Abstract
Scope
To determine if whole-grain (WG) flour with resistant starch (RS) will produce greater fermentation than isolated RS in obese Zucker Diabetic Fatty (ZDF) rats, and whether greater fermentation results in different microbiota, reduced abdominal fat, and increased insulin sensitivity.
Methods and results
This study utilized four groups fed diets made with either isolated digestible control starch, WG control flour (6.9% RS), isolated RS-rich corn starch (25% RS), or WG corn flour (25% RS). ZDF rats fermented RS and RS-rich WG flour to greatest extent among groups. High-RS groups had increased serum glucagon-like peptide 1 (GLP-1) active. Feeding isolated RS showed greater Bacteroidetes to Firmicutes phyla among groups, and rats consuming low RS diets possessed more bacteria in Lactobacillus genus. However, no differences in abdominal fat were observed, but rats with isolated RS had greatest insulin sensitivity among groups.
Conclusions
Data demonstrated ZDF rats (i) possess a microbiota that fermented RS, and (ii) WG high-RS fermented better than purified RS. However, fermentation and microbiota changes did not translate into reduced abdominal fat. The defective leptin receptor may limit ZDF rats from responding to increased GLP-1 and different microbiota for reducing abdominal fat, but did not prevent improved insulin sensitivity.
Keywords: Fermentation, Leptin signaling, Microbiota, Obese ZDF rats, Resistant starch, Whole-grain
1 Introduction
The health aspects of whole grains (WG) and their potential health benefits were recently reviewed, and epidemiological studies have demonstrated beneficial health effects for WG products [1]. WG contain a variety of compounds including resistant starch (RS) type 1 [2] and other fermentable (e.g. arabinoxylans and oligosaccharides) and nonfermentable (e.g. cellulose and hemicellulose) fibers [3, 4].
Consumption of RS, a carbohydrate that bypasses digestion and absorption in the small intestine and is fermented by colonic bacteria, results in increased empty cecum weight, and reduced cecal pH (<7.0) due to increased short chain fatty acids (SCFAs) [5]. Generation of peptide YY and glucagon-like peptide-1 (GLP-1) may also occur [6]. These products contribute to improved insulin sensitivity, energy expenditure, and beta-oxidation of fatty acids, often leading to reduced body fat [7, 8]. However, Zhou et al. observed no reduction in abdominal fat for polygenic obese mouse models fed low-fat and moderate-fat diets enriched with RS [8].. Identifying rodent models that respond to dietary treatments like RS may have important implications for human health. Our research group proposes that the inability to ferment prebiotic fibers well may be an important attribute of dysbiosis, which is defined as a microbiota associated with obesity and insulin resistance [9].
In rodent research studies, RS is often used as a low glycemic replacement for most of the higher glycemic starches [10]. In many studies, such as one conducted by Belobrajdic et al., experimental diets containing RS have lower energy density compared to control diets. Specifically, they showed that obese-prone Sprague Dawley rats fed RS at 8% of the weight of diet had reduced body fat [11]. Our group believes it is important to test the effect of fermentation alone. The addition of any nondigestible substance can dilute the energy of a diet [12]. We removed the effect of energy dilution in our feeding trials by using isocaloric diets containing high-amylose starch to isolate the effects of fermentation alone without caloric dilution of the diet [5, 7, 13]. We demonstrated that fermentation of high-amylose starch can reduce body fat, improve insulin sensitivity, and affect the microbiota [7].
The obese Zucker Diabetic fatty (ZDF) rat was developed by inbreeding Zucker obese rats with hyperglycemia [14]. Both Zucker and ZDF rats possess a mutation in the extracellular domain of the leptin receptor (Leprfa) that causes them to develop obesity and insulin resistance between 7 and 10 wk of age [15, 16]. However, ZDF rats develop greater insulin resistance and hyperglycemia than their parent strain [14]. Characterization of the ZDF fermentation response has been limited. Two studies in which Zucker obese rats were fed oligofructose indicated that the fermentation response was not strong [17, 18]. An important question is whether this animal model’s genotype has altered the gastrointestinal microbiota making it unresponsive to RS. We tested if the obese ZDF would ferment RS and compared isolated RS with a whole grain form of RS.
Our first aim was to compare a WG source of RS with an isolated source of RS to determine if WG would promote greater fermentation in ZDF rats. In parallel we also determined the composition of the cecal microbiota of the ZDF rats fed high-RS diets or low RS control diets using next generation sequencing. Finally, we measured the percent abdominal fat and insulin sensitivity in order to determine RS-mediated changes in the host phenotype.
2 Materials and methods
2.1 Animal models
The study protocol was approved by the IACUC as 11–049. Four-week-old male ZDF rats (n = 48; Charles River) were fed a standard chow diet during a 7-day quarantine (one rat died after arrival due to renal failure). Rats were housed in wire-mesh cages in a climate-controlled environment (21–22°C, 55% humidity) with a 12:12 h light-dark cycle. Rats were acclimated over 2 wk to a powdered control diet before stratification and administration of experimental diets. Blood glucose and insulin values were determined after a 12-h fast (retroorbital bleed) using glucometer and Millipore’s insulin kit. The homeostatic model of assessment-insulin resistance (HOMA-IR), which equals fasting plasma glucose (mg/dL) multiplied by fasting plasma insulin (µU/mL) and then divided by 2430 [19], was employed to stratify animals into four groups based on body weight and insulin sensitivity [19].
