Abstract
Purpose of review
The purpose of this article is to review recent literature regarding the use of Hepatitis C virus (HCV) positive donor livers in liver transplantation. Given the prevalence of HCV-positive patients on the waitlist coupled with high waitlist mortality, use of HCV-positive livers may be a means to meet patient needs. This review seeks to primarily answer the following questions: can HCV-positive livers be used safely and effectively? Are new direct acting antiviral medications safe and effective in HCV-positive liver recipients?
Recent findings
Use of HCV-positive donor livers for liver transplantation in HCV-positive recipients is increasing. These donor livers have equivalent patient and graft survival when compared to HCV-negative donor livers in HCV-positive liver transplant recipients. Recent studies suggest that use of direct acting antiviral medications in HCV-positive liver transplant recipients can be successful, although there is insufficient data for their use in recipients of HCV-positive donor livers.
Summary
HCV-positive donor livers may be safely and effectively used in HCV-positive liver transplant recipients. Direct acting antiviral medications appear safe and effective in HCV-positive liver transplant recipients, but data on their efficacy in HCV-positive liver transplant recipients are limited. Future research should focus on the use of HCV-positive donor livers in HCV-negative liver transplant recipients.
Keywords: Hepatitis C virus, liver transplantation, direct acting antivirals, HCV-positive organs, HCV-positive livers, HCV-positive liver transplantation
Introduction
Approximately 2.7–3.9 million people are infected with chronic hepatitis C virus (HCV) in the United States.1 Consequentially, end stage liver disease due to HCV has been the leading indication for liver transplantation in this country (see Table 1).2 Although new direct acting antiviral (DAA) medications have improved safety and efficacy over prior therapies in treating HCV infection, access to these medications is limited by cost. Given the high waitlist mortality for patients with end stage liver disease and an inadequate supply of donor livers to meet this need, there has been increased interest in the use of extended criteria or marginal donor livers. This has included utilizing livers with increased donor age, hepatic steatosis, prolonged ischemia time, donation after cardiac death and hepatitis B seropositivity.3 Given the prevalence of HCV-positive patients on the waitlist, HCV-positive donor livers have also been considered as a means to bridge the gap between donor shortage and patient need. This review will explore the recent evidence for the use of HCV-positive donor livers, including current trends, limitations and future directions in this growing area of transplant hepatology.
Table 1.
Indications for liver transplantation in the United States in 2014 in descending order2
| Leading Indications for liver transplantation |
| Hepatitis C virus (39%) |
| NASH/cryptogenic (17%) |
| Alcoholic liver disease (16%) |
| Other (14%) |
| Primary sclerosing cholangitis (4%) |
| Autoimmune hepatitis (4%) |
| Primary biliary cholangitis (3%) |
Outcomes of HCV-positive donor livers in liver transplantation
Initially, enthusiasm for the use of HCV-positive donor livers in HCV-positive liver transplant (LT) recipients was tempered by concerns over poor graft function and risk of severe viral hepatitis. Yet as the number of HCV-negative donors remained limited and inadequate to service the large number of HCV-positive patients on the waiting list, use of HCV-positive donor livers has become more common.4 Recent studies prior to the advent of DAA medications have demonstrated that use of HCV-positive donor livers offers comparable graft function and patient survival to use of HCV-negative livers in HCV-positive LT recipients.
