Summary
Objectives
We sought to determine the association between previous daptomycin exposure and daptomycin non-susceptible Enterococcus faecium (DNSEf) bloodstream infections (BSI) in adult leukemia patients.
Methods
We retrospectively identified adult (≥ 18 years old) leukemia patients with Enterococcus spp. bacteremia at The University of Texas MD Anderson Cancer Center (MDACC) from 6/1/2013 to 7/22/2015. Antimicrobial susceptibility and previous antibiotic exposure within the 90 days prior to bacteremia were collected. Classification and Regression Tree (CART) analysis was used to identify the most significant breakpoint between daptomycin exposure and DNSEf.
Results
Any amount of daptomycin received within the 90 days preceding BSI was significantly associated with isolation of DNSEf compared to daptomycin susceptible E. faecium (DSEf) (88% vs. 44%, respectively, p<0.01). CART analysis identified receiving ≥ 13 days of daptomycin in the preceding 90 days as most significantly correlated with DNSEf (60% vs. 11%, relative risk [RR] 5.31, 95% Confidence interval [CI] 2.36–11.96, p < 0.01).
Conclusions
Prior daptomycin exposure for ≥ 13 days within 90 days preceding BSI was significantly associated with isolation of DNSEf BSI in adult leukemia patients at our institution. Antimicrobial stewardship initiatives aimed at minimizing daptomycin exposure in high-risk patients may be of significant benefit in limiting the emergence of DNSEf.
Keywords: Risk factor, antibiotic exposure, linezolid, resistance, vancomycin
Introduction
Bloodstream infections (BSI) caused by enterococci present a major challenge for clinicians due to a marked increase in resistance to the agents of choice (1). Enterococci are increasing in prevalence as a nosocomial pathogen, particularly in immunocompromised patients such as those with hematologic malignancies, and are associated with increased mortality, hospital length of stay, and cost (2, 3).
Daptomycin is a lipopeptide antibiotic with bactericidal activity that is frequently used off-label for infections caused by vancomycin-resistant enterococci (VRE) (1). Although overall susceptibility to daptomycin in enterococci remains high, the clinical breakpoint has been recently questioned and the increasing prevalence of resistance in select populations threatens its clinical utility (1, 3, 4). The emergence of daptomycin non-susceptible Enterococcus (DNSE) while on therapy is well-described, however the clinical pattern of de novo DNSE has yet to be elucidated (3, 5). It is reasonable to assume that previous daptomycin exposure (i.e., antimicrobial selection pressure) increases risk for the development of DNSE BSI, however to date there has been conflicting reports of this phenomena (6, 7). Additionally, it is unknown whether the total extent of previous daptomycin exposure correlates with increased risk for DNSE BSI.
The purpose of this study was to identify clinical factors predictive of daptomycin non-susceptibility among leukemia patients with enterococcal BSI. We specifically sought to test the hypothesis that the risk of de novo daptomycin non-susceptibility increased with increasing total prior daptomycin exposure.
Patients and methods
Study Design
This was a retrospective, observational cohort study of adult (≥ 18 years old) leukemia patients with a blood culture positive for Enterococcus spp. at The University of Texas MD Anderson Cancer Center (MDACC) from 6/1/2013 to 7/22/2015. Patients were identified using records maintained by the microbiology department. The only exclusion criteria was lack of daptomycin susceptibility testing. Clinical information was collected from the electronic health record, including antimicrobial susceptibility data. Previous antibiotic exposure was assessed using pharmacy records capturing all inpatient and outpatient doses of antibiotics administered at MDACC during the pre-specified time period. Records were manually reviewed to ensure that any written prescriptions or medications administered through home-health agencies were also recorded. Patients with more than one episode of Enterococcus spp. BSI were included only once.
Microbiologic testing
Species identification with Vitek 2 (bioMérieux, Marcy-L'Étoile, France) and susceptibility testing were performed in the MDACC clinical microbiology laboratory. The daptomycin minimum inhibitory concentration (MIC) was determined via Etest (bioMérieux) with daptomycin non-susceptibility defined as an MIC of >4 mg/L, in accordance with current Clinical Laboratory and Standards Institute (CLSI) definitions (http://clsi.org/m100/). We chose Etest since compelling evidence suggests that Etest more accurately identifies DNSE compared to broth microdilution (4, 8). The linezolid MIC was determined via Etest, while ampicillin and vancomycin MICs were determined via automated broth microdilution (Vitek 2). Resistance for these agents was assessed according to CLSI breakpoints.
