Abstract
Background
Systemic thrombolysis (ST) and catheter-directed intervention (CDI) are both used in the treatment of acute pulmonary embolism (PE), but the comparative outcomes of these two therapies remain unclear. The objective of this study was to compare short-term mortality and safety outcomes between the two treatments using a large national database.
Methods
Patients presenting with acute PE were identified in the National Inpatient Sample from 2009–2012. Comorbidities, clinical characteristics, and invasive procedures were identified using International Classification of Diseases version 9 (ICD-9) codes and the Elixhauser comorbidity index. To adjust for anticipated baseline differences between the two treatment groups, propensity score matching was used to create a matched ST cohort with clinical and comorbid characteristics similar to the CDI cohort. Subgroups of patients with and without hemodynamic shock were analyzed separately. Primary outcomes were in-hospital mortality, overall bleeding risk, and hemorrhagic stroke risk.
Results
Of 263,955 subjects with acute PE, 1.63% (n=4272) received ST and 0.55% (n=1455) received CDI. ST subjects were older, had more chronic comorbidities, and higher rates of respiratory failure (ST: 27.9%, n=1192; CDI: 21.2%, n=308; P<.001) and shock (ST: 18.2%, n=779; CDI: 12%, n=174; P<.001). CDI subjects had higher rates of concurrent deep venous thrombosis (ST: 35.8%, n=1530; CDI 45.9%, n=668; P<.001) and vena cava filter placement (ST: 31.1%, n=1328; CDI: 57%, n=830; P<.001). In the unmatched cohort, ST subjects had higher in-hospital mortality (ST: 16.7%, n=714; CDI: 9.4%, n=136, P<.001) and hemorrhagic stroke rates (ST: 2.2%, n=96; CDI: 1.4%, n=20; P=.041). After propensity matching, 1434 patients remained in each cohort; baseline characteristics of the matched cohorts did not differ significantly using standardized difference comparisons. Analysis of the matched cohorts did not demonstrate a significant effect of CDI on in-hospital mortality or overall bleeding risk but did show a significant protective effect against hemorrhagic stroke compared to ST (OR 0.47, 95% CI 0.27–0.82, P=.01). Subgroup analysis showed decreased odds of hemorrhagic stroke for CDI in the non-shock subgroup, and increased procedural bleeding for CDI but no difference in hemorrhagic stroke risk in the shock subgroup.
Conclusions
Systemic thrombolysis for acute pulmonary embolism may not improve in-hospital mortality compared to CDI but increases the overall risk of hemorrhagic stroke compared to catheter-directed intervention. Further prospective studies should examine the comparative effectiveness and safety of these two treatments.
INTRODUCTION
Acute pulmonary embolism (PE) is a morbid condition with a wide severity spectrum, ranging from asymptomatic incidentally detected emboli to large PE causing hemodynamic instability and even death. Acute PE is classified into risk categories based on the presence of hemodynamic shock (high-risk or massive PE) or absence of shock but with evidence of myocardial necrosis or right heart strain (intermediate-risk or submassive PE)1–3. Systemic thrombolysis (ST) remains the current standard of care for high-risk PE and has been advocated4 as the treatment of choice for select intermediate-risk patients.
Catheter-directed interventions (CDI) have recently become popular for the treatment of acute high and intermediate-risk PE due to the potential for decreased bleeding complications compared to systemic thrombolysis, while providing similar efficacy in mortality and improvement in imaging parameters of heart strain. However a paucity of direct comparative studies of CDI and ST exist. The expectation of lower rates of complications and similar effectiveness are based primarily on mechanistic similarities between CDI and ST but with less thrombolytic exposure with CDI, with supporting data from single-arm studies or comparisons with anticoagulation. Therefore the objective of our study is to compare outcomes of CDI and ST in patients with massive or sub-massive PE using a large national database.
METHODS
This study of deidentified national database data was approved and exempted from informed consent by the university institutional review board prior to data acquisition and analysis (IRB# PRO15060452).
Data for the study were acquired from the National Inpatient Sample (NIS) from 2009–2012. The NIS is a dataset containing a 20% sample of nationwide inpatient discharges from US hospitals collected and curated by the Agency for Healthcare Research Quality’s Healthcare Cost and Utilization Project (AHRQ HCUP5). Prior to 2011, this was a collection of all discharges from a 20% sample of hospitals but transitioned to a 20% sample of all discharges with the 2012 dataset, precluding further analysis of center volume data. Diagnoses and procedures were identified using International Classification of Disease, version 9 (ICD-9-CM) coding. Admission and discharge information including in-hospital mortality, length-of-stay, and hospital characteristics are hard-coded in the data. Nationwide population estimates were calculated using sampling weights provided by the AHRQ in order to approximate nationwide hospital prevalence and incidence of these interventions.