The largest study to report on outcomes for HCV-positive donor livers in HCV-positive LT recipients analyzed data from the US Organ Procurement and Transplantation Network Scientific Registry.5 Northup et al, in a multivariable analysis that included 934 HCV-positive LT recipients from 1994–2008, demonstrated that there was no difference in overall patient survival between those receiving HCV-positive and HCV-negative donor livers (adjusted hazard ratio for death 1.176 v. 1.165, p=0.91). The donor risk index between the two groups was statistically but not clinically significant (1.82 for HCV-positive donors v. 1.78 for HCV-negative donors, p=0.005). Liver biopsy results were not recorded, so it is not possible to determine if there was more active inflammation or fibrosis in the HCV-positive donor livers. These findings have been replicated in numerous other studies showing no difference in overall survival between HCV-positive and HCV-negative donor livers in HCV-positive LT recipients.6,7
In a multicenter European, case-control study, Ballarin et al compared 63 HCV-positive LT recipients receiving HCV-positive donor livers with 63 LT recipients receiving HCV-negative donor livers. 8 They also found no difference in patient survival at 1 and 5 years (p=0.22 and 0.11, respectively). Allograft pathology showed that HCV-positive donor livers had higher histologic activity index scores (17.5% v. 3.2% for scores 5–8, p=0.01) and stage I fibrosis (52.4% v. 17.5%, p<0.001). They also found that only 42.9% of HCV+ donors were viremic, and these viremic grafts were more likely to have stage I fibrosis (100% v. 16.7%, p<0.001). There was no survival difference between viremic and aviremic grafts. Another study sought to determine the effect of viremia and genotype dominance in HCV-positive LT recipients: O’Leary et al examined HCV viral load in 32 LT recipients from HCV-positive donor grafts.9 Fifteen donor grafts were HCV RNA positive, and nine had discordant HCV genotypes or subtypes between donor and recipient. Analysis of graft survival and fibrosis found no difference between viremic and aviremic grafts. In an analysis of genotype dominance, in 4 of 6 recipients, genotype 1 was found to be dominant when competing with a non-1 genotype. In 2 of 3 recipients, genotype 1a was found to be dominant when competing with mismatched genotype 1 subtypes.
These studies suggest that there is no difference between viremic and aviremic HCV-positive grafts on survival. They also suggest no difference in HCV-positive donors compared to HCV-negative donors in HCV-positive recipients. Historically, the United Network for Organ Sharing (UNOS) has required HCV antibody testing in all organ donors, but over the last several years, the concomitant use of HCV RNA (nucleic acid testing [NAT]) has become nearly uniform across all organ procurement organization. This is in part due to the availability of rapid HCV NAT testing, coupled with reports of transmission of HCV from HCV antibody negative (and even HCV NAT negative) donors.10
Although HCV-positive LT recipients receiving HCV-positive donor livers have comparable survival to those receiving HCV-negative livers, there has been concern about increased risks of graft damage due to accelerated progression of HCV infection. In a retrospective multicenter study, Lai et al found HCV-positive LT recipients had a 58% overall adjusted increased risk of advanced fibrosis (95% CI=1.05–2.36, p=0.03) in patients receiving an HCV-positive liver in comparison to those receiving an HCV-negative liver.11 Further stratification showed that this risk was only seen in donors greater than 45 years-old. HCV infection has also been associated with insulin resistance, and studies have shown that HCV-positive LT recipients have high incidences of post-transplant diabetes.12 These studies highlight the importance of careful donor selection based on hepatic and non-hepatic comorbidities in HCV-positive donor LT recipients. However, treatment with DAA medications in the early post-transplant period may mitigate or eliminate this increased risk of graft damage.
DAA medications for HCV-positive liver transplant recipients
In HCV-positive LT recipients with active infection, HCV recurrence is universal. Such recurrence is associated with worse post-LT survival, as HCV-positive LT recipients have decreased survival in comparison to HCV-negative LT recipients.13 In fact, rates of allograft fibrosis are higher than native liver fibrosis in HCV-positive LT recipients.14 Studies have shown that 20–54% of HCV infected LT recipients develop bridging fibrosis or cirrhosis within 5 years post-transplant in the absence of therapy.15 However, until recently, treatment of recurrent HCV in LT recipients had been limited by available pharmacotherapies. Treatment with pegylated-interferon and ribavirin was poorly tolerated in HCV-positive LT recipients; a systematic review of 19 studies including 611 patients found that the mean SVR in this patient population was only 30.2%, owing in part to adverse events resulting in dose reduction and treatment discontinuation.16 The advent of new DAA medications, however, has revolutionized treatment of HCV, and there has been heightened interest in their utility in treating HCV infection in HCV-positive LT recipients.
