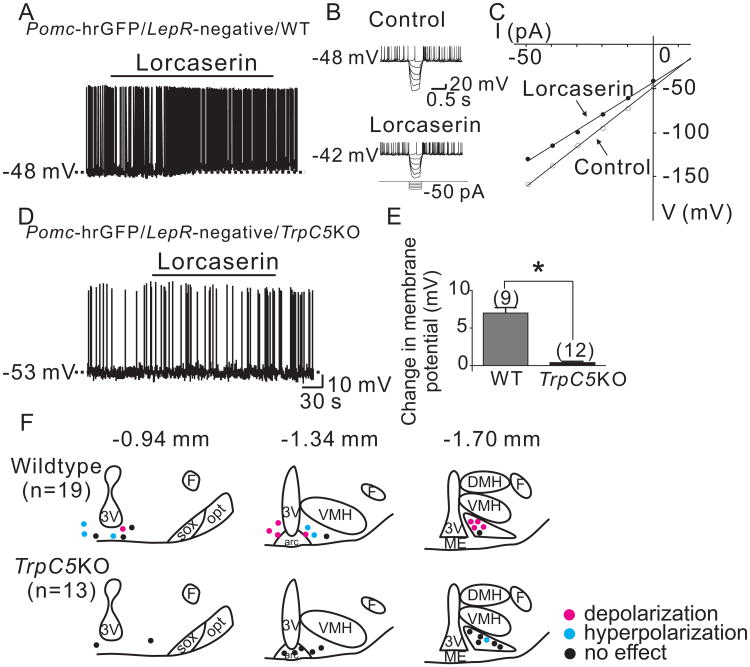
Whole-cell patch-clamp recordings were made in 58 arcuate Pomc neurons from Pomc-hrGFP mice (Parton et al., 2007; Ramadori et al., 2010; Sohn et al., 2011). 26 Pomc neurons were from wildtype and, 32 were in Pomc neurons deficient for TrpC5 subunits (Riccio et al., 2009). Pomc neurons from wildtype mice (n= 26) had a resting membrane potential of −41.5 ± 1.3 mV, a mean input resistance of 1260 ± 80 MΩ, and overshooting action potentials. When compared to values obtained from wildtype Pomc neurons, Pomc neurons deficient for TrpC 5 exhibited a hyperpolarized mean resting membrane potential (−46.3± 0.9 mV; 1085 ± 53 MΩ; n=32; p<0.05; supplemental figure 1A). Analogous results were obtained in Pomc neurons from Pomc-creERT2 vs Pomc-creERT2∷TrpC5lox/Y mice (RMP = -44.3 ± 1.0mV from wildtype Pomc neurons, n=24; and RMP = -48.0 ± 1.4mV in Pomc neurons from Pomc-creERT2∷Trpc5lox/Y mice n = 22; p<0.05; supplemental figure 1A).
Similar to previous reports (Hill et al., 2008; Sohn et al., 2011), Pomc neurons that express LepRs were targeted from Pomc-hrGFP∷LepR-cre-tdtomato (PLT) mice (Sohn et al., 2011; Sun et al., 2016; Williams et al., 2014) to test the acute cellular effects of leptin (Figures 3A to 3E). Leptin (100 nM) depolarized 70.6 % (12 out of 17) of wildtype Pomc neurons that express LepRs by 6.7 ± 0.5 mV (n=12, Figures 3F, 3J and supplemental table 1). We found 1 cell (5.9 %) that was hyperpolarized by -10 mV, while the remaining 4 cells (23.5 %) were not responsive to leptin (0.3 ± 0.3 mV, n=4). Analysis of current-voltage relationships revealed that leptin decreased input resistance by 21.4 ± 3.0 % (n=12, from 1.2 ± 0.1 GΩ in control ACSF to 0.9 ± 0.1 GΩ in leptin) with a reversal potential of -26.0 ± 2.2 mV (n=12) (Figures 3G and 3H). These results confirmed that leptin activates a non-selective cation conductance to depolarize Pomc neurons.
Figure 3.