At 7 wk of age, stratification was into four (n = 11–12) isocaloric (3.2 kcal/g) diet groups (Table 1). The diets varied in RS and WG content. Two diets contained isolated starches varying in RS; one was formulated with highly-digestible, amylopectin corn starch (Low-RS-S, n = 12) and the other with high-amylose (approximately 70%) maize RS (Hi-RS-S, n = 12). Two WG flour diets that also varied in RS content were formulated using a dent WG corn flour with approximately 27% amylose content (Low-RS-WG, n = 12), and WG high-amylose corn flour comprised of approximately 70% amylose (Hi-RS-WG, n = 11). The starch and WG ingredients were analyzed by proximate analysis to allow formulation of all the diets with AIN-93M [20] macronutrient amounts, and modified Englyst assay for RS dietary amounts [21]. Energy values for RS were determined previously [22]. Formulation is described in Table 1. The RS contents of the diets were 0% (Low-RS-S), 25% (Hi-RS-S), 6.9% (Low-RS-WG), and 25% (Hi-RS-WG). The amounts of RS used in this study were well-tolerated by rodents in previous studies as evidenced by unaltered or greater food intake [23]. Body weight, food intake, and spillage were measured twice weekly for 11 wk. Fasting serum collections for HOMA-IR calculations for assessing insulin sensitivity were performed at 8 wk after beginning the trial.
Table 1.
Diets used in the study
| Ingredients (g) | Low-RS-Sa) | Hi-RS-S | Low-RS-WG | Hi-RS-WG |
|---|---|---|---|---|
| Waxy corn starchb) | 533.6 | 61.7 | 83.7 | 169.4 |
| High-amylose corn starchc) | 0 | 619 | 0 | 0 |
| Whole-grain high-amylose corn flourd) | 0 | 0 | 0 | 576 |
| Whole-grain dent corn floure) | 0 | 0 | 550 | 0 |
| Sucrose | 100.0 | 100.0 | 100.0 | 100.0 |
| Caseinf) | 136.6 | 135.1 | 93.7 | 84.6 |
| Cellulose | 140.0 | 0 | 108.0 | 15.8 |
| Soybean oilf)with TBHQg) | 40 | 34.4 | 14.8 | 4.4 |
| Mineral mix | 35 | 35 | 35 | 35 |
| Vitamin mix | 10 | 10 | 10 | 10 |
| Choline chlorideh) | 3 | 3 | 3 | 3 |
| L-cystine | 1.8 | 1.8 | 1.8 | 1.8 |
| Total | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Kcal/g | 3.2 | 3.2 | 3.2 | 3.2 |
| RS (% weight)i) | 0 | 25 | 6.9 | 25 |
Acronym letters for the diets include: Low-RS-S, isolated starch control diet; Hi-RS-S, high isolated RS diet that contains high-amylose maize RS type 2; Low-RS-WG, low RS whole-grain control diet that contains whole-grain dent corn flour; Hi-RS-WG, high RS whole-grain flour diet that contains high-amylose whole-grain flour.
AMIOCA® corn starch.
HI-MAIZE® resistant corn starch.
HI-MAIZE® whole-grain corn flour.
Waxy corn starch, high-amylose corn, whole-grain high-amylose corn flour and whole-grain dent corn were all gifts from Ingredion Incorporated (Bridgewater, NJ, USA).
Casein and soybean oil were reduced from AIN-93M amounts (140 and 40 g/kg, respectively) based on the protein and fat in the four experimental diets (as analyzed by proximate analysis performed by Medallion Labs, Minneapolis, MN, USA).
Antioxidant added to soybean oil by Dyets Incorporated at 0.0002 g/g oil.
Choline chloride added at three times the level of choline chloride for the AIN-93M diet (3g/kg versus 1 g/kg of diet).
RS content of the four experimental starch ingredients determined by Ingredion Incorporated using the modified Englyst assay [19].
2.2 Tissue collection
After the 11- wk study, nonfasting rats were euthanized by isoflurane inhalation. Blood was collected in tubes containing DPP-4 inhibitor for measurement of GLP-1 active using ALPCO ELISA kit. The GI tract was removed from base of the esophagus to the anus. The stomach, small intestine, cecum, and rest of large intestine were weighed full, divested of their contents, rinsed, and then reweighed. Peritoneal, retroperitoneal, and epididymal fat pads were collected and weighed, and the sum of all three is referred to as total abdominal fat. Emboweled body weight was calculated as disemboweled body weight (final body weight minus weight of full GI tract) plus the weight of the empty GI tract. Percent abdominal fat was calculated as total abdominal fat divided by emboweled body weight. We used emboweled body weight as the normalizer for abdominal fat rather than body weight with gut contents or disemboweled body weight (removal of gut and gut contents) because it best reflects body mass. Cecal contents were preserved in liquid nitrogen. After thawing, pH was assayed using Mettler Toledo SevenEasy™ pH meter (Model S20), and SCFA concentrations measured using gas–liquid chromatography as described in Charrier et al. [24].
2.3 Microbiota analysis
DNA from 0.2 g of cecal contents was extracted using fecal kits from MP Biomedical. To further purify the DNA extracts they were run through a “Gene Clean Turbo Clean Up” column (MP Biomedical). However, the DNA purity remained suboptimal. Therefore, additional clean-up with 70% alcohol solution and the PureLink® PCR Purification kit from Life Technologies was performed, which produced high-quality DNA.