Several studies have demonstrated the safety and efficacy of DAAs in HCV-positive LT recipients with compensated HCV infection. In a prospective open-label pilot study, Charlton et al found that in 40 HCV-positive LT recipients with recurrent and compensated HCV infection, treatment with sofosbuvir and ribavirin resulted in sustained virological response at 12 weeks (SVR12) in 70% of patients.17 Sixty-three percent of patients had METAVIR F3-F4 fibrosis, and most (83%) were infected with genotype 1. A phase II study of 34 LT recipients with recurrent genotype 1 HCV infection achieved SVR12 in 96% of patients following treatment with paritaprevir, dasabuvir, ombitasvir and ribavirin.18 All patients had F0-F2 fibrosis. Another multicenter study demonstrated that in 123 HCV-positive LT recipients treated with simeprevir and sofosbuvir with or without ribavirin, 90% achieved SVR with the majority of patients experiencing only mild adverse events.19 Similar to the aforementioned studies, most patients were infected with genotype 1 (95%) and had compensated disease: 30% had F3-F4 fibrosis, and only 4% had decompensated disease.
DAA medications have also been studied in LT recipients with more severe liver disease. In a study of 104 patients with limited life expectancy and severe liver disease including fibrosing cholestatic hepatitis (FCH) and decompensated cirrhosis, Forns et al found that treatment with sofosbuvir and ribavirin, with or without pegylated-interferon, was associated with SVR12 in 59% of patients.20 The investigators found that 57% also reported improved clinical status, although treatment was associated with severe adverse events in 47% of patients, which may have been in part due to the severity of their underlying liver disease. In a study of 125 patients with F3-F4 fibrosis, Dumortier et al found that in patients treated primarily with sofosbuvir and daclatasvir with or without ribavirin (a number of other DAA treatment combinations were also studied), 93% of HCV-positive LT recipients achieved SVR12.21 In an open label study of 229 HCV-positive LT recipients that included 45% with F4 fibrosis (22% CTP A, 23% CTP B), Charlton et al found that 93% of patients achieved SVR12.22 Of the six patients with FCH, all achieved SVR12. The efficacy of DAA medications for FCH in HCV-positive LT recipients was also demonstrated by Leroy et al, in which 22 of 23 patients (96%) with FCH achieved SVR12 following treatment with sofosbuvir and ribavirin with or without PEG-IFN or sofosbuvir and daclatasvir with or without ribavirin.23 There were no deaths after SVR12 during the study period. Fifteen patients (65%) had a serious adverse event, with infection being the most common, but these were thought to be directly related to FCH rather than the DAA medications. These findings have been replicated in other studies showing high rates of SVR12 in HCV-positive LT recipients with varying degrees of fibrosis.2425
Use of DAA medications in LT recipients with recurrent HCV infection is promising (see Table 2). However, the efficacy of these medications in recipients of HCV-positive livers in particular has yet to be established. Additionally, more data are needed for different genotypes, broader spectrums of liver disease severity and use of ribavirin-free regimens.
Table 2.
Evidence for use of direct acting antiviral medications in HCV-positive liver transplant recipients