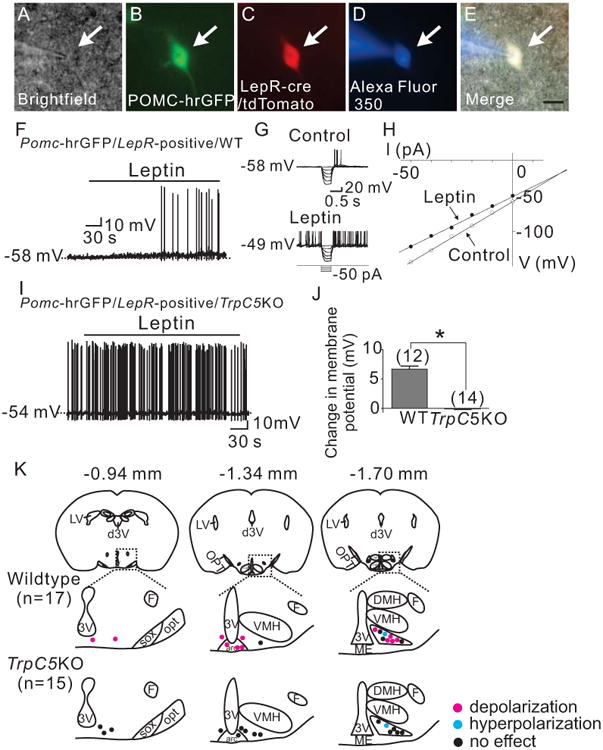
Trpc5 subunits are required for the acute leptin-induced depolarization of arcuate Pomc neurons. (A) Brightfield illumination of Pomc-hrGFP∷Lepr-cre∷tdtomato neuron from PLT mice. (B) and (C) The same neuron under FITC (hrGFP) and Alexafluor 594 (tdtomato) illumination. (D) Complete dialysis of Alexa Fluor 350 from the intracellular pipette. (E) Merge image illustrates colocalization of hr-GFP, tdtomato, and Alexa Fluor 350 indicative of a Pomc neuron which expresses Leprs. (F) Electrophysiological study demonstrates a Pomc-hrGFP∷Lepr-cre∷tdtomato (green/red) neuron that is depolarized in response to leptin (100nM). (G) Traces showing decreased voltage deflection and increased action potential frequency after leptin application. (H) Current versus voltage (I-V) plot from same WT neuron illustrating a characteristic decrease in input resistance subsequent to leptin application. Shown are responses before (control) and during leptin application. (I) demonstrates a current clamp recording of a Pomc-hrGFP∷Lepr-cre∷tdtomato∷TrpC5KO (green/red) neuron in which leptin fails to induce a depolarization. (J) Histogram summarizing the acute effect of leptin on the membrane potential of Pomc neurons which express leptin receptors as well as express or do not express Trpc5 subunits (n= 12-14 per group). (K) Rostro-caudal and medio-lateral distribution of electrophysiological responses to leptin from Pomc neurons which express leptin receptors as well as express or do not express Trpc5 subunits.
In contrast, leptin failed to depolarize any of 15 Pomc neurons which express LepRs from TrpC5 knockout (PLT5KO) mice (Figures 3I, 3J and supplemental table 2): 14 cells (93.3 %) remained unresponsive to leptin (-0.1 ± 0.5 mV), while 1 cell (6.7 %) was hyperpolarized by -9 mV. Analogous results were obtained in recordings from Pomc neurons selectively deficient for TrpC5 subunits (from Pomc-creERT2∷TrpC5 mice; supplemental figure 1B). These data suggest that TrpC5 subunits underlie the leptin-induced activation of a non-selective cation conductance resulting in the depolarization of Pomc neurons. Notably, the hyperpolarizing effects of leptin remained unchanged in Pomc neurons lacking TrpC5 subunits and these effects were accompanied by decreased input resistance with a reversal potential close to EK. Similar results were obtained in ventral premammillary nucleus (PMv) neurons which express leptin receptors (supplemental material and supplemental figure 2). Thus, the null TrpC5 gene does not affect the functional expression of leptin receptors or other channels in our model.
After recordings, the sections were fixed in formalin to determine the location of recorded cells within the arcuate nucleus, as described previously (Sohn et al., 2011). Since acute leptin responses show a distinct distribution pattern (Williams et al., 2010), we targeted cells of similar region within the arcuate nucleus in both wildtype and TrpC5 knockout mice (Figure 3K).
The acute mCPP-induced activation of Pomc neurons requires TrpC5 subunits
To test the acute cellular effects of Ht2Crs, we targeted Pomc neurons that do not express LepRs from PLT mice (Figures 4A to 4E), as previously described (Sohn et al., 2011). We confirmed that mCPP (4 μM) depolarized 55 % (5 out of 9) of wildtype Pomc neurons that do not express LepRs by 6 ± 0.6 mV (n=5, Figures 4F, 4J, and supplemental table 1). The remaining 4 cells (45 %) were not responsive to mCPP (0.4 ± 0.2 mV, n=4). Application of current steps revealed that mCPP decreases input resistance by 14.8 ± 4.8 % (n=5, from 1.5 ± 0.1 GΩ in control ACSF to 1.3 ± 0.1 GΩ in mCPP) with a reversal potential of -14 ± 4.8 mV (n=5) (Figures 4G and 4H). In contrast, mCPP failed to depolarize all Pomc neurons tested from Trpc5 knockout (PLT5KO) mice (0.1 ± 0.2 mV, n=19, Figures 4I, 4J, and supplemental table 2). Similar to the acute leptin effects in the current study, we targeted cells in similar region and responses were mapped within the arcuate nucleus (Figure 4K).