Sequencing and informatics were performed by the LSU Microbial Genomics Resource Group (http://metagenomics.lsuhsc.edu). The 16S rDNA hypervariable regions V3–V4 were PCR amplified using primers with the gene-specific sequences (V3F CCTACGGGAGGCAGCAG; V4R GGACTACHVGGGTWTCTAAT), Illumina adaptors, and molecular barcodes as described [25] to produce 430bp amplicons that were sequenced on an Illumina MiSeq using the V3 sequencing kit (300 bp paired end reads). The read files were processed through the UPARSE pipeline [26], merging forward and reverse reads and truncating reads to a uniform length of 400 bp, and then removing reads with quality scores less than 11. The pipeline also (i) removed reads that appeared only once throughout all samples (singletons) and remaining unique reads were clustered into Operational Taxonomic Units (OTU) at 97% similarity; (ii) chimeric OTUs were removed as identified against a gold standard reference database of nonchimeric sequences; and finally, the original filtered reads (before dereplication) were mapped to the OTUs using USEARCH at 97% identity. QIIME 1.8 was used to pick and align a representative set. The Ribosomal Database Project (RDP) classifier was used to assign taxonomic classification to each read in the representative set and phylogenetic tree was constructed from representative sequences. Among samples, the minimum read count after filtering was 21 182, with a median read count of 57 537. Relative abundance of each OTU was examined at Phylum, Class, Order, Family, Genus and Species levels. Alpha (within a community) and beta (between communities) diversity metrics as well as taxonomic community assessments were produced using QIIME 1.8 scripts.
2.4 Statistical analysis
Data were analyzed by one-way ANOVA followed by F-protected LSD mean comparison tests using the MIXED procedure of SAS® Version 9.3. A two-way ANOVA was not used because the Low-RS-WG group contained some RS2, which confounded the analysis. The MIXED procedure was used to determine equal or nonequal variance and normal distribution. Data were log transformed if normality assumption was not met. Results were considered significant at p < 0.05 and expressed as means ± pooled SE. For microbiota taxa assessments, the p value for significance was reduced by a Bonferoni adjustment and reported as p < 0.008 because of the many taxa. Correlations were run using PROC CORR.
3 Results
3.1 Markers of degree of fermentation in cecum
Empty cecum weights were different among groups (p < 0.0001) based on increased RS and WG content (Table 2). RS-containing groups (Low-RS-WG, Hi-RS-S, and Hi-RS-WG) had significantly lower cecal contents pH values compared to the non-RS control, and the Hi-RS-WG group had lower pH than the low-RS-WG group, but the Low-RS-WG group pH approached significance compared to the Hi-RS-S group at p < 0.052 (Table 2). All three groups that had some RS in their diets had significantly greater amounts of the three short-chain fatty acids: acetate, propionate, and butyrate (min. of p < 0.002). However, the Hi-RS-WG group had greater amounts than both the Low-RS-WG and Hi-RS-S groups (Table 2).
Table 2.
Dependent variable results from the studya)
| Treatment groupsb) | Low-RS-S | Hi-RS-S | Low-RS-WG | Hi-RS-WG | Pooled SEM |
|---|---|---|---|---|---|
| Empty cecum weights (g) | 0.461 | 0.872 | 0.553 | 1.144 | 0.04 |
| Cecal contents pH | 7.921 | 6.722 | 7.362 | 6.283 | 0.15 |
| Acetate (millimoles/cecum) | 0.0721 | 0.2592 | 0.1622 | 0.5623 | 0.050 |
| Propionate (millimoles/cecum) | 0.0121 | 0.0502 | 0.0262 | 0.1153 | 0.011 |
| Butyrate (millimoles/cecum) | 0.0201 | 0.1432 | 0.0502 | 0.3133 | 0.033 |
| Glucagon-like peptide 1, pM | 0.0511 | 0.1492 | 0.0811 | 0.1742 | 0.028 |
| Energy intake (kcal) | 5550.41 | 6302.42 | 5898.93 | 6352.32 | 112.3 |
| Emboweled body weight (EBW, g)c) | 450.01 | 464.11,2 | 484.32 | 480.82 | 9.5 |
| Abdominal fat (AF, %)AF/EBW × 100 | 7.1 | 7.0 | 7.2 | 7.0 | 0.1 |
| HOMA-IRd) | 31.31 | 17.22 | 30.11 | 26.81,2 | 3.2 |
Significant differences for treatment group dependent variable values with p < 0.05 are indicated by different superscript numbers. Note that the p-value for the difference between the cecal contents pH for Hi-RS-S and Low-RS-WG approached significance at p = 0.052.
Acronym letters for the diets include: Low-RS-S, isolated starch control diet; Hi-RS-S, high isolated RS diet that contains high-amylose maize RS type 2; Low-RS-WG, low-RS whole-grain control diet that contains whole-grain dent corn flour; Hi-RS-WG, high-RS whole-grain flour diet that contains high-amylose whole-grain flour.
Emboweled body weight was calculated as disemboweled body weight (final body weight minus weight of full GI tract) plus the weight of the empty GI tract.
The homeostatic model of assessment-insulin resistance (HOMA-IR), which equals fasting plasma glucose (mg/dL) multiplied by fasting plasma insulin (µU/mL) and then divided by 2430.