Patients with acute PE were identified by ICD-9-CM coding (ICD-9 codes 415.11/13/19): both primary and secondary codes were utilized, increasing sensitivity of identification in order to include patients who may have developed acute PE after admission for another primary diagnosis. Clinical characteristics such as respiratory failure and hemodynamic shock were also identified using ICD-9 diagnosis codes.
Procedures were also identified using ICD-9-CM volume 3 coding. CDI does not have a unique ICD-9 code; the coding for endoluminal intervention (39.79) in the presence of a diagnosis of PE has been previously utilized6 and so was incorporated in this study to identify CDI. In addition, procedure codes for invasive pulmonary angiography (88.43) in conjunction with a same-day administration of thrombolytic (99.10), in the absence of coronary or electrophysiology procedures, were used to identify catheter-directed thrombolysis.
Comparative Analysis
The primary outcomes were in-hospital mortality, overall hemorrhagic complications, and hemorrhagic stroke. Secondary outcomes included additional hemorrhagic events such as gastrointestinal bleed and clinically significant hematoma, as well as hospital length of stay and total charges.
Propensity matching was used in order to balance clinical and comorbid conditions7,8. A propensity model was specified using logistic regression on the odds of receiving CDI compared to ST (Supplement Table I). The predicted probability was used as a propensity score in order to match CDI patients to ST patients with the same clinical and comorbid characteristics using 1:1 greedy matching. Validity of the model to create covariate balance between the matched groups was analyzed using significance testing and standardized differences, demonstrating adequate balancing across propensity-matched treatment groups (Supplemental Figure 1). Sensitivity of the propensity model to an unmeasured confounder was assessed using the bounding method described by Rosenbaum9,10.
Exploratory subgroup analyses of high and intermediate-risk patients were performed. High-risk PE patients were identified using ICD-9 coding of hemodynamic shock. Intermediate-risk patients, however, are more difficult to identify as classification is based on disease and imaging-specific information not present in the NIS. By assuming that those receiving systemic thrombolysis or CDI were either at high or intermediate-risk (i.e, no or very few low-risk patients would receive thrombolysis), removing the high-risk patients, then matching ST patients to those remaining in the lysis cohort, matched subgroups were created approximating those at intermediate-risk.
Statistical Methods
Statistical analyses were performed using Stata SE 13.1 (StataCorp, College Station, TX). Normality was assessed qualitatively. Unadjusted demographic and outcome comparisons ere performed using chi-square, student t-test, Fisher exact, Wilcoxon rank-sum, and Kruskal-Wallis testing where appropriate. Paired t-testing, McNemar test, standardized differences, and binomial-family generalized estimating equation regression with logit link and robust standard errors were used for analysis of matched outcomes.
RESULTS
Using NIS datasets from 2009–2012, we identified 263,955 patient admissions with a diagnosis of acute PE. A minority (n=5727) underwent treatment with ST or CDI: 4272(75%) received ST and 1455(25%) CDI. The average age was 57.1±16.7 years, and 50% were male. A quarter of patients had respiratory failure (n=1500, 26%) and 953 (17%) carried an ICD-9 diagnosis of hemodynamic shock. Compared to patients receiving systemic thrombolysis, patients receiving CDI were significantly younger, were more often male, and had lower rates of respiratory failure, hemodynamic shock, and overall chronic comorbidities including heart failure, hypertension, diabetes, and renal failure (Table I). The median time to initiation of therapy was 1 day for both the CDI (IQR 1–1 days) and ST (IQR 0–2 days; P<.001).
Table 1.