Figure 4.
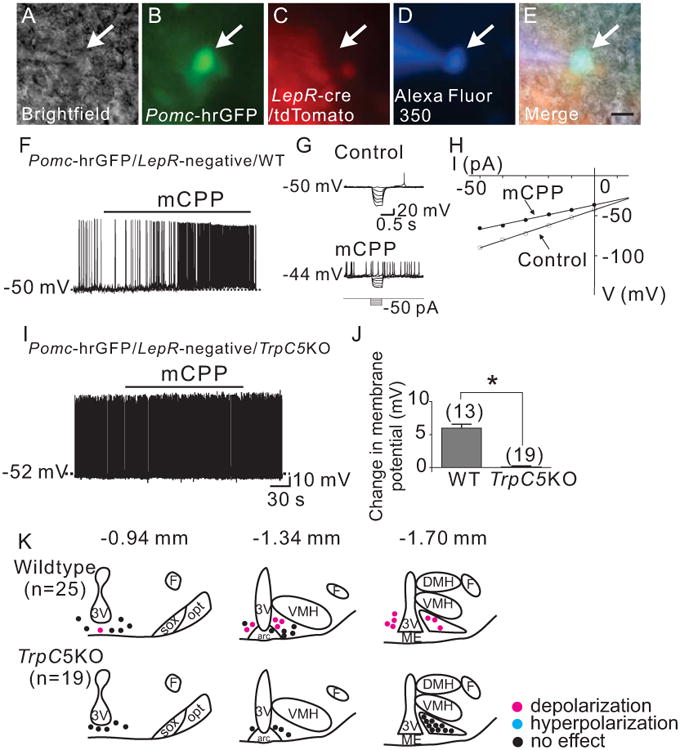
Trpc5 subunits are required for the acute mCPP-induced depolarization of arcuate Pomc neurons. (A) Brightfield illumination of Pomc-hrGFP neuron from PLT mice. (B) and (C) The same neuron under FITC (hrGFP) and Alexafluor 594 (tdtomato) illumination. (D) Complete dialysis of Alexa Fluor 350 from the intracellular pipette. (E) Merge image illustrates colocalization of hr-GFP and Alexa Fluor 350 indicative of a Pomc neuron which does not express Leprs. (F) Electrophysiological study demonstrates a Pomc-hrGFP (green) neuron from PLT mice that depolarized in response to mCPP (4μM). Traces showing decreased voltage deflection and increased action potential frequency after mCPP application. (H) Current versus voltage (I-V) plot from same WT neuron illustrating a characteristic decrease in input resistance subsequent to mCPP application. Shown are responses before (control) and during mCPP application. (I) demonstrates a current clamp recording of a Pomc-hrGFP∷Trcp5 -/Y (green) neuron in which mCPP fails to induce a depolarization. (J) Histogram summarizing the acute effect of mCPP on the membrane potential of Pomc neurons which do not express leptin receptors as well as express or do not express Trpc5 subunits (n= 13-19 per group). (K) Rostro-caudal and medio-lateral distribution of electrophysiological responses to mCPP from Pomc neurons which do not express leptin receptors as well as express or do not express Trpc5 subunits.
The lorcaserin-induced depolarization of Pomc neurons is dependent upon TrpC5
We previously confirmed that the acute effects of mCPP on Pomc neurons were mediated by Ht2Crs (Berglund et al., 2013; Xu et al., 2010a). Thus, the observed cellular effects of mCPP in the present study should depend upon Ht2Cr activation, and the lack of mCPP effects on Pomc neurons from the TrpC5 knockout mice indicate that Trpc5 is required for the acute activation of Pomc neurons by Ht2Crs.
However, to further examine this activity, we used lorcaserin (a specific Ht2Cr agonist recently approved for chronic weight management). Similar to results obtained with mCPP, lorcaserin (4 μM) depolarized 50 % (6 out of 12) of wildtype Pomc neurons that do not express LepRs by 7.0 ± 0.7 mV (n=6, Figures 5A, 5E, and supplemental table 1). The remaining 6 cells (50 %) were not responsive to lorcaserin (0.3 ± 0.2 mV, n=6). Application of current steps confirmed that lorcaserin decreased the input resistance by 25.6 ± 6.5 % (n=6, from 1.6 ± 0.1 GΩ in control ACSF to 1.2 ± 0.2 GΩ in lorcaserin) with a reversal potential of -13.1 ± 3.2 mV (n=6) (Figures 5B and 5C). Similar results were observed in Pomc neurons which express Ht2Crs (from Pomc∷ Ht2Cr-cre∷tdtomato [P2CT] mice; supplemental material, supplemental figures 3, 4, and supplemental table 3).