3.2 Serum GLP-1 active, energy intake, abdominal fat, insulin sensitivity, body weight
GLP-1 active was greater in groups fed Hi-RS-S and Hi-RS-WG (p < 0.01) compared to the two low-RS groups, but within the two low-RS groups and Hi-RS groups there were no differences (Table 2). All three RS-containing groups consumed more food and energy compared to the Low-RS-S group over the entire study period (p < 0.03, energy intake in Table 2). Rats supplemented with Hi-RS-S or Hi-RS-WG consumed more food and energy than the WG-control group (Low-RS-WG), but not more than each other (energy intake in Table 2). Despite increased food and energy intakes and GLP-1 activity, there were no differences in percent abdominal fat (Table 2). The group with the isolated high RS (Hi-RS-S) had a lower insulin resistance (greater insulin sensitivity as HOMA-IR, Table 2) measured 8 wk into the 11-wk study (Low-RS-S versus Hi-RS-S, p < 0.004; Low-RS-WG versus Hi-RS-S, p < 0.006) than the two control groups, but not lower than the Hi-RS-WG group with the latter comparison approaching significance at p = 0.05. The two control groups were not different from each other. For emboweled body weights the ANOVA F-value approached significance (p = 0.05) among groups (Table 2). However, the three groups consuming RS had numerically greater weights and WG groups were numerically greater than no WG.
3.3 Microbiota assessments
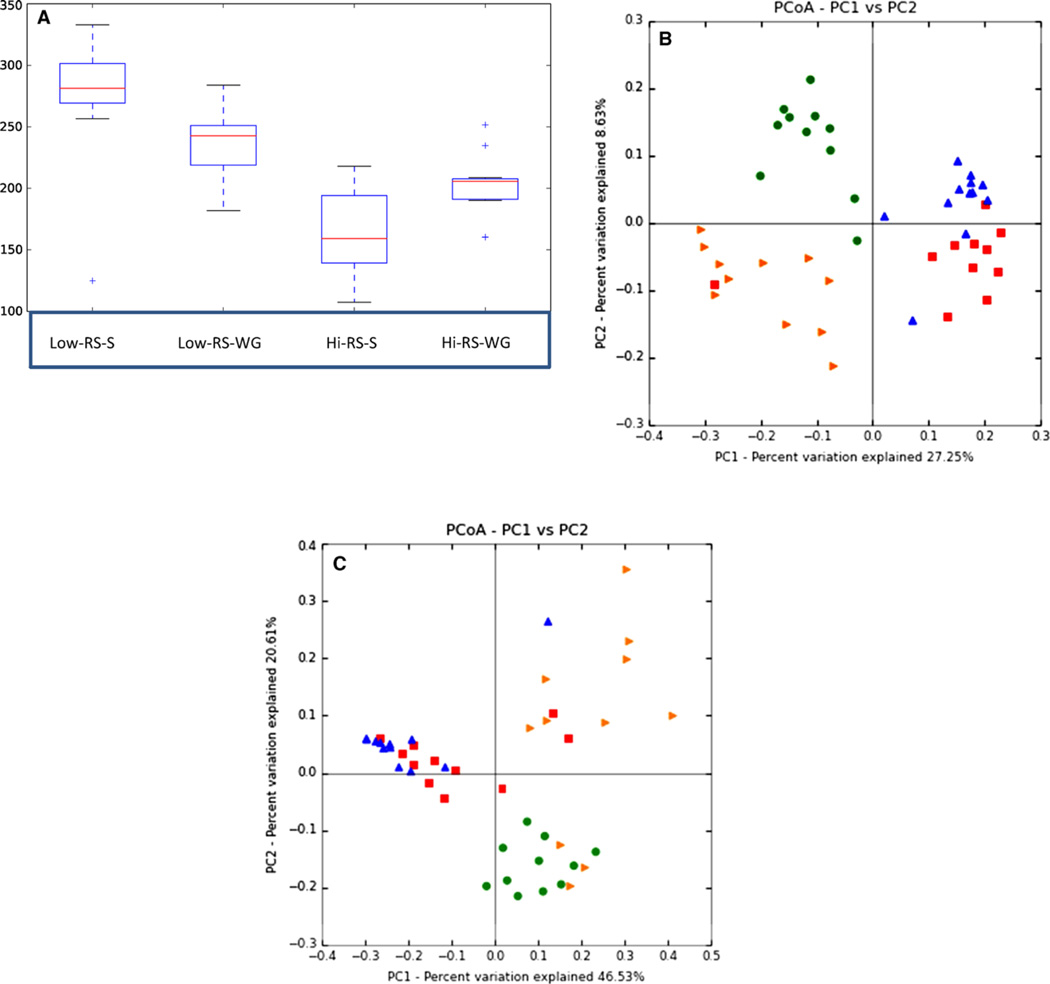
After 11 wk, the microbial community structure of the high-RS groups differed compared to the two low-RS control groups. There were significant diet effects on α-diversity using common metric measures for estimating total species richness that showed lower diversity in the high-RS groups with the mean rank numerical order being Low-RS-S, Low-RS-WG, Hi-RS-WG, and Hi-RS-S (Fig. 1A).
Figure 1.
α-Diversity (chao1) and principal coordinate analysis of the UniFrac β-diversity metrics of the microbiota. Treatments listed in all figure components are: Low-RS-S (diet with amylopectin corn starch), Low-RS-WG (diet with dent whole-grain corn flour), Hi-RS-S (diet with high amylose starch), and Hi-RS-WG (diet with high amylose whole-grain corn flour). (A) α-Diversity in box plots form. Statistical differences were Low-RS-S and Low-RS-WG versus Hi-RS-S p < 0.0006; Low-RS-S versus Hi-RS-WG p < 0.005, and Low-RS-WG versus Hi-RS-WG p < 0.04). (B) Unweighted β-diversity p < 0.0001. (C) Weighted β-diversity p < 0.0001. The legend for β-diversity is: Low-RS-S (squares), Low-RS-WG (upright triangles), Hi-RS-S (right-tilted triangles), Hi-RS-WG (circles).