Unmatched Cohort Characteristics
| Total | ST | CDI | P | |
|---|---|---|---|---|
| N=5727 | N=4272 | N=1455 | ||
| Age | 57.13±16.7 | 57.41±16.7 | 56.32±16.7 | .032 |
| Female | 2849 (49.8) | 2195 (51.4) | 654 (44.9) | <.001 |
| Hypertension | 2890 (50.5) | 2198 (51.5) | 692 (47.6) | .010 |
| Congestive Heart Failure | 776 (13.5) | 621 (14.5) | 155 (10.7) | <.001 |
| Diabetes Mellitus | 1185 (20.7) | 932 (21.8) | 253 (17.4) | <.001 |
| Chronic Renal Failure | 516 (9.0) | 421 (9.9) | 95 (6.5) | <.001 |
| Emphysema | 1029 (18.0) | 777 (18.2) | 252 (17.3) | .46 |
| Cancer | 618 (10.8) | 446 (10.4) | 172 (11.8) | .14 |
| Respiratory Failure | 1500 (26.2) | 1192 (27.9) | 308 (21.2) | <.001 |
| Hypotension | 953 (16.6) | 779 (18.2) | 174 (12.0) | <.001 |
| Deep Venous Thrombosis | 2198 (38.4) | 1530 (35.8) | 668 (45.9) | <.001 |
| Vena Cava Filter Placement | 2158 (37.7) | 1328 (31.1) | 830 (57.0) | <.001 |
all values are mean±sd or N(%).
The overall unadjusted in-hospital mortality rate was 15% (n=850), and was significantly higher for the ST group (ST: 17%, n=714; CDI: 9%, n=136; P<.001). Overall unadjusted rates of major hemorrhagic complications did not differ between groups (ST: 7.8%, n=335; CDI: 8.5%, n=123; P=.5), but CDI carried a lower rate of intracranial hemorrhage (CDI: 1.4%, n=20; ST: 2.2%, n=96; P=.04) and higher rates of gastrointestinal bleed (CDI: 4.8%, n=70; ST: 3.5%, n=151; P=.03) and hematoma (CDI: 3.8%, n=55; ST: 2.6%, n=111; P=.02) compared to ST (Table II). CDI patients also had higher unadjusted length of stay (CDI: 8d [5–14]; ST: median 7d, IQR [4–12]; P<.001) and higher total charges (CDI: $103,919 [$64,760–$180,638]; ST: median $73,757, IQR[$46,051–$128,641]; P<.001). CDI subjects had higher rates of concurrent deep venous thrombosis (ST: 35.8%, n=1530; CDI 45.9%, n=668; P<.001) and vena cava filter placement (ST: 31.1%, n=1328; CDI: 57%, n=830; P<.001)
Table 2.
Unmatched Cohort Outcomes
| ST | CDI | P | |
|---|---|---|---|
| N=4272 | N=1455 | ||
| In-Hospital Mortality | 714 (16.7) | 136 (9.4) | <.001 |
| Any Major Bleed | 335 (7.8) | 123 (8.5) | .46 |
| Intracranial Hemorrhage | 96 (2.2) | 20 (1.4) | .041 |
| GI Bleed | 151 (3.5) | 70 (4.8) | .029 |
| Procedural Hematoma | 111 (2.6) | 55 (3.8) | .020 |
| Discharge Location | <.001 | ||
| Home | 2674 (62.6) | 1025 (70.4) | |
| Skilled or Rehab Facility | 882 (20.7) | 293 (20.2) |
all values are N(%).
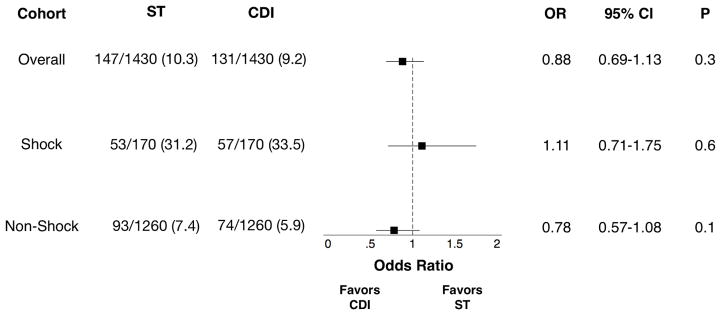
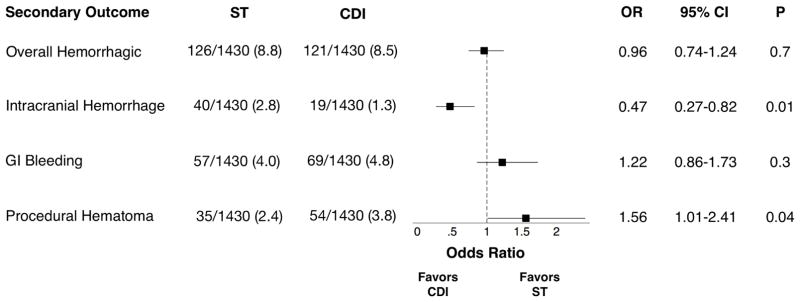
After propensity matching, 1430 patients remained in each group (Table III). Matched mortality did not differ between CDI and ST overall, or in the high-risk or intermediate-risk subgroups (Figure 1). Odds of overall hemorrhagic complication also did not differ between CDI and ST; however, the odds of intracranial hemorrhage were significantly lower for CDI compared to ST, at an expense of higher odds of hematoma in the CDI group (Figure 2). Length of stay remained higher in the CDI group (CDI: median 8d, IQR [5–14]; ST: 7d [5–12]; P=.004) as did total charges (CDI: median $103,934, IQR [$64,593–$180,636]; ST: $82,025 [$50,501–$151,698]; P<.001).