Figure 5.
Trpc5 subunits are required for the acute lorcaserin-induced depolarization of arcuate Pomc neurons. (A) Electrophysiological study demonstrates a Pomc-hrGFP (green) neuron from PLT mice that depolarized in response to lorcaserin (4μM). (B) Traces showing decreased voltage deflection and increased action potential frequency after lorcaserin application. (C) Current versus voltage (I-V) plot from same WT neuron illustrating a characteristic decrease in input resistance subsequent to lorcaserin application. Shown are responses before (control) and during lorcaserin application. (D) Demonstrates a current clamp recording of a Pomc-hrGFP∷Trcp5lox/Y (green) neuron from PLT mice in which lorcaserin fails to induce a depolarization. (E) Histogram summarizing the acute effect of lorcaserin on the membrane potential of Pomc neurons which do not express leptin receptors as well as express or do not express TrpC5 subunits (n= 9-12 per group). (F) Rostro-caudal and medio-lateral distribution of electrophysiological responses to lorcaserin from Pomc neurons which do not express leptin receptors as well as express or do not express TrpC5 subunits.
As expected, lorcaserin failed to depolarize any of 12 Pomc neurons from TrpC5 knockout (PLT5KO) mice (Figures 5D, 5E, and supplemental table 2). 11 cells (91.7 %) remained unresponsive to lorcaserin (0.4 ± 0.2 mV), while 1 cell (8.3 %) was hyperpolarized by -8 mV. A summary of the location of targeted cells was mapped throughout the rostro-caudal extent of the arcuate nucleus (Figure 5F). Similar results were obtained in recordings from Pomc neurons selectively deficient for TrpC5 subunits (from Pomc-creERT2∷TrpC5 mice; supplemental figure 1C and supplemental table 4). These data confirm that TrpC5 subunits underlie the Ht2Cr-mediated activation of non-selective cation conductance and the depolarization of Pomc neuron membrane potential.
Discussion
Neuronal or Pomc-specific TrpC5 deficiency resulted in a positive energy balance which contributed to excess weight gain. In addition to these physiological aberrations, global deficiency of TrpC5 as well as neuron- or Pomc-specific deficiency of TrpC5 alone abrogated the acute anorexigenic effects of both leptin and serotonin 2C receptor agonists. TrpC5 subunits were also required for the acute effects of lorcaserin to improve both glucose and insulin tolerance (Supplemental Figures 5 and 6). On a cellular level, deficiency of TrpC5 subunits abrogates the acute effects of both leptin and serotonin 2C receptors to activate arcuate Pomc neurons. Together, these data demonstrate that TrpC5 subunits in arcuate Pomc neurons are important regulators of the pharmacological effects of both leptin and serotonin as well as link critical molecular mechanisms with recently developed anti-obesity pharmacotherapeutics, such as lorcaserin.
Multiple signaling cascades are activated in response to leptin receptor activation. In particular, phosphoinositol 3 kinase (PI3K) has been suggested to be required for the acute effects of leptin to stimulate Pomc neuronal activity via a putative activation of TrpC channels (Al-Qassab et al., 2009; Hill et al., 2008; Qiu et al., 2010). Pharmacological inhibition of PI3K or targeted disruption of PI3K regulatory subunits in Pomc neurons alone blunted the suppression of feeding elicited by central leptin administration (Hill et al., 2008; Niswender et al., 2001). Moreover, mice deficient for PI3K catalytic activity exhibited central leptin resistance, increased adiposity, diet-induced obesity, and impaired glucose regulation (Al-Qassab et al., 2009; Hill et al., 2009). Importantly, these data are analogous to the observations detailed in the current study, supporting a role for the PI3K-TrpC5 pathway in the acute and chronic regulation of energy balance and glucose homeostasis.
It should be noted that, a previous report demonstrated that global deficiency of TrpC5 subunits (Trpc5KO) fails to alter body weight (Riccio et al., 2009). However, numerous reports have demonstrated that global deficiency of various genes may result in compensatory adaptation (Luquet et al., 2007; Zeltser et al., 2012). In the current study, global TrpC5 deficiency resulted in improved glucose tolerance whereas neuronal- or Pomc-specific loss of TrpC5 failed to alter basal glucose tolerance (Supplemental Figures 5 and 6). Despite the improved glucose tolerance, global TrpC5 deficiency did not improve insulin tolerance, suggesting that Trpc5 subunits might ameliorate only specific components of the glucose-stimulated insulin response. Together, these data may suggest that inhibition of TrpC5 subunits in the periphery (possibly including TrpC5 subunits in adipose tissue) may improve glucose homeostasis; however loss of TrpC5 subunits in Pomc neurons might impair energy balance and blunt targeted improvements in glucose metabolism. While these data support possible mechanisms for improved energy balance and glucose metabolism via TrpC5 subunits, they also highlight the utility of cell type targeted strategies to better identify roles for cellular signaling in energy balance and glucose metabolism.