Principal coordinate analysis of the Unifrac β-diversity metric, which organizes the treatment groups according to the properties of the microbiota, demonstrated that the two high-RS groups (i.e. Hi-RS-WG and Hi-RS-S) clustered separately. The two control groups, Low-RS-S and Low-RS-WG, were different from the high-RS groups, but more similar to each other (especially when displayed weighted). These findings were significant for both the unweighted (presence or absence of an OTU) and weighted (accounts for relative abundance of OTUs) Unifrac analyses (ANOSIM R = 0.6713, p < 0.0001; and R = 0.5913, p < 0.0001, respectively (Fig. 1B and C).
Within the isolated starch groups the high RS had increased bacteria (p < 0.0001) in Bacteroidetes phylum and decreased bacteria in Firmicutes phylum (Table 3 and Supporting Information Fig. 1). In the high-RS groups, WG decreased (p < 0.0001) the Bacteroidetes:Firmicutes ratio, and the Hi-RS-WG had less (p < 0.0001) Firmicutes than the Low-RS-WG group. There were no phyla differences seen in the low-RS groups. At the genus level, several significant differences were noted (Supporting Information Table 1 and Supporting Information Fig. 2). Amounts of Lactobacillus were different among all four groups (all, p < 0.0001). The two control groups had greater amounts than the two high-RS groups and whole-grain groups had greater amounts than purified starches. Other differences included family Lacnospiraceae genus other (p < 0.001, Low-RS-S greater than the other three groups), family S24-7 genus unknown (p < 0.0001, Hi-RS-S greater than the other three groups and Hi-RS-WG was greater than Low-RS-WG, Supporting Information Table 1), family Ruminococcaceae genus other (p < 0.0001, the two high-RS groups were greater than the control groups and Hi-RS-WG was highest), genus SMB53 (p < 0.001, Hi-RS-S greater than other three groups), genus Ruminococcus (p < 0.0001, the two high-RS groups were greater than Low-RS-S, Low-RS-WG = Hi-RS-S, and Hi-RS-WG was greater than Hi-RS-S), and Bacteroides (p < 0.0001, Hi-RS-S group greater than all other groups).
Table 3.
| Taxonomy at phylum level | Percentage of total sequences of all groups | Percentage of total sequences within groups | |||
|---|---|---|---|---|---|
| Low-RS-S | Low-RS-WG | Hi-RS-S | Hi-RS-WG | ||
| Actinobacteria | 2.0 | 1.5 | 1.5 | 0.8 | 4.2 |
| Bacteroidetes | 13.6 | 7.7 | 5.3 | 29.8 | 11.5 |
| Cyanobacteria | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 |
| Deferribacteres | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 |
| Firmicutes | 82.3 | 88.7 | 91.4 | 66.2 | 82.8 |
| Fusobacteria | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Proteobacteria | 0.9 | 0.6 | 0.2 | 1.9 | 0.9 |
| Spirochaetes | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| TM7 | 0.4 | 0.8 | 0.3 | 0.4 | 0.0 |
| Tenericutes | 0.2 | 0.0 | 0.1 | 0.1 | 0.5 |
| Verrucomicrobia | 0.5 | 0.3 | 1.2 | 0.6 | 0.1 |
Treatments listed as: Low-RS-S (diet with amylopectin corn starch), Low-RS-WG (diet with dent whole-grain corn flour), Hi-RS-S (diet with high amylose starch), and Hi-RS-WG (diet with high amylose whole-grain corn flour).
Statistical differences were: bacteroidetes (p < 0.0001, Low-RS-S versus Hi-RS-S, Hi-RS-WG versus Hi-RS-S, Low-RS-WG versus Hi-RS-S); and firmicutes (p < 0.0001, Low-RS-WG versus Hi-RS-WG). The p < 0.05 value was divided by 6 as a Bonferoni adjustment.
A few correlations were run. Serum GLP-1 active was negatively correlated with the Genus Lactobacillus (R = −0.431, p < 0.003) and phylum Firmicutes (R= −0.676, p < 0.0001); and positively correlated with empty cecum weights (R = 0.391, p < 0.009). Also the Genus Lactobacillus was negatively correlated with Phylum Bacteroidetes (R = −0.603, p < 0.0001) and Phylum Firmicutes (R = −0.685, p < 0.0001), but the correlation of empty cecum weight with Phylum Bacteroidetes (R = 0.268, p = 0.08) and Firmicutes (R = 0.255, p < 0.098) only approached significance.
Core microbiota was defined as taxa present in all samples within an experimental group. The groups differed as to which core taxa were represented (Supporting Information Table 2). A core taxa, Genus Staphylococcus identified only in both low-RS groups and Genus Phascolarctobacterium only in high-RS groups; Family Ruminococcaceae as a core taxa, was associated with WG groups. Other core taxa results were as follows: Low-RS-S: Genera Bifidobacterium, Bilophila, and Akkermansia; Low-RS-WG: Genera Anaerotruncus and Oscillospora; Hi-RS-S: Genera SMB53, Ruminococcus, Holdemania, and Sutterella; Hi-RS-WG: Genera Parabacteroides, Prevotella, Collinsella, and rc4-4.
