ABSTRACT
ATM is a 350 kDa serine/threonine kinase best known for its role in DNA repair and multiple cellular homeostasis pathways. Mutation in ATM causes the disease ataxia telangiectasia (A-T) with clinical features including ataxia, severe cerebellar atrophy and Purkinje cell loss. In a cross-species study, using primary rat neurons, the roundworm C. elegans, and a mouse model of A-T, we showed that loss of ATM induces mitochondrial dysfunction and compromised mitophagy due to NAD+ insufficiency. Remarkably, NAD+ repletion mitigates both the DNA repair defect and mitochondrial dysfunction in ATM-deficient neurons. In C. elegans, NAD+ repletion can clear accumulated dysfunctional mitochondria through restoration of compromised mitophagy via upregulation of DCT-1. Thus, NAD+ ties together DNA repair and mitophagy in neuroprotection and intimates immediate translational applications for A-T and related neurodegenerative DNA repair-deficient diseases.
KEYWORDS: ataxia telangiectasia, ATM, autophagy, DNA damage, mitophagy
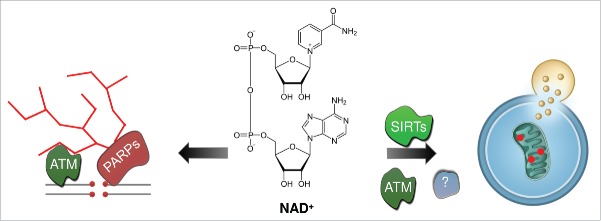
Nicotinamide adenine dinucleotide (NAD+) is an important coenzyme in all human cells. NAD+ modulates mitochondrial bioenergetics, which plays major roles in mitochondrial metabolism, neuroprotection, and aging. It also serves as a critical coenzyme for many proteins, including SIRTs/sirtuins, PARPs/poly(ADP-ribose polymerases), and the cyclic ADP-ribose synthases (CD38, and BST1/CD157). For example, NAD+ is a rate-limiting co-substrate for SIRT1 (sirtuin 1), a deacetylase that regulates many cellular pathways and forestalls the progression of aging and age-related disease. The most abundant PARP member, PARP1, senses DNA damage and consumes NAD+ to synthesize poly(ADP-ribose) in order to recruit DNA repair proteins and perform other functions. Both of these important processes require NAD+ as a coenzyme for proper activity, so PARP1 and SIRT1 may reciprocally limit each other's activity.
These NAD+-dependent enzymes also regulate important steps for autophagy. SIRT1 can bind to and deacetylate several major autophagy proteins including Atg5, Atg7, and LC3/Atg8, and the absence of SIRT1 inhibits autophagy. Among DNA repair proteins, ATM/ataxia telangiectasia mutated is a master regulator of the DNA damage response by directly or indirectly modifying (e.g., phosphorylating) over 1000 proteins. In postmitotic neurons, following induction of DNA double-strand breaks, ATM facilitates repair with the MRE11-RAD50-NBN/Nbs1 (MRN) complex at the site of DNA damage. Recent studies now show that ATM regulates autophagy and many other cell signaling pathways involved in cellular homeostasis. Mechanistically, upregulation of autophagy by ATM has been suggested to occur through MTOR inhibition via the ATM-STK11/LKB1-AMPK-TSC2 pathway or the ATM-HIF1A/HIF1α-DDIT4/REDD1-TSC2 pathway. Of note, SIRT1 and ATM can also interact with each other. In neurons, SIRT1 can recruit to breaks to stimulate ATM autophosphorylation and stabilize ATM at double-strand break sites.
In our recent paper, we reported that in the absence of ATM, NAD+ depletion inhibits mitophagy, leading to accumulation of damaged mitochondria and mitochondrial malfunction. In A-T laboratory animal models including C. elegans and mice, persistent activation of PARP1 depletes NAD+, inactivating SIRT1 and leading to compromised mitophagy. We showed that in C. elegans, SIR-2.1 (ortholog to mammalian SIRT1) binds to DAF-16 (ortholog to the mammalian FOXO3 [Forkhead box O3]) to upregulate DCT-1 (DAF-16/FOXO controlled, germline tumor affecting) expression. An ortholog to the mammalian BNIP3L/NIX, DCT-1 is a mitochondrial outer membrane protein and has recently been shown to be a major player in mitophagy in worms by the Tavernarakis group. In line with the importance of NAD+ in mitophagy, NAD+ repletion through either nicotinamide riboside or nicotinamide mononucleotide, can increase life span and health span in both atm-1 worms and an Atm−/− mouse model. At the molecular level, activation of the NAD+-SIRT1 pathway can restore mitophagy through upregulation of DCT-1 (in worms) and possibly activation of general autophagic proteins. The NAD+-SIRT1 pathway can also improve DNA repair through activation of the PRKDC/DNA-dependent protein kinase pathway in neurons.
Taken together, these results suggest a complicated and interconnected mitophagy regulatory network involving NAD+, SIRT1, PARP1 and ATM (Figure 1). Further molecular mechanistic studies on how NAD+ regulates mitophagy are necessary, including the roles of the NAD+-SIRT3 pathway in this process. Laboratory studies of NAD+ supplementation have shown profound health benefits to halt aging and age-related diseases. This finding provides potential for further investigation of NAD+ in autophagy/mitophagy to develop into translational applications.
Figure 1.
NAD+ links DNA repair and mitochondrial maintenance. The cellular metabolite NAD+ is essential for PARP-dependent generation of poly(ADP-ribose), a process needed to recruit DNA repair proteins at the site of DNA damage sites. NAD+ is also a limiting cofactor for SIRTs/sirtuins, such as SIRT1 and the mitochondrial SIRT3. In A-T, persistent PARylation leads to NAD+ insufficiency and SIRT1 inactivation, resulting in compromised mitophagy. Other proteins involved in NAD+-dependent mitophagy need to be further studied.
Acknowledgments
We thank Drs. Beverly Baptiste and Deborah Croteau for reading of the manuscript.
Funding
This research was supported by the Intramural Research Program of the NIH (VAB), National Institute on Aging, including a 2014–2015 NIA intra-laboratory grant (EFF, VAB).