Endpoints
The primary aim of this study was to assess the association between previous daptomycin exposure and the emergence of primary DNSE isolates (i.e., DNSE BSI in a patient with no previous Enterococcus spp. BSI). The main variable of interest was total days of previous daptomycin treatment in the 90 days prior to the onset of BSI. We also analyzed any daptomycin exposure, as a dichotomous variable, within 14, 30, and 90 days prior to recovery of the isolate. These specific time points were chosen as potentially clinically relevant time periods for bedside decision-making in addition to facilitating comparisons with other antimicrobial resistance literature. Secondary variables included other antibiotic use within 90 days, presence of neutropenia (ANC < 500 cells/mm3), and length of hospitalization prior to isolate recovery. This study was approved by the MDACC institutional review board with a waiver of informed consent.
Statistical Analyses
Data were managed using REDCap (Vanderbilt University, Nashville, TN) electronic data capture tools hosted at MDACC (9). Differences in categorical variables were assessed with the χ2 test while the Wilcoxon rank-sum test was used for continuous variables after confirming that all followed non-normal distributions. Classification and Regression Tree (CART) analysis was utilized to identify the duration of daptomycin exposure most significantly correlated with DNSE. The relative risk for isolation of DNSE in patients with daptomycin exposures above and below the CART-derived breakpoint was calculated using the Mantel-Haenszel method. The area under the receiver operator characteristic (ROC) curve was used to confirm the categorization ability of the CART-derived breakpoint (10). Stata version 14.1 (StataCorp LP, College Station, TX) and SAS JMP Pro v11 (SAS Systems, Cary, NC) were used for statistical analysis.
Results
Demographics
Over the 25-month study period, 272 patients with Enterococcus spp. BSI were identified and 139 leukemia patients (95 E. faecium, 44 E. faecalis) met the pre-specified inclusion criteria. Primary daptomycin non-susceptibility was found to occur exclusively in E. faecium isolates, therefore, the subsequent analysis was focused on patients with E. faecium bacteremia. After excluding 17 patients without daptomycin susceptibility data, 77 patients with primary E. faecium BSIs were included.
Demographic characteristics were similar between patients with daptomycin susceptible E. faecium (DSEf) compared to daptomycin non-susceptible E. faecium (DNSEf), including a median age of 53 years (interquartile range [IQR] 36-64) vs. 48 years (IQR 40-62, p=0.80) and leukemia type, with a majority of patients in both groups having acute myeloid leukemia; 61% vs. 69%, p=0.73). The majority of patients in both groups were profoundly neutropenic at the onset of BSI (80% vs. 94%, p=0.20). Four of 16 patients (25%) with primary DNSEf had isolation of E. faecium from other sources within 90 days prior to bloodstream isolate collection. Sources of isolation included urine (3 patients) and both urine and skin or soft tissue (1 patient). Each of these 4 patients had a stool screen positive for VRE during the same time period. Demographic and clinical characteristics stratified by daptomycin susceptibility are shown in Table 1.
Table 1.