Another salient finding is that TrpC5 subunits in Pomc neurons alter basal metabolism and feeding behavior. Several reports using chemo- and opto- genetic strategies have highlighted the role of acutely modulating melanocortin cellular activity and resulting changes in feeding behavior and body weight (Aponte et al., 2011; Zhan et al., 2013). However, identification of humoral signals linked to endogenous signaling cascades and channels which may acutely alter metabolism has remained undefined. Moreover, leptin's and serotonin's acute beneficial effects on metabolism are due at least in part to the acute activity of melanocortin neurons. In the current study we propose that activation of TrpC5 in arcuate Pomc neurons, as occurs in response to LepRs or Ht2Crs, represents a physiological cellular correlate to the light/chemical-induced activation of channelrhodopsins/DREADDs in Pomc neurons previously demonstrated to stimulate a negative energy balance (Aponte et al., 2011; Zhan et al., 2013). Moreover, in addition to the pharmacological activity of leptin and Ht2Cr agonists; Trpc5 subunits contribute to a basal activity of melanocortin neurons which alters metabolic rate and feeding behavior.
Ht2Crs are known to regulate energy homeostasis and body weight. This regulatory impact is perhaps most notable due to the anti-obesity drug d-fenfluramine (d-Fen) which was identified to act via Ht2Cr (Vickers et al., 1999; Xu et al., 2008). However, the cellular mechanism required for Ht2Cr-expressing neurons to mediate effects on energy homeostasis remains unclear. This is topical due to the recent approval by the FDA of the anti-obesity drug, lorcaserin, which targets Ht2Cr for chronic weight management (Harlan et al., 2011; Martin et al., 2011). Additionally, other Ht2Cr agonists are also being tested in humans to treat obesity further reinforcing a need for mechanistic insights on how these compounds exert their effects (Rodgers et al., 2012). Importantly, lorcaserin has also been associated with improvements of blood glucose levels in patients with type 2 diabetes (O'Neil et al., 2012). Notably, in the current study lorcaserin-induced acute improvements in glucose and insulin tolerance were observed and required TrpC5 subunits in Pomc neurons. This observation suggests potential weight-independent effects of lorcaserin and TrpC channels on blood glucose levels, which should be studied further.
It should be noted that in addition to classic neuropeptides (α-MSH, β-endorphin, NPY, AgRP, etc.), there is an emerging role for the fast acting neurotransmitters (glutamate and GABA) in regulating energy balance and glucose metabolism(Krashes et al., 2014; Liu et al., 2012; Pinto et al., 2004; Tong et al., 2008; Yang et al., 2011). Arcuate Pomc neurons co-express glutamate (Collin et al., 2003; Kiss et al., 2005); independent of NPY/AgRP and to a variable extent GABA (Hentges et al., 2004; Hentges et al., 2009; Horvath et al., 1997; Ovesjo et al., 2001; Wittmann et al., 2013; Yee et al., 2009). It's currently unclear how TrpC5 dependent activation of arcuate Pomc neurons might differentiate between the release of either neurotransmitters or neuropeptides. Similarly, recent work suggest a sensory component of Pomc or NPY/AgRP neuronal activity which is likely independent of endogenous peptidergic signals (Betley et al., 2015; Chen et al., 2015). Although currently unclear, involvement of pharmacological levels of leptin and serotonin 2C receptor agonists might act on these sensory cues influencing feeding behavior. Thus the role of either neurotransmitters or neuropeptides in the Pomc specific TrpC5 dependent regulation of metabolism warrants further investigation.
In summary, activation of arcuate Pomc neurons results in a negative energy balance (Aponte et al., 2011; Zhan et al., 2013). Numerous peptides and neurotransmitters have been demonstrated to acutely stimulate the activity of arcuate Pomc neurons, including leptin and serotonin. Although several signaling mechanisms have been identified, the channel required for these activities has remained largely undefined. In the current study we demonstrate that TrpC5 subunits are essential for the negative energy balance associated with Pomc neuronal activation. TrpC5 subunits not only link the acute activities of leptin and serotonin receptors in Pomc neurons, but also modify direct effects on basal metabolism. Similar to opto- and chemo-genetic strategies, TrpC5 subunits may provide an endogenous target to manipulate the activity of key neurons involved in the regulation of energy balance and glucose metabolism.