| Authors | Date | Medications | Genotype 1 | Patient population | SVR12 |
|---|---|---|---|---|---|
| Charlton et al17 | 2015 | Sofosbuvir and ribavirin |
83% | 63% F3-F4 fibrosis, no decompensations |
70% |
| Kwo et al18 | 2014 | Ombitasvir– ABT-450/r, dasabuvir and ribavirin |
100% | All F0-F2 | 97% |
| Pungapong et al19 | 2015 | Simeprevir and sofosbuvir +/− ribavirin |
95% | 30% F3-F4 fibrosis |
90% |
| Forns et al20 | 2015 | Sofosbuvir and ribavirin +/− PEG-IFN |
82% | 50% F4 (compensated or decompensated) or severe hepatitis |
59% |
| Dumortier et al21 | 2016 | Primarily sofosbuvir and daclatasvir +/− ribavirin |
78% | All F3-F4 fibrosis | 93% |
| Charlton et al22 | 2015 | Ledipasvir, sofosbuvir and ribavirin |
99% | 45% F4 fibrosis (22% CTP A, 23% CTP B) |
93% |
| Leroy et al23 | 2015 | Sofosbuvir and ribavirin +/− PEG-IFN or sofosbuvir and daclatasvir +/− ribavirin |
96% | All fibrosing cholestatic hepatitis |
78% |
| Guttierez et al24 | 2015 | Sofosbuvir and simeprevir +/− ribavirin |
100% | 38% F3-F4 fibrosis |
93% |
| Coilly et al25 | 2016 | Sofosbuvir and daclatasvir +/− ribavirin |
80% | 44% F0-F2 fibrosis |
96% |
Liver transplantation for HIV/HCV co-infected patients
Advances in antiretroviral therapy have resulted in significant improvements in long-term survival for patients with HIV. Concurrent with this rising life expectancy has been a rising incidence of chronic comorbidities, including liver disease secondary to HCV. This has brought attention to liver transplantation in HIV/HCV co-infected patients, who have higher mortality rates in comparison to HCV mono-infected patients.26 In fact, patient and graft survival following LT in HIV/HCV co-infected patients has been found to be worse in comparison to HCV mono-infected patients.27
Use of HCV-positive livers in HIV/HCV co-infected patients has been studied. In a prospective study of 89 HIV/HCV co-infected patients, Terrault et al found that 3-year graft and patient survival were significantly lower in these co-infected patients in comparison to HCV mono-infected LT recipients (p<0.001).28 Thirteen percent of patients in the HIV/HCV co-infected cohort received an HCV-positive donor liver, and in this cohort, receipt of an HCV-positive donor liver was a significant predictor of reduced graft survival (HR=2.5, p=0.03). Further data are needed in the use of HCV-positive donor livers in these HIV/HCV co-infected patients, as well as the role of DAA medications in both the pre- and post-transplant setting.
Trends and future directions in HCV-positive donor liver transplantation
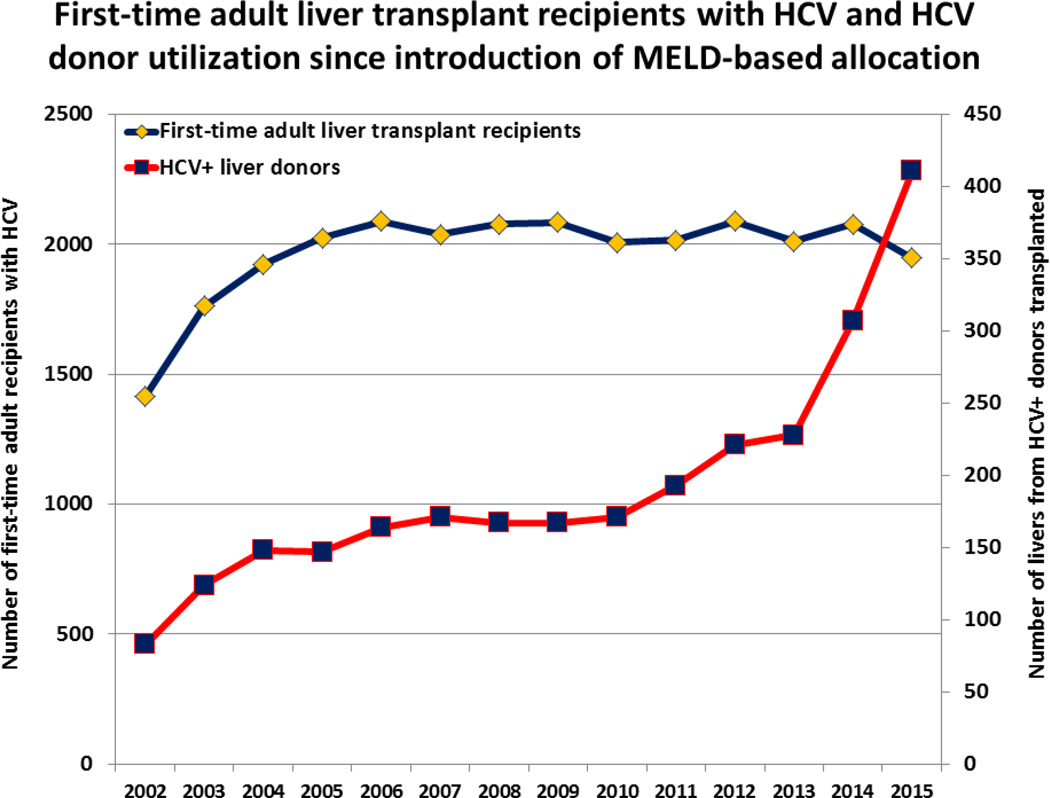
Given the rising consensus that HCV-positive donor livers offer similar efficacy to HCV-negative donor livers in HCV-positive LT recipients, and with the advent of DAA medications showing efficacy in HCV-positive LT recipients, there has been increasing use of HCV-positive donor livers in the United States. Bowring et al examined these trends and found that the proportion of HCV-positive LT recipients receiving an HCV-positive donor liver increased from 6.9% in 2010 to 16.9% in 2015.4 Yet they also found that after 2013, HCV-positive livers were still 1.7 times more likely to be discarded than HCV- livers (p<0.001), highlighting the persistent hesitation limiting utilization. Additionally, more data on genotype dominance in cases of donor and recipient mismatch, including the necessity of pangenotypic therapy in these patients, are needed.