4 Discussion
Several studies have shown different gut microbiota profiles in obese versus lean people or rodents [27–29]. These results may indicate dysbiosis (a dysfunctional microbiota) in the obese [9]. We have previously reported that fermentation of RS occurs in several rodent models [5, 7, 8], but not in two obese mouse models of polygenic obesity, New Zealand Obese (NONcNZO10/LtJ) and NON/ShiLtJ [8]. Considering these results, we hypothesized that an animal model of obesity and diabetes, obese ZDF rats, may be dysbiotic and poorly ferment RS (poor fermentation of RS would be indicated by cecal contents pH usually above 7 and good fermentation below 6.5) [24]. However, our alternate hypothesis was that if obese ZDF rats could ferment RS, then the fermentation would be associated with some reduction in abdominal fat and increased insulin sensitivity in the rats. Several of our previous studies demonstrated reduced body fat was associated with fermentation of RS in studies with Sprague Dawley rats [5, 23, 24], C57BL/6J mice [8], and Goto-Kakazaki (GK) rats [7]. There was also increased insulin sensitivity and pancreatic mass in the (GK) rat, which is a lean model of type 2 diabetes [7]. In the current study, our results, demonstrated that obese ZDF rats possessed an intestinal microbiota capable of fermenting RS. Based on beta-diversity and principal coordinate analysis, we found high-RS and low-RS diets induced a distinctly different microbiota and that WG led to more robust fermentation, greater SCFA production, and lower cecal contents pH, than treatments without WG. There was also a distinctly different microbiota between the two high-RS groups, isolated and WG. WG RS is a source of two types of RS, i.e. RS1 (due to WG structure) and RS2 (granular structure of starch), compared to Hi-RS-S, which only provides RS2. This difference as well as corn bran in WG corn flour having other components, such as arabinoxylans, may have increased the amount of fermentation in the large intestine [3].
Microbiota data from the current study are similar to those from our previous study in agedC57Bl/6J mice supplemented with isolated RS at 0, 18, and 36% of the weight of the diet [13]. Both showed that the isolated high-RS diet induces a higher Bacteroidetes to Firmicutes ratio as is found in lean versus obese rodents and humans [29]. The current study also adds demonstration of greater fermentation with WG products (especially Hi-RS-WG) beyond the isolated starches.
With increased fermentation and changes in microbiota in obese ZDF rats, phenotypic changes such as decreased body fat and improved insulin sensitivity were expected. In previous studies these phenotypic changes were associated with a larger cecum [5, 23, 24, 30] and changes in the microbiota [13,23]. However, we did not observe reduced abdominal fat in the current study in obese ZDF rats. The obese ZDF rats have a genotype that results in a deficiency in leptin signaling; thus, the current study suggests a functional leptin receptor is required to translate the fermentation and microbiota changes into reduced abdominal fat. However, we did not compare the obese ZDF rat with the lean ZDF rat in this study to be able to confirm this speculation. Our results were not totally unexpected because the related obese Zucker rats have previously been reported to lose body weight with energy restriction, but to not lose adipose tissue volume [31].
The mechanism for reduced abdominal fat in our previous studies appears to be increased energy expenditure and fat burning in response to dietary RS [8], possibly through proopiomelanocortin gene expression in the arcuate nucleus of the hypothalamus [32]. The metabolic effects appear related to sustained elevation of serum GLP-1 [6], and elevated GLP-1 active was observed here for RS-fed obese ZDF rats. GLP-1 is reported to regulate food consumption through an interaction with leptin [33], but the interaction in our previous studies may involve the regulation of energy expenditure. In the current study food and energy intake were greater for all three groups fed RS, but may have been offset by an increase in energy expenditure as we previously reported for mice fed RS [8]. However, this would appear to be by some other mechanism than leptin signaling. All the diets were formulated to be isocaloric and we previously determined the metabolizable energy value for the isolated starch product to be 2.8 kcal/g [22]. This consists of about half of the dietary RS not fermented when fed at levels >20% of weight of diet (calculation and experimental data that is unpublished) and the generally accepted approximation of 50% of the energy from fermentable fiber available to the host [34, 35]. The ability of GLP-1 to reduce body fat appears lost with leptin receptor signaling deficiency. The leptin signal is required for weight loss effect in another model, Roux-en-Y gastric bypass surgery. We recently reported leptin-deficient ob/ob mice were resistant to weight loss with Roux-en-Y gastric bypass surgery despite elevated plasma GLP-1 levels [36]. We also demonstrated high levels of plasma GLP-1 in response to DPP4 inhibition by the drug sitigliptin that promoted weight loss in C57Bl/6J mice [37]. The weight loss appears to be dependent on GLP-1 receptor as the effect was not observed in the receptor knockout mice. These results suggest that GLP-1 is able to reduce body weight in wild-type mice when leptin signaling is intact.
In the current study, we did observe improved insulin sensitivity (lower HOMA-IR) with feeding of the isolated RS product (Hi-RS-S) to the obese ZDF rats. The HOMA-IR of this group was significantly lower than the values for the two low RS groups, Low-RS-S and Low-RS-WG, but statistically similar to the Hi-RS-WG group. The Hi-RS-WG group was only numerically lower than the two low RS groups. It is surprising that the effect was not stronger in the Hi-RS-WG group because of a greater fermentation response and a distinctly different microbiota identified by next generation sequencing compared to the Hi-RS-S group. We speculate that one possible reason for the increased insulin sensitivity in the obese ZDF rats fed the isolated high RS product compared to the low RS groups was the reduced diversity of the microbiota. The reduction in diversity could suggest a more-focused bacterial population for fermentation of the single type of fermentable fiber. Also, a major difference in the study for the Hi-RS-S group was a greater amount of bacteria from phylum Bacteroidetes and a lower amount from phylum Firmicutes compared to the other three groups.
Reimer et al. reported a delay in diabetes progression in obese male ZDF rats treated with a viscous and fermentable fiber, PolyGlycopleX (PGX), combined with two drugs, sitagliptin and metformin [38]. Our current study and the Reimer et al. study indicated that improved insulin sensitivity is possible in obese ZDF rats and an isolated prebiotic is effective in improving insulin sensitivity.
