Abstract
Objective:
The purpose of the study was to analyze the prevalence of hyponatremia and related 1-year outcomes of patients hospitalized for decompensated heart failure with reduced ejection fraction (HFrEF) in Turkish patients.
Methods:
A total of 500 hospitalized patients with HFrEF were consecutively included in a retrospective study at 19 participating hospitals. Patients were categorized according to their serum sodium levels (sNa) on admission day as normonatremic (135–145 mEq/L) and hyponatremic (<135 mEq/L). One-year all-cause mortality, re-hospitalization rates, and the impact of the changes in sNa at the time of discharge to clinical outcomes were examined.
Results:
Hyponatremia was observed in 29% of patients. Patients with hyponatremia had lower blood pressures, creatinine clearance, and left ventricular ejection fraction and higher serum creatinine and BUN levels on admission compared with those with normonatremia. Hyponatremia was associated with higher 1-year all-cause mortality (14% vs. 2.6%, p<0.001) and re-hospitalization rates (46.9% vs. 33.7%, p=0.005). After adjustment for covariates, hyponatremia was independently associated with 1-year all-cause mortality (adjusted HR, 4.762; 95% CI, 1.941–11.764; p=0.001). At discharge, only 50.8% of hyponatremic patients were corrected to normonatremia (≥135 mEq/L). Those with persistent hyponatremia had the highest all-cause mortality (p<0.001).
Conclusion:
In this study, it is demonstrated that hyponatremia is relatively common and is associated with increased 1-year all-cause mortality and re-hospitalization rates among Turkish patients hospitalized with HFrEF. Approximately 50% of the patients with initial low sNa had persistent hyponatremia at discharge, and these patients had the worst clinical outcomes.
Keywords: heart failure, hyponatremia, mortality, rehospitalization
Introduction
Hyponatremia is a frequent finding in patients hospitalized for heart failure (HF), with a prevalence of 8%–28% (1–7). Besides being frequent, it also has been established as a poor prognostic factor for both out- and inpatients with HF (2–13). It is associated with both short- and long-term adverse outcomes, including all-cause death. This adverse effect is not only for those patients with reduced EF but also for those with HF and preserved EF (5, 10). Most of North American, European, and some Asian countries reported their relevant data for hyponatremia and its impact on clinical outcomes in patients with HF. Data on the prevalence of hyponatremia and its relation with clinical outcomes in Turkish patients with HF does not exist.
Therefore, in this study, we examined the prevalence of hyponatremia and related 1-year clinical outcomes (mortality and rehospitalization) in patients hospitalized for decompensated HF