The advent of highly effective DAA medications has raised the possibility of utilizing HCV-positive donor livers for HCV-negative LT recipients. Use of HCV-positive donor kidneys in HCV-negative kidney transplant is currently under investigation in an open-label pilot clinical trial.29,30 There are potential risks in using HCV-positive donor livers in HCV-negative LT recipients. HCV-positive livers could be associated with a higher risk of acute hepatitis and fibrosing cholestatic HCV than in HCV-positive kidney transplant recipients, although immediate post-transplant treatment could mitigate, if not eliminate, this risk. There is insufficient data on outcomes regarding patient and graft survival, and hepatic and non-hepatic comorbidities in this setting. Moreover, the efficacy of DAA medications has yet to be established in HCV-positive donor liver recipients. High cost may also pose a significant barrier for some patients.
However, there has been an increase in donor utilization of HCV-positive livers (see Figure 1). This may in part be attributed to the tragic consequences of the opioid epidemic, in which there has been an increase in the number of deceased donors due to drug overdose. Organs transplanted from these patients, many of whom have risk factors for HCV infection, are underutilized; the mean number of organs transplanted per donor was lower for donors who died from drug overdose than for those who died from gunshot wound, asphyxiation and blunt trauma (P<0.001).31 Use of HCV-positive livers may allow for increased utilization of organs from this growing potential donor group. However, any use of HCV-positive livers in HCV-negative LT recipients would clearly require a robust informed-consent process with careful patient selection, but given the high wait list mortality for patients with end stage liver disease, these risks may be acceptable for some.
Figure 1.
First-time adult liver transplant recipients with HCV and HCV donor utilization since introduction of MELD-based allocation
Conclusions
The prevalence of end stage liver disease due to chronic HCV, concurrent with a shortage of donor livers, underscores the importance of considering the utilization of HCV-positive livers in liver transplantation. Numerous studies have demonstrated that use of HCV-positive livers yields equivalent outcomes to use of HCV-negative livers in HCV-positive LT recipients. Recent studies in specific patient cohorts suggest that DAA medications can be successful in HCV-positive LT recipients, although data on their use for different genotypes, in patients with more severe liver disease, and in recipients of HCV-positive livers are needed. Utilization of HCV-positive donor livers has been increasing, but challenges remain. Outcomes for HCV-positive donor livers in HIV/HCV co-infected patients are worse than in HCV-negative donor livers, and access to DAA medications for LT recipients may be constrained by high cost. Use of HCV-positive livers in HCV-negative LT recipients is a potential source of donor livers, and data on patient outcomes are needed. Yet given the burden of end stage liver disease coupled with a shortage of donor livers, use of HCV-positive livers with proper donor and recipient selection, including protocols to optimize the timing and type of DAA medications in the post-transplant period, should be encouraged.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Daniel Bushyhead and David Goldberg each declare no potential conflicts of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
- 1.Viral Hepatitis – Statistics & Surveillance. [Accessed 11 Nov 2016];Center for Disease Control and Prevention. 2016 http://www.cdc.gov/hepatitis/statistics/index.htm.