Methods
Animals
Male (4- 16-week-old) pathogen-free CamkIIα-cre, Pomc-creERT2, Pomc-hrGFP∷LepR-cre∷tdtomato (PLT), Pomc-hrGFP∷Ht2Cr-cre∷tdtomato (P2CT), TrpC5KO, and TrpC5fl/y (described below) mice (Berglund et al., 2013; Parton et al., 2007; Ramadori et al., 2010; Riccio et al., 2009; Sohn et al., 2011; Sun et al., 2016) were used for all experiments. All mice were housed under standard laboratory conditions (12 hr on/off; lights on at 7:00 a.m.) and temperature-controlled environment with food and water available ad libitum. All experiments were performed in accordance with the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of Texas Institutional Animal Care and Use Committee.
Generation of a cre-conditional TrpC5 allele (TrpC5fl/y)
The TrpC5 targeted clone obtained from EUCOMM was injected into blastocysts to obtain highly chimeric mice. These mice were then bred to obtain germline transmission of the targeted TrpC5 allele. The TrpC5lox/Y mice were generated following flp-mediated removal of the neo cassette leaving two loxP sequences flanking exons 5 of the TrpC5 gene. Deletion of exon 5 creates a frameshift mutation.
Tamoxifen treatment to induce adult-onset ablation of TrpC5 in Pomc neurons
Tamoxifen (0.15mg/g; Sigma-Aldrich) dissolved in corn oil (Sigma-Aldrich) was administered i.p. for 5 consecutive days to 6-weekold male TrpC5fl/y mice (controls) and TrpC5fl/y × Pomc-cre:ERT2 littermate mice. Corn oil was used as a vehicle control.
Electrophysiology
Whole-cell patch-clamp recordings from Pomc-hrGFP neurons with or without leptin receptors were maintained in hypothalamic slice preparations and data analysis were performed as previously described (Hill et al., 2008). Briefly, 4- to 16-week-old male mice were anesthetized and transcardially perfused with a modified ice-cold artificial CSF (ACSF) (described below), in which an equiosmolar amount of sucrose was substituted for NaCl. The mice were then decapitated, and the entire brain was removed, and immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) ACSF (126 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 5 mM glucose). Coronal sections (250 μm) were cut with a Leica VT1000S Vibratome and then incubated in oxygenated ACSF at room temperature for at least 1 hr before recording. Slices were transferred to the recording chamber and allowed to equilibrate for 10–20 min before recording. The slices were bathed in oxygenated ACSF (32°C–34°C) at a flow rate of ~2 ml/min.
The pipette solution for whole-cell recording was modified to include an intracellular dye (Alexa Fluor 594 or Alexa Fluor 350) for whole-cell recording: 120 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, and 2 mM MgATP, 0.03 mM Alexa Fluor 594 or Alexa Fluor 350 hydrazide dye (pH 7.3). Epifluorescence was briefly used to target fluorescent cells, at which time the light source was switched to infrared differential interference contrast imaging to obtain the whole-cell recording (Zeiss Axioskop FS2 Plus or Nikon FN1 equipped with a fixed stage and a QuantEM:512SC electron-multiplying charge-coupled device camera). Electrophysiological signals were recorded using an Axopatch 700B amplifier (Molecular Devices), low-pass filtered at 2–5 kHz, and analyzed offline on a PC with pCLAMP programs (Molecular Devices). Recording electrodes had resistances of 2.5–5 MΩ when filled with the K-gluconate internal solution. Input resistance was assessed by measuring voltage deflection at the end of the response to a hyperpolarizing rectangular current pulse steps (500 ms of −10 to −50 pA).
Leptin (100 nM; provided by A.F. Parlow, through the National Hormone and Peptide Program), mCPP (4 μM, Sigma Aldrich), and Lorcaserin (4 μM, provided by Kathryn Cunningham), were added to the ACSF for specific experiments. Solutions containing leptin, mCPP, or lorcaserin were typically perfused for 2–4 min. A drug effect was required to be associated temporally with peptide application, and the response had to be stable within a few minutes. A neuron was considered depolarized or hyperpolarized if a change in membrane potential was at least 2 mV in amplitude.
Analyses of leptin-, mCPP- and lorcaserin-induced hypophagia
Leptin (5 mg/kg; provided by A.F. Parlow, through the National Hormone and Peptide Program)), mCPP (3 mg/kg; Sigma-Aldrich), lorcaserin (1, 3, and 6 mg/kg; proved by Kathryn Cunningham), and vehicle (sterile saline) were administered i.p. in a counterbalanced manner to chow-fed 18-hour overnight-fasted mice as previously described (Berglund et al., 2013; Williams et al., 2014; Xu et al., 2010b). Food intake was measured hourly for 6 hours and then a single measurement at 24 hours.