Demographic and clinical characteristics for patients with E. faecium isolates
| Characteristic (n, %) | Daptomycin susceptible (n=61) | Daptomycin non-susceptible (n=16) | p value |
|---|---|---|---|
| Age, years (median [IQRa]) | 53 (36-64) | 48 (40-62) | 0.80Ŧ |
| Male gender | 37 (61) | 12 (75) | 0.29¶ |
| Cancer type | 0.73¶ | ||
| AML | 37 (61) | 11 (69) | |
| ALL | 14 (23) | 4 (25) | |
| Other leukemia | 10 (16) | 1 (6) | |
| Active chemotherapy | 58 (95) | 16 (100) | 0.37¶ |
| Salvage chemotherapy | 46 (79) | 13 (81) | 0.86¶ |
| ANC < 500 (cells / mm3) | 49 (80) | 15 (94) | 0.20¶ |
| Number of hospital days in previous 90 days (median [IQRa]) | 37 (17-52) | 36 (22-67) | 0.61Ŧ |
| History of stem cell transplant | 22 (36) | 4 (25) | 0.41¶ |
| Positive VREb stool screen within 90 days prior to bacteremia | 38 (62) | 9 (56) | 0.66¶ |
| Select antibiotic use within 90 days prior to bacteremia | |||
| Vancomycin | 21 (34) | 4 (25) | 0.47¶ |
| Linezolid | 57 (93) | 16 (100) | 0.29¶ |
| Levofloxacin | 42 (69) | 13 (81) | 0.33¶ |
| Cefepime | 55 (90) | 12 (75) | 0.11¶ |
| Piperacillin/ tazobactam | 40 (66) | 13 (81) | 0.23¶ |
| Meropenem | 48 (79) | 15 (94) | 0.16¶ |
| Daptomycin usage | |||
| Number of daptomycin days in previous 90 days (median [IQRa]) | 0.0 (0.0-7.0) | 13.0 (4.5-16.0) | <0.01Ŧ |
| Any daptomycin use within 14 days of isolate | 15 (25) | 7 (44) | 0.13¶ |
| Any daptomycin use within 30 days of isolate | 19 (31) | 10 (63) | 0.02¶ |
| Any daptomycin use within 90 days of isolate | 27 (44) | 14 (88) | 0.01¶ |
| ≥ 13 days of daptomycin use within 90 days of isolate | 7 (11) | 9 (60) | 0.01¶∫ |
Interquartile range
Vancomycin-resistant Enterococcus faecium
Wilcoxon rank-sum test
χ2 test
Relative risk 5.31 (95% CI 2.36–11.96, p <0.01)
Microbiologic characteristics
Among the 77 E. faecium isolates, 16 (21%) were daptomycin non-susceptible, 75 (97%) were ampicillin resistant, 53 (69%) were vancomycin resistant, and 13 (17%) were linezolid resistant. Three (4%) isolates were resistant to ampicillin, linezolid, and vancomycin and non-susceptible to daptomycin. The MIC50 / MIC90 for daptomycin and linezolid were 3/8 mg/L and 2/32 mg/L, respectively.
Daptomycin exposure
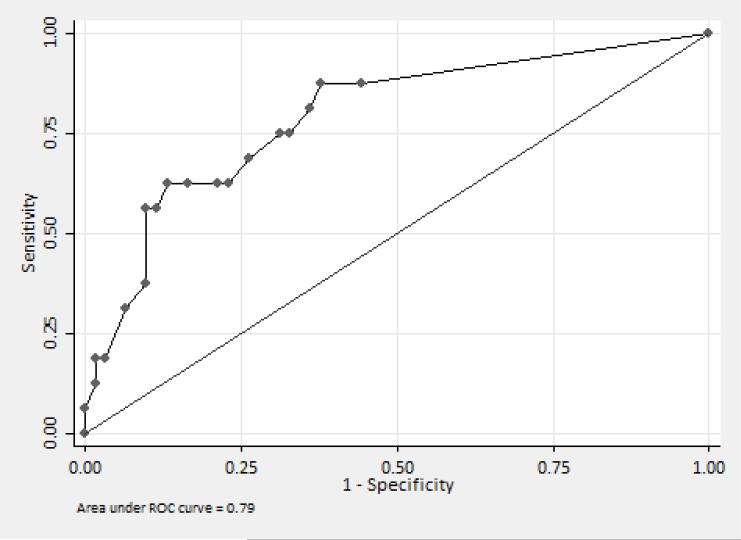
Overall, 53% of patients had received at least one dose of daptomycin in the 90 days preceding BSI onset. Any daptomycin exposure within the 90 days preceding BSI onset was significantly associated with isolation of DNSEf compared to DSEf (88% vs. 44%, respectively, p<0.01). No other antibiotic exposure correlated significantly with DNSEf (Table 1). CART analysis identified the receipt of ≥ 13 days of daptomycin in the preceding 90 days as being most significantly correlated with DNSE (60% vs. 11%, relative risk [RR] 5.31, 95% Confidence interval [CI] 2.36–11.96, p < 0.01). The area under the ROC curve (AUC ROC) for the CART-derived breakpoint was 0.79 (95% CI 0.67 – 0.92) (Figure 1). This breakpoint correctly classified 83% of patients at a sensitivity of 56% and specificity of 92%.
Figure 1.