Table 3.
Matched Cohort Characteristics
| ST | CDI | P | Std Diff | |
|---|---|---|---|---|
| N=1430 | N=1430 | |||
| Age | 55.80 ± 16.5 | 56.39 ± 16.7 | .34 | 0.02 |
| Female | 647 (45.2) | 641 (44.8) | .82 | 0.01 |
| HTN | 671 (46.9) | 683 (47.8) | .65 | 0.04 |
| CHF | 121 (8.5) | 153 (10.7) | .042 | 0.03 |
| DM | 227 (15.9) | 247 (17.3) | .31 | 0.04 |
| Chronic Renal Failure | 88 (6.2) | 94 (6.6) | .65 | 0.01 |
| COPD | 242 (16.9) | 248 (17.3) | .77 | 0.01 |
| Any Cancer | 170 (11.9) | 170 (11.9) | 1.00 | 0.01 |
| Respiratory Failure | 293 (20.5) | 302 (21.1) | .68 | 0.02 |
| Hypotension | 161 (11.3) | 170 (11.9) | .60 | 0.07 |
| DVT | 655 (45.8) | 658 (46.0) | .91 | 0.01 |
| IVC Filter Placement | 804 (56.2) | 819 (57.3) | .57 | 0.03 |
all values are mean±sd or N(%). A standardized difference of <0.1 suggests adequate variable balance after propensitymatching. Std Diff: standardized differences.
Figure 1. Matched In-Hospital Mortality.
OR: odds ratio. CI: confidence interval. ST: systemic thrombolysis. CDI: catheter-directed intervention. ST and CDI columns are mortalities/total cohort (N%).
Figure 2. Matched Secondary Outcomes.
OR: odds ratio. CI: confidence interval. ST: systemic thrombolysis. CDI: catheter-directed intervention. ST and CDI columns are events/total cohort (N%)
DISCUSSION
Systemic thrombolysis has been established as the standard of care for patients with high-risk PE, but the role of CDI for high risk patients and ST for intermediate-risk patients is poorly defined1–3. Regardless, the usage of ST even in patients with hemodynamic shock is inconsistent and is though to be underutilized nationwide6, potentially due to the risk of severe hemorrhagic complications including intracranial hemorrhage. Catheter-directed interventions provide a viable alternative based on the presumption that these interventions provide a similar benefit as ST while decreasing the risk of major hemorrhagic complication by decreasing thrombolytic dose, increasing the thrombolytic infusion time, or utilizing mechanical thrombectomy in place of or adjunctive to thrombolysis11,12. However, these differences have yet to be consistently demonstrated as few head-to-head studies have been performed.
Our study compared mortality and major hemorrhagic complications of CDI to ST using a propensity-matched cohort to create comparable cohorts balanced in clinical and comorbid characteristics. Our results showed a decreased mortality for CDI compared to ST in the unmatched cohort due to the overall higher acuity of patients receiving ST. However, after matching, mortality did not differ between CDI and ST in the overall cohort or in either of the subgroups (high-risk, intermediate-risk).
Our finding of comparable mortality after adjustment by propensity-matching is not surprising based on mechanistic similarities between CDI and ST13; however, this has not been well demonstrated in the literature as comparative effectiveness studies of CDI are limited14. A recent study by Patel and colleagues15 using NIS data found that CDI provided a significant benefit for in-hospital mortality compared to ST, with nearly a 40% relative risk reduction and significant decrease in odds (CDI: 13.36%; ST: 21.81%; OR 0.55, 95% CI [0.36–0.85]; P=.007). Our methodology differed from theirs in terms of patient selection, resulting in a larger amount of patients in our study and reflecting a higher sensitivity of identification of acute PE.