Analyses of lorcaserin-induced alterations in glucose tolerance test (GTT) and insulin tolerance test (ITT)
For GTTs mice were intraperitoneally injected with 1.5 g/kg body weight of dextrose after an overnight fast. Mice were injected with lorcaserin (1.5 mg/kg) or vehicle 45 min prior to glucose injection. We drew blood samples at 0, 15, 30, 60, 90, and 120 min time points and measured glucose levels with a Bayer contour glucometer.
For ITTs, mice were fasted for 3 hours with water ad libitum. Mice were injected with lorcaserin (1.5 mg/kg) or vehicle 45 min prior to insulin injection. After measurement of basal levels of glucose, insulin (1.2 U/kg, Eli Lilly and Company) was administrated i.p. Blood glucose levels were monitored at given time points after insulin injection.
Statistics
Statistical analysis was carried out using GraphPad 5 (GraphPad) software. All data were evaluated using a 2-tailed Student's t test or ANOVA where appropriate with a P value of less than 0.05 being considered significant. In all instances, data are presented as mean ± SEM. Degrees of freedom (DF) for t statistics are marked as t(DF).
Supplementary Material
Highlights.
Leptin- and serotonin-induced activation of arcuate Pomc neurons requires TrpC5 subunits
Acute effects of leptin and serotonin on food intake require TrpC5 subunits
TrpC5 subunits are a physiological mechanism in the CNS to regulate metabolism
Acknowledgments
We thank Dr. Joel K. Elmquist (The University of Texas Southwestern Medical Center, Dallas, Texas, USA) for kindly providing us with the LepR-cre and Pomc-hrGFP mice. We thank Dr. David E. Clapham (Howard Hughes Medical Institute, Boston Children's Hospital, Boston, Massachusetts, USA) for generously providing us with the TrpC5KO mice (Riccio et al., 2009). This work was supported by grants to T.Y. (China Scholarship Council 201406280111), J-W.S (The National Research Foundation of Korea NRF-2015M3A9E7029177 and NRF-2016R1C1B2006614 and from the Korean Ministry of Health and Welfare HI14C1946), J.S (National Natural Science Foundation of China No.81300689 & No.81670783), X.K. (K99 DK106550), K.A.C (K05 DA020087), Y.C. (The National Natural Science Foundation of China #81471049) T.L. (AHA 14SDG20370016), and K.W.W. (R01 DK100699).
Footnotes
Author contributions: K.W.W. conceived and designed the study. Y.G., T.Y., Z.D. and J-W.S. are co-first authors. Y.G. and J-W.S. performed electrophysiological experiments, analyzed data, and wrote the manuscript. T.Y. and Z.D. designed and performed all experiments except electrophysiological experiments, analyzed the data, and wrote the manuscript. J.S. and Y.H. assisted performing experiments. X.K., K.Y., R.W., H.C., H.G. and J.Y. designed experiments and edited the manuscript. K.A.C. provided lorcaserin and edited the manuscript. Y.C., T.L. and K.W.W. are corresponding authors that supervised development of the mouse models, designed experiments, and edited the manuscript. K.W.W. is the lead contact of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, et al. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, Vianna CR, Williams KW, Xu Y, Elmquist JK. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest. 2013;123:5061–5070. doi: 10.1172/JCI70338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Vianna CR, Donato J, Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog Horm Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF, Schutz G. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 2001;31:37–42. doi: 10.1002/gene.1078. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lin YC, Kuo TW, Knight ZA. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, O'Rahilly S. The hormonal control of food intake. Cell. 2007;129:251–262. doi: 10.1016/j.cell.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M, Backberg M, Ovesjo ML, Fisone G, Edwards RH, Fujiyama F, Meister B. Plasma membrane and vesicular glutamate transporter mRNAs/proteins in hypothalamic neurons that regulate body weight. Eur J Neurosci. 2003;18:1265–1278. doi: 10.1046/j.1460-9568.2003.02840.x. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Flier JS. Neuroscience. Regulating energy balance: the substrate strikes back. Science. 2006;312:861–864. doi: 10.1126/science.1127971. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Modern science versus the stigma of obesity. Nat Med. 2004;10:563–569. doi: 10.1038/nm0604-563. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Tecott LH. Contributions of 5-HT(2C) receptors to multiple actions of central serotonin systems. Eur J Pharmacol. 2004;488:1–9. doi: 10.1016/j.ejphar.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circulation research. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Zigman JM, et al. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann N Y Acad Sci. 2003;994:169–174. doi: 10.1111/j.1749-6632.2003.tb03177.x. [DOI] [PubMed] [Google Scholar]