Area under the receiver operator characteristic (ROC) curve for daptomycin exposure and isolation of daptomycin non-susceptible E. faecium
Discussion
Due to broad activity against common multidrug-resistant gram-positive organisms, such as VRE and methicillin-resistant Staphylococcus aureus, daptomycin is frequently used as a front line agent for proven or suspected infections due to these organisms. As delayed appropriate therapy for VRE BSI is associated with increased morbidity and mortality and daptomycin non-susceptibility in enterococci is increasingly reported, understanding the key risk factors for the development of DNSEf BSI in high-risk patients is critical (3, 11). Here, we show that previous daptomycin exposure is highly correlated with isolation of DNSEf in high-risk leukemia patients with enterococcal BSI.
The two most likely paths of infection with DNSE are prolonged daptomycin exposure selecting for DNSE in a patient already colonized with Enterococcus spp. and de novo acquisition of DNSE in a susceptible host. Previous data are consistent with both pathways being clinically relevant (6, 7, 12). Although we did not perform epidemiologic typing and VRE colonization rates were similar in patients with DNSEf and DSEf, we speculate that differences in local flora and our heavily immunosuppressed patient population, primarily with relapsed leukemia, predispose our patients to the development of DNSE in the setting of prolonged daptomycin exposure. As DNSE was highly correlated with daptomycin exposure in our patients, our data suggest that antimicrobial stewardship efforts may be of substantial benefit in limiting the emergence of DNSE.
Given the relatively low virulence of enterococci, the clinical impact of timely, effective therapy for enterococcal BSI has long been debated. A recent analysis of non-neutropenic patients with enterococcal BSI found that delayed administration of microbiologically-active antibiotics greater than 48 hours after isolate collection was associated with a 3-fold increased risk in 30-day mortality (13). Additionally, our group has shown that patients with enterococcal BSI caused by isolates with elevated, yet still susceptible, MICs (i.e., 3 – 4 mg/L) have increased risk of microbiologic failure (4). Thus, together with previously published data, our findings suggest that patients with previous daptomycin exposure ≥ 13 days presenting with enterococcal BSI not only have increased risk of DNSEf, but potentially increased mortality and prolonged bacteremia resulting from delayed appropriate treatment (13).
This study has several limitations, including its retrospective nature. We were unable to capture information on antibiotic usage at other institutions, however, as a comprehensive cancer center, many of our patients exclusively receive care within our institution. The single center nature of our study may limit the generalizability of our findings, but DNSE infections have been broadly reported in the United States and worldwide. Since this study was designed to detect risk factors for DNSE, the secondary time points chosen for daptomycin exposure (i.e. 90, 30, and 14 days) were selected based on presumed clinical relevance. It is possible, however, that other time points have been associated with differing risk profiles. Also, since we defined DNSE as isolates with daptomycin MIC > 4 mg/L via Etest based on current CLSI breakpoints, we did not evaluate the correlation of previous daptomycin exposure and E. faecium isolated with MICs of 3 – 4mg/L.
In summary, DNSE occurred exclusively in E. faecium isolates at our institution. We were able to define the extent of prior exposure to daptomycin (≥ 13 days) most significantly associated with DNSEf BSI in our population, with a nearly six-fold increased risk of DNSEf BSI. Taken together, our findings suggest that antimicrobial stewardship initiatives aimed at minimizing daptomycin exposure in high-risk patients may be of significant benefit in limiting the emergence of DNSEf. Finally, these findings indicate that previous daptomycin exposure is an important consideration when selecting empiric therapy for multidrug-resistant enterococcal infections.
Highlights.
Daptomycin non-susceptible E. faecium (DNSE) bloodstream infection was found in 21% of cases.
Receipt of ≥ 13 days of daptomycin was significantly correlated with DNSE.
Stewardship interventions to minimize daptomycin exposure may limit the emergence of DNSE.
Acknowledgments
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases [grants R01-AI093749; R21 AI114961; R21/R33 AI121519; K24-AI114818 to CAA and R01-AI089891 to SAS] and the National Cancer Institute [Cancer Center Support Grant P30 CA016672 funds for the Bioinformatics Shared Resource to MD Anderson].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Transparency Declarations
All authors: no conflicts to declare.