- 2. [Accessed 3 June 2016];Organ Procurement and Transplantation Network – 2014 Data Report. 2016 https://optn.transplant.hrsa.gov/
- 3.Hashimoto K, Miller C. The use of marginal grafts in liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:92. doi: 10.1007/s00534-007-1300-z. [DOI] [PubMed] [Google Scholar]
- 4.Bowring MG, Kucirka LM, Massie AB, Luo X, Cameron A, Sulkowski M, et al. Changes in Utilization and Discard of Hepatitis C–Infected Donor Livers in the Recent Era. Am J Transplant. 2016 doi: 10.1111/ajt.13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Northup PG, Argo CK, Nguyen DT, McBride MA, Kumer SC, Schmitt TM, et al. Liver allografts from hepatitis C positive donors can offer good outcomes in hepatitis C positive recipients: a US National Transplant Registry analysis. Transplant International. 2010;23:1038–1044. doi: 10.1111/j.1432-2277.2010.01092.x. This is the largest study to analyze outcomes for HCV-positive donor livers in HCV-positive liver transplant recipients.
- 6.Marroquin CE, Marino G, Kuo PC, Plotkin JS, Rustgi VK, Lu AD, et al. Transplantation of hepatitis C-positive livers in hepatitis C-positive patients is equivalent to transplanting hepatitis C-negative livers. Liver Transpl. 2001;7:762–768. doi: 10.1053/jlts.2001.27088. [DOI] [PubMed] [Google Scholar]
- 7.Álvaro E, Abradelo M, Fuertes A, Manrique A, Colina F, Alegre C, et al. Liver transplantation from anti-hepatitis C virus-positive donors: our experience. Transplant Proc. 2012;44:1475–1478. doi: 10.1016/j.transproceed.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Ballarin R, Cucchetti A, Spaggiari M, Montalti R, Di Benedetto F, Nadalin S, et al. Long-Term Follow-Up and Outcome of Liver Transplantation From Anti-Hepatitis C Virus-Positive Donors: A European Multicentric Case-Control Study Transplantation. 2011;91:1265–1272. doi: 10.1097/TP.0b013e318219eb8f. [DOI] [PubMed] [Google Scholar]
- 9.O’Leary JG, Neri MA, Trotter JF, Davis GL, Klintmalm GB. Utilization of hepatitis C antibody-positive livers: genotype dominance is virally determined. Transplant International. 2012;25:825–829. doi: 10.1111/j.1432-2277.2012.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tugwell BD, Patel PR, Williams IT, Hedberg K, Chai F, Nainan O, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Annals of Internal Medicine. 2005;143:648–654. doi: 10.7326/0003-4819-143-9-200511010-00008. [DOI] [PubMed] [Google Scholar]
- 11.Lai JC, O'Leary JG, Trotter JF, Verna EC, Brown RS, Stravitz RT, et al. Risk of advanced fibrosis with grafts from hepatitis C antibody–positive donors: A multicenter cohort study. Liver Transpl. 2012;18:532–538. doi: 10.1002/lt.23396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gane EJ. Diabetes mellitus following liver transplantation in patients with hepatitis C virus: risks and consequences. Am J Transplant. 2012;12:531–538. doi: 10.1111/j.1600-6143.2011.03854.x. [DOI] [PubMed] [Google Scholar]
- 13.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver Transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 14.Coilly A, Roche B, Samuel D. Current management and perspectives for HCV recurrence after liver transplantation. Liver Int. 2013;33:56–62. doi: 10.1111/liv.12062. [DOI] [PubMed] [Google Scholar]
- 15.Berenguer M, Schuppan D. Progression of liver fibrosis in post-transplant hepatitis C: Mechanisms, assessment and treatment. J Hepatol. 2013;58:1028–1041. doi: 10.1016/j.jhep.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Berenguer M. Systematic review of the treatment of established recurrent hepatitis c with pegylated interferon in combination with ribavirin. J Hepatol. 2008;49:274–287. doi: 10.1016/j.jhep.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Charlton M, Gane E, Manns MP, Brown RS, Curry MP, Kwo PY, et al. Sofosbuvir and Ribavirin for Treatment of Compensated Recurrent Hepatitis C Virus Infection After Liver Transplantation. Gastroenterology. 2015;148:108–117. doi: 10.1053/j.gastro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R, et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med. 2014;371:2375–2382. doi: 10.1056/NEJMoa1408921. [DOI] [PubMed] [Google Scholar]
- 19.Pungpapong S, Aqel B, Leise M, Werner KT, Murphy JL, Henry TM, et al. Multicenter experience using simeprevir and sofosbuvir with or without ribavirin to treat hepatitis C genotype 1 after liver transplant. Hepatology. 2015;61:1880–1886. doi: 10.1002/hep.27770. [DOI] [PubMed] [Google Scholar]
- 20. Forns X, Charlton M, Denning J, McHutchison JG, Symonds WT, Brainard D, et al. Sofosbuvir compassionate use program for patients with severe recurrent hepatitis C after liver transplantation. Hepatology. 2015;61:1485–1494. doi: 10.1002/hep.27681. This article examined the safety and efficacy of direct acting antiviral medications in patients with severe liver disease.