Other studies with obese ZDF or obese Zucker rats showed a prebiotic improves phenotypic effects. Koh et al. demonstrated improved kidney health in ZDF rats fed 20% of diet weight as RS [39]. In another study using a different fermentable fiber than RS, Daubioul et al. found that body fat was reduced in obese Zucker rats fed 10% w/w oligofructose [17]. However, they reported significantly lower food intake in the first 4 wk of oligofructose feeding. This may have represented a time effect for acceptance of dietary oligofructose. In the current study, there was no reduction in food intake at the beginning of the study (data not shown) with the feeding of RS; and the obese ZDF rats fed RS had significantly greater food intake for the entire study than the rats fed the control diets. The obese Zucker rats fed oligofructose had the reduced body fat in spite of the defective leptin signaling, which appears to be caused by an energy deficit.
In conclusion, this is the first study to compare a WG and an isolated source of RS. The authors of a recent review conclude from epidemiological studies that WGs have health benefits if cereal fiber or bran is included in the WG treatment [1]. Enhanced fermentation of WG compared to the isolated RS supports this conclusion. Obese ZDF rats are an intriguing model as they produced increased fermentation markers with feeding of RS that included increased serum GLP-1 active. These rats also demonstrated distinctly different microbiota between WG and purified high-RS groups; and between high- and low-RS groups. Normally, high levels of fermentation have resulted in reduced accretion of abdominal fat. The genetics of the ZDF rat appear to have a stronger influence on host body fat than the intestinal ecosystem created by fermentation of RS. Additionally, the isolated high-RS group had improved insulin sensitivity compared to the low-RS treatments and this may be the result of an increase in the phyla Bacteroidetes to Firmicutes ratio.
Supplementary Material
Acknowledgments
This research study was funded by Ingredion Incorporated and LSU AgCenter. The starches and whole-grain flours were gifts from Ingredion Incorporated. This work is partially supported by the Major International (Regional) Joint Research Project of National Natural Science Foundation of China (81220108006). Also, this work was partially supported by a NORC Center Grant #P30DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions.” The authors wish to thank Rebecca Elzer for her assistance with care of the rats used in the study.
Conflict of interest statement: M. J. K. and R. J. M. received funding from Ingredion Incorporated and C. P. is an employee of Ingredion Incorporated.
Abbreviations
- GLP-1
glucagon-like peptide 1
- HOMA-IR
homeostatic model of assessment-insulin resistance
- OTU
operational taxonomic units
- RS
resistant starch
- SCFAs
short chain fatty acids
- WG
whole-grain
- ZDF
Zucker Diabetic Fatty
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site
Colour online: See the article online to view Fig. 1 in colour.
F. G., R. J.M., C. P., and M. J. K. designed the research; F. G., M. J. K., J. G, R. P., A.M. R., R. W. S., A.G., D. C.-A., and D. C. conducted research; J. Y. provided essential ideas for the study; F. G., J. G., H. A. D., and M. J. K. performed statistical analysis; D. A. W., G. B., C. M. T. and M. L. performed the bacterial DNA amplification, sequencing, and bioinformatics, and F. G., M. J. K., and C. P. had primary responsibility for final content. All authors have read and approved the final manuscript.
References
- 1.Cho SS, Qi L, Fahey GC, Jr, Klurfeld DM. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am. J. Clin. Nutr. 2013;98:594–619. doi: 10.3945/ajcn.113.067629. [DOI] [PubMed] [Google Scholar]
- 2.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 3.Maki KC, Gibson GR, Dickmann RS, Kendal CWC, et al. Digestive and physiologic effects of a wheat bran extract, arabino-xylan-oligosaccharide, in breakfast cereal. Nutrition. 2012;28:1115–1121. doi: 10.1016/j.nut.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Costabile A, Klinder A, Fava F. Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br. J. Nutr. 2008;99:110–120. doi: 10.1017/S0007114507793923. [DOI] [PubMed] [Google Scholar]
- 5.Keenan MJ, Zhou J, McCutcheon K, Raggio AM. Effects of resistant Starch, a non-digestible fermentable fiber, on reducing body fat. Obesity. 2006;14:1523–1534. doi: 10.1038/oby.2006.176. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Martin RJ, Tulley RT, Raggio AM, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1160–E1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen L, Keenan MJ, Raggio AM, Williams C, et al. Dietary-resistant starch improves maternal glycemic control in Goto-Kakizaki rat. Mol. Nutr. Food Res. 2011;55:1499–1508. doi: 10.1002/mnfr.201000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou J, Martin RJ, Tulley RT, Raggio AM, et al. Failure to ferment dietary resistant starch in specific mouse models of obesity results in no body fat loss. J. Agric. Food. Chem. 2009;57:8844–8851. doi: 10.1021/jf901548e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen W, Gaskins R, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function, and metabolic outcomes. J. Nutr. Biochem. 2014;25:270–280. doi: 10.1016/j.jnutbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Pawlak DB, Bryson JM, Denyer GS, Brand-Miller JC. High glycemic index starch promotes hypersecretion of insulin and higher body fat in rats without affecting insulin sensitivity. J. Nutr. 2001;131:99–104. doi: 10.1093/jn/131.1.99. [DOI] [PubMed] [Google Scholar]