References
- 1.Arias CA, Murray BE. Emergence and management of drug-resistant enterococcal infections. Expert Rev Anti Infect Ther. 2008 Oct;6(5):637–55. doi: 10.1586/14787210.6.5.637. PubMed PMID: 18847403. Epub 2008/10/14. eng. [DOI] [PubMed] [Google Scholar]
- 2.Butler AM, Olsen MA, Merz LR, Guth RM, Woeltje KF, Camins BC, et al. Attributable costs of enterococcal bloodstream infections in a nonsurgical hospital cohort. Infect Control Hosp Epidemiol. 2010 Jan;31(1):28–35. doi: 10.1086/649020. PubMed PMID: 19951200. Pubmed Central PMCID: 3608393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias CA, Panesso D, McGrath DM, Qin X, Mojica MF, Miller C, et al. Genetic basis for in vivo daptomycin resistance in enterococci. N Engl J Med. 2011 Sep 8;365(10):892–900. doi: 10.1056/NEJMoa1011138. PubMed PMID: 21899450. Pubmed Central PMCID: PMC3205971. Epub 2011/09/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shukla BS, Shelburne S, Reyes K, Kamboj M, Lewis JD, Rincon SL, et al. Influence of Minimum Inhibitory Concentration in Clinical Outcomes of Enterococcus faecium Bacteremia Treated With Daptomycin: Is it Time to Change the Breakpoint? Clin Infect Dis. 2016 Jun 15;62(12):1514–20. doi: 10.1093/cid/ciw173. PubMed PMID: 27045126. Pubmed Central PMCID: 4885651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis JS, 2nd, Owens A, Cadena J, Sabol K, Patterson JE, Jorgensen JH. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents Chemother. 2005 Apr;49(4):1664–5. doi: 10.1128/AAC.49.4.1664-1665.2005. PubMed PMID: 15793168. Pubmed Central PMCID: 1068653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamboj M, Cohen N, Gilhuley K, Babady NE, Seo SK, Sepkowitz KA. Emergence of daptomycin-resistant VRE: experience of a single institution. Infect Control Hosp Epidemiol. 2011 Apr;32(4):391–4. doi: 10.1086/659152. PubMed PMID: 21460492. Pubmed Central PMCID: 3676937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storm JC, Diekema DJ, Kroeger JS, Johnson SJ, Johannsson B. Daptomycin exposure precedes infection and/or colonization with daptomycin non-susceptible enterococcus. Antimicrob Resist Infect Control. 2012;1(1):19. doi: 10.1186/2047-2994-1-19. PubMed PMID: 22958379. Pubmed Central PMCID: 3436660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis JD, Enfield KB, Cox HL, Mathers AJ, Sifri CD. A single-center experience with infections due to daptomycin-nonsusceptible Enterococcus faecium in liver transplant recipients. Transpl Infect Dis. 2016 Mar 8; doi: 10.1111/tid.12523. PubMed PMID: 26953719. [DOI] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. PubMed PMID: 18929686. Pubmed Central PMCID: 2700030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes NJ, O'Donnell JN, Lizza BD, McLaughlin MM, Esterly JS, Scheetz MH. Tree-Based Models for Predicting Mortality in Gram-Negative Bacteremia: Avoid Putting the CART before the Horse. Antimicrob Agents Chemother. 2016 Feb;60(2):838–44. doi: 10.1128/AAC.01564-15. PubMed PMID: 26596934. Pubmed Central PMCID: 4750664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zasowski EJ, Claeys KC, Lagnf AM, Davis SL, Rybak MJ. Time Is of the Essence: The Impact of Delayed Antibiotic Therapy on Patient Outcomes in Hospital-Onset Enterococcal Bloodstream Infections. Clin Infect Dis. 2016 May 15;62(10):1242–50. doi: 10.1093/cid/ciw110. PubMed PMID: 26945013. Pubmed Central PMCID: 4845789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judge T, Pogue JM, Marchaim D, Ho K, Kamatam S, Parveen S, et al. Epidemiology of vancomycin-resistant enterococci with reduced susceptibility to daptomycin. Infect Control Hosp Epidemiol. 2012 Dec;33(12):1250–4. doi: 10.1086/668438. PubMed PMID: 23143365. [DOI] [PubMed] [Google Scholar]
- 13.Zasowski EJ, Claeys KC, Lagnf AM, Davis SL, Rybak MJ. Time Is of the Essence: The Impact of Delayed Antibiotic Therapy on Patient Outcomes in Hospital-Onset Enterococcal Bloodstream Infections. Clin Infect Dis. 2016 Mar 3; doi: 10.1093/cid/ciw110. PubMed PMID: 26945013. [DOI] [PMC free article] [PubMed] [Google Scholar]