- 21.Dumortier J, Leroy V, Duvoux C, de Ledinghen V, Francoz C, Houssel-Debry P, et al. Sofosbuvir-based treatment of hepatitis C with severe fibrosis (METAVIR F3/F4) after liver transplantation. Liver Transpl. 2016;22:1367–1378. doi: 10.1002/lt.24505. [DOI] [PubMed] [Google Scholar]
- 22.Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Leroy V, Dumortier J, Coilly A, Sebagh M, Fougerou-Leurent C, Radenne S, et al. Efficacy of Sofosbuvir and Daclatasvir in Patients With Fibrosing Cholestatic Hepatitis C After Liver Transplantation. Clin Gastroenterol Hepatol. 2015;13:1993–2001. doi: 10.1016/j.cgh.2015.05.030. e1-2. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez JA, Carrion AF, Avalos D, O'Brien C, Martin P, Bhamidimarri KR, et al. Sofosbuvir and simeprevir for treatment of hepatitis C virus infection in liver transplant recipients. Liver Transpl. 2015;21:823–830. doi: 10.1002/lt.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coilly A, Fougerou-Leurent C, de Ledinghen V, Houssel-Debry P, Duvoux C, Di Martino V, et al. Multicentre experience using daclatasvir and sofosbuvir to treat hepatitis C recurrence – The ANRS CUPILT study. J Hepatol. 2016;65:711–718. doi: 10.1016/j.jhep.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 26.Araiz JJ, Serrano MT, García-Gil FA, Lacruz EM, Lorente S, Sánchez JI, et al. Intention-to-treat survival analysis of hepatitis C virus/human immunodeficiency virus coinfected liver transplant: Is it the waiting list? Liver Transpl. 2016;22:1186–1196. doi: 10.1002/lt.24474. [DOI] [PubMed] [Google Scholar]
- 27.Stock PG, Terrault NA. Human immunodeficiency virus and liver transplantation: Hepatitis C is the last hurdle. Hepatology. 2015;61:1747–1754. doi: 10.1002/hep.27553. [DOI] [PubMed] [Google Scholar]
- 28.Terrault NA, Roland ME, Schiano T, Dove L, Wong MT, Poordad F, et al. Outcomes of liver transplant recipients with hepatitis C and human immunodeficiency virus coinfection. Liver Transpl. 2012;18:716–726. doi: 10.1002/lt.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reese PP, Abt PL, Blumberg EA, Goldberg DS. Transplanting Hepatitis C-Positive Kidneys. N Engl J Med. 2015;373:303–305. doi: 10.1056/NEJMp1505074. [DOI] [PubMed] [Google Scholar]
- 30.Zepatier For Treatment Of Hepatitis C-Negative Patients Who Receive Kidney Transplants From Hepatitis C-Positive Donors (HCV) [Accessed 20 November 2016]; ClinicalTrials.gov. 2016
- 31.Goldberg DS, Blumberg E, McCauley M. Abt P and Levine M5. Improving Organ Utilization to Help Overcome the Tragedies of the Opioid Epidemic. Am J Transplant. 2016 doi: 10.1111/ajt.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]