- Hentges ST, Nishiyama M, Overstreet LS, Stenzel-Poore M, Williams JT, Low MJ. GABA release from proopiomelanocortin neurons. J Neurosci. 2004;24:1578–1583. doi: 10.1523/JNEUROSCI.3952-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Otero-Corchon V, Pennock RL, King CM, Low MJ. Proopiomelanocortin expression in both GABA and glutamate neurons. J Neurosci. 2009;29:13684–13690. doi: 10.1523/JNEUROSCI.3770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, Balthasar N, Coppari R, Cantley LC, Kahn BB, et al. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150:4874–4882. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Bechmann I, Naftolin F, Kalra SP, Leranth C. Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res. 1997;756:283–286. doi: 10.1016/s0006-8993(97)00184-4. [DOI] [PubMed] [Google Scholar]
- Kiss J, Csaba Z, Csaki A, Halasz B. Glutamatergic innervation of neuropeptide Y and pro-opiomelanocortin-containing neurons in the hypothalamic arcuate nucleus of the rat. Eur J Neurosci. 2005;21:2111–2119. doi: 10.1111/j.1460-9568.2005.04012.x. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, et al. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature. 2014;507:238–242. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides. 2007;28:214–225. doi: 10.1016/j.peptides.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Martin CK, Redman LM, Zhang J, Sanchez M, Anderson CM, Smith SR, Ravussin E. Lorcaserin, a 5-HT(2C) receptor agonist, reduces body weight by decreasing energy intake without influencing energy expenditure. J Clin Endocrinol Metab. 2011;96:837–845. doi: 10.1210/jc.2010-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- O'Neil PM, Smith SR, Weissman NJ, Fidler MC, Sanchez M, Zhang J, Raether B, Anderson CM, Shanahan WR. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- Ovesjo ML, Gamstedt M, Collin M, Meister B. GABAergic nature of hypothalamic leptin target neurones in the ventromedial arcuate nucleus. J Neuroendocrinol. 2001;13:505–516. doi: 10.1046/j.1365-2826.2001.00662.x. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Ronnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Li Y, Moon J, Kim KS, Smith KS, Rudolph U, Gapon S, Yao GL, Tsvetkov E, Rodig SJ, et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Tschop MH, Wilding JP. Anti-obesity drugs: past, present and future. Disease models & mechanisms. 2012;5:621–626. doi: 10.1242/dmm.009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Sun J, Gao Y, Yao T, Huang Y, He Z, Kong X, Yu KJ, Wang RT, Guo H, Yan J, et al. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol Metab. 2016 doi: 10.1016/j.molmet.2016.08.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Clifton PG, Dourish CT, Tecott LH. Reduced satiating effect of d-fenfluramine in serotonin 5-HT(2C) receptor mutant mice. Psychopharmacology (Berl) 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- Williams KW, Liu T, Kong X, Fukuda M, Deng Y, Berglund ED, Deng Z, Gao Y, Liu T, Sohn JW, et al. Xbp1s in Pomc Neurons Connects ER Stress with Energy Balance and Glucose Homeostasis. Cell Metab. 2014 doi: 10.1016/j.cmet.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Hrabovszky E, Lechan RM. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol. 2013;521:3287–3302. doi: 10.1002/cne.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Berglund ED, Sohn JW, Holland WL, Chuang JC, Fukuda M, Rossi J, Williams KW, Jones JE, Zigman JM, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate insulin sensitivity in liver. Nat Neurosci. 2010a;13:1457–1459. doi: 10.1038/nn.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60:582–589. doi: 10.1016/j.neuron.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Lauzon DA, Anderson JG, Balthasar N, Heisler LK, Zinn AR, Lowell BB, Elmquist JK. A serotonin and melanocortin circuit mediates D-fenfluramine anorexia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010b;30:14630–14634. doi: 10.1523/JNEUROSCI.5412-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CL, Wang Y, Anderson S, Ekker M, Rubenstein JL. Arcuate nucleus expression of NKX2.1 and DLX and lineages expressing these transcription factors in neuropeptide Y(+), proopiomelanocortin(+), and tyrosine hydroxylase(+) neurons in neonatal and adult mice. J Comp Neurol. 2009;517:37–50. doi: 10.1002/cne.22132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltser LM, Seeley RJ, Tschop M. Synaptic plasticity in circuits regulating energy balance. Nat Neurosci. 2012 doi: 10.1038/nn.3219. [DOI] [PubMed] [Google Scholar]
- Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33:3624–3632. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.