The finding of improved mortality in the Patel study is difficult to interpret considering the difficulty of demonstrating improved efficacy of systemic thrombolysis just in comparison to anticoagulation alone in previous trials. These studies have definitively shown improvement after ST in clinical surrogates such as RV function and RV/LV ratio16. However, they have not consistently individually demonstrated improvement in mortality attributable to ST when compared to anticoagulation alone, requiring meta-analysis of pooled data from randomized trials to demonstrate a mortality benefit for ST17,18. An analysis of NIS data prior to 2009 by Stein and colleagues6 found lower case-fatality rates for those with high-risk PE receiving ST, but the study design did not include adjustment for confounding covariates, limiting the ability to draw causal inference.
Likewise, benefits for CDI in improving right heart function have been quantified in several studies11,12,14,19,20 but improvement in mortality compared to anticoagulation has not been demonstrated. This casts into doubt the accuracy of results claiming significant improvement in in-hospital mortality for CDI compared to ST in matched cohorts, especially when the efficacy difference between the two treatments is yet unknown but is unlikely to be large, if presuming efficacy of CDI based on mechanistic similarities13. Our results showing no difference between the two treatments for improvement of in-hospital mortality suggest that benefit, if it exists, may be found in outcome measures other than mortality.
Conversely, our results showed a higher rate of intracranial hemorrhage for those receiving ST. This is supported by rough historical comparison of single-arm and comparative anticoagulation studies suggesting that CDI carries a decreased risk of bleeding relative to ST, although head-to-head prospective studies comparing ST and CDI have not been performed. Previous studies pooling randomized trials of thrombolytic therapy for acute PE have showed an intracranial bleeding risk17,18,21 between 1.5–2.2%, compared to almost no reported intracranial hemorrhagic episodes in retrospective or prospective single-arm studies of catheter-directed intervention11,12,14,19,20. This is offset by a reportedly higher risk of major and moderate hemorrhagic complications, as demonstrated in our results and supported by recent findings in the SEATTLE II trial19, a single-arm study evaluating the effects of catheter-directed thrombolysis in 149 patients. This study showed a 10% rate of major bleeding complications overall (1% GUSTO22 major and 9% GUSTO moderate).
Our findings suggest that pursuit of a CDI-first strategy in patients stable enough to tolerate the time required to initiate a catheter-directed intervention may be beneficial, due to similar mortality results with systemic thrombolysis with a decreased risk of intracranial hemorrhage. Although this is offset somewhat by the higher incidence of other bleeding complications, previous evidence has shown that these hemorrhagic events tend to be mild and of minimal clinical consequences19,20.
This study is limited by the dataset used for analysis. Although the NIS contains a wealth of clinical data, the granularity and temporal specifics are limited by the use of ICD-9 coding. The limitations of NIS for diagnosis of PE has been previously reported, and the usage of secondary coding positions for identification of acute diagnoses carries a known tradeoff of specificity for sensitivity. In addition, certain selection biases may be present, as bias due to unmeasured disease-specific covariates may confound any analysis despite adjustment. Selection bias may also arise from the types of procedures and patient populations performed, as many patients with severe cardiogenic shock and hypotension may not have had time to receive catheter-directed intervention and so received systemic thrombolysis as primary therapy. The accuracy of classification of high-risk and intermediate-risk groups is predisposed to some unknown level of misclassification despite being performed to the best extent possible given data coding. The extent of risk identification for ICH in the clinical setting is also unable to be determined, leaving potentially relevant factors such as magnitude of hypertension and medication or anticoagulant usage unable to be assessed. The two cohorts demonstrated several important differences at baseline, notably the increased rate of deep venous thrombosis and placement of vena cava filters. The increased rate of DVT in the CDI group is difficult to interpret in the absence of further data granularity but may potentially be due to selection bias from DVT screening, or even due to the effect of systemic thrombolytics on deep venous thrombus. The increased rate of vena cava filter placement likewise is difficult to explain or interpret but may be due in part to filter placement for protection during CDI cases. Despite these limitations, we believe that the study design, large sample size, the continued proliferation of catheter-directed interventions, and use of hard endpoints and propensity-based adjustment mechanisms allows for a reasonable interpretation of these results to be used in clinical practice.