- 11.Belobrajdic DP, King RA, Christerpherson CT, Bird AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr. Metab. 2012;9:93. doi: 10.1186/1743-7075-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterson AD, Baumgardt BR. Influence of level of energy demand on the ability of rats to compensate for diet dilution. J. Nutr. 1971;101:1069–1074. doi: 10.1093/jn/101.8.1069. [DOI] [PubMed] [Google Scholar]
- 13.Tachon S, Zhou J, Keenan MJ, Martin RJ, et al. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2013;83:299–309. doi: 10.1111/j.1574-6941.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan K, Ramarao P. Animalmodels in type 2 diabetes research: an overview. Indian J. Med. Res. 2007;125:451–472. [PubMed] [Google Scholar]
- 15.Pickavance L, Widdowson PS, King P, Ishii S, et al. The development of overt diabetes in young Zucker Diabetic Fatty (ZDF) rats and the effect of chronicMCC-555 treatment. Br. J. Pharmacol. 1998;125:767–770. doi: 10.1038/sj.bjp.0702158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhlmann J, Neumann-Haefelin C, Belz U, Kalisch J, et al. Intramyocellular lipid and insulin resistance: a longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes. 2003;52:138–144. doi: 10.2337/diabetes.52.1.138. [DOI] [PubMed] [Google Scholar]
- 17.Daubioul CA, Taper HS, De Wispelaere LD, Delzenne NM. Dietary oligofructose lessens hepatic steatosis, but does not prevent hypertriglyceridemia in obese zucker rats. J. Nutr. 2000;130:1314–1319. doi: 10.1093/jn/130.5.1314. [DOI] [PubMed] [Google Scholar]
- 18.Daubioul CA, Rousseau N, Demeure R, Gallez B, et al. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J. Nutr. 2002;132:967–973. doi: 10.1093/jn/132.5.967. [DOI] [PubMed] [Google Scholar]
- 19.Cacho J, Sevillano J, de Castro J, Herrera E, et al. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Phyiol. Endrocrinol. Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- 20.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 21.Englyst HN, Kingman SM, Hudson GJ, Cummings JH. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 1996;75:749–755. doi: 10.1079/bjn19960178. [DOI] [PubMed] [Google Scholar]
- 22.Tulley RT, Appel MJ, Enos TG, Hegsted M, et al. Comparative methodologies for measuring metabolizable energy of various types of resistant high amylose corn starch. J. Agric. Food Chem. 2009;57:8474–8479. doi: 10.1021/jf900971c. [DOI] [PubMed] [Google Scholar]
- 23.Keenan MJ, Janes M, Robert J, Martin RJ, et al. Resistant starch from high amylose maize (HM260) reduces body fat and increases gut bacteria in ovariectomized (OVX) rats. Obesity. 2013;21:981–984. doi: 10.1002/oby.20109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charrier JA, Martin RJ, McCutcheon KL, Raggio AM, et al. High fat diet partially attenuates fermentation responses in rats fed resistant starch from high-amylose maize. Obesity. 2013;21:2350–2355. doi: 10.1002/oby.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, et al. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edgar RC. UPARSE: highly accurate OUT sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 27.Kim K-A, Gu W, Lee I-A, Joh E-H, et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. Plos One. 2012;7:e47713. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiBiase JK, Frank DN, Mathur R. Impact of the gut microbiota on the development of obesity: current concepts. Am. J. Gastroenterol. 2012;1:22–27. [Google Scholar]
- 29.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keenan MJ, Martin RJ, Raggio AM, McCutcheon KL, et al. High-amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: amicroarray study. J. Nutrigenet. Nutrigenomics. 2012;5:26–44. doi: 10.1159/000335319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hausman DB, Fine JB, Tagra K, Fleming SS, et al. Regional fat pad growth and cellularity in obese Zucker rats: modulation by caloric restriction. Obes. Res. 2003:674–682. doi: 10.1038/oby.2003.96. [DOI] [PubMed] [Google Scholar]
- 32.Shen L, Keenan MJ, Martin RJ, Tulley RT, et al. Dietary resistant starch increase hypothalamic POMC expression in rats. Obesity. 2009;17:40–45. doi: 10.1038/oby.2008.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–3393. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 34.Nutrition Council of The Netherlands. The Energy Value of Sugar Alcohols, Recommendations of the Committee on Polyalcohols. Voedinsgraad: The Hague; 1987. pp. 1–17. [Google Scholar]
- 35.Livesey G. The energy values of dietary fibre and sugar alcohols for man. Nutr. Res. Rev. 5:61–84. doi: 10.1079/NRR19920007. [DOI] [PubMed] [Google Scholar]
- 36.Hao Z, Munzberg H, Rezai-Zadeh K, Keenan M, et al. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int. J. Obes. 2015;39:798–805. doi: 10.1038/ijo.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldsmith F, Keenan MJ, Raggio AM, Ye X, et al. Induction of energy expenditure by sitigliptin is dependent on GLP-1 receptor. Plos One. 2015;10(5):e0126177. doi: 10.1371/journal.pone.0126177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reimer RR, Grover GJ, Koetzner L, Gahler RJ, et al. Combining sitigliptin/metformin with a functional fiber delays diabetes progression in Zucker rats. J. Endocrinol. 2014;220:361–373. doi: 10.1530/JOE-13-0484. [DOI] [PubMed] [Google Scholar]
- 39.Koh GY, Whitley EM, Mancoskey K, Mancosky K, et al. Dietary resistant starch prevents urinary excretion of vitamin D metabolites and maintains circulating 25-hydroxycholecalciferol concentrations in Zucker diabetic fatty rats. J. Nutr. 2014;144:1667–1673. doi: 10.3945/jn.114.198200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.