CONCLUSIONS
This study does not demonstrate any differences for in-hospital mortality between systemic thrombolysis and catheter-directed intervention in the treatment of acute massive or submassive pulmonary embolism in a propensity matched cohort of patients. However, systemic thrombolysis carries significantly increased odds of intracranial hemorrhagic complications compared to catheter-directed intervention. Based on the results of this study, a first-line strategy of catheter-directed intervention should be considered an acceptable option whenever feasible and safe. Further prospective studies should attempt to corroborate these findings in patients suitable for either intervention.
Supplementary Material
Footnotes
Presented at the plenary session of the 2016 American Venous Forum meeting, Orlando, FL, February 2016.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–830. doi: 10.1161/CIR.0b013e318214914f. [DOI] [PubMed] [Google Scholar]
- 2.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–69. 3069a–3069k. doi: 10.1093/eurheartj/ehu283. [DOI] [PubMed] [Google Scholar]
- 4.Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer-Westendorf J, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–11. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 5.Healthcare Cost and Utilization Project (HCUP) Rockville M: HCUP National Inpatient Sample (NIS); 2012. [Google Scholar]
- 6.Stein PD, Matta F. Thrombolytic Therapy in Unstable Patients with Acute Pulmonary Embolism: Saves Lives but Underused. Am J Med. 2012;125(5):465–70. doi: 10.1016/j.amjmed.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Imbens GW, Rubin DB. Causal Inference for Statistics, Social, and Biomedical Sciences. 2015. [Google Scholar]
- 8.Austin PC, Schuster T. The performance of different propensity score methods for estimating absolute effects of treatments on survival outcomes: A simulation study. Stat Methods Med Res. 2014:1–24. doi: 10.1177/0962280213519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenbaum PR. Observational Studies. 2002. [Google Scholar]
- 10.Becker S, Caliendo M. Sensitivity analysis for average treatment effects. stata J. 2007;7(1):71–83. [Google Scholar]
- 11.Engelberger RP, Kucher N. Ultrasound-assisted thrombolysis for acute pulmonary embolism: a systematic review. Eur Heart J. 2014;35(12):758–64. doi: 10.1093/eurheartj/ehu029. [DOI] [PubMed] [Google Scholar]
- 12.Kuo WT, Gould MK, Louie JD, Rosenberg JK, Sze DY, Hofmann LV. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Interv Radiol. 2009;20(11):1431–40. doi: 10.1016/j.jvir.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Engelhardt TC, Taylor AJ, Simprini LA, Kucher N. Catheter-directed ultrasound-accelerated thrombolysis for the treatment of acute pulmonary embolism. Thromb Res. 2011;128(2):149–54. doi: 10.1016/j.thromres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Kucher N, Boekstegers P, Müller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014;129(4):479–86. doi: 10.1161/CIRCULATIONAHA.113.005544. [DOI] [PubMed] [Google Scholar]
- 15.Patel N, Patel NJ, Agnihotri K, Panaich SS, Thakkar B, Patel A, et al. Utilization of catheter-directed thrombolysis in pulmonary embolism and outcome difference between systemic thrombolysis and catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2015;1227(August) doi: 10.1002/ccd.26108. n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 16.ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med. 2014;164(15):1685–9. doi: 10.1001/archinte.164.15.1685. [DOI] [PubMed] [Google Scholar]
- 17.Wan S, Quinlan DJ, Agnelli G, Eikelboom JW. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation. 2004;110(6):744–9. doi: 10.1161/01.CIR.0000137826.09715.9C. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA. 2014;311(23):2414–21. doi: 10.1001/jama.2014.5990. [DOI] [PubMed] [Google Scholar]
- 19.Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism. JACC Cardiovasc Interv. 2015;8(10):1382–92. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Kuo WT, Banerjee A, Kim PS, DeMarco FJ, Levy JR, Facchini FR, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results from a Prospective Multicenter Registry. Chest. 2015:1–33. doi: 10.1378/chest.15-0119. [DOI] [PubMed] [Google Scholar]
- 21.Kanter DS, Mikkola KM, Patel SR, Parker JA, Goldhaber SZ. Thrombolytic Therapy for Pulmonary Embolism. Chest. 1997;111(5):1241–5. doi: 10.1378/chest.111.5.1241. [DOI] [PubMed] [Google Scholar]
- 22.The GUSTO Investigators. An International Randomized Trial Comparing Four Thrombolytic Strategies for Acute Myocardial Infarction. N Engl J Med. 1993;329(10):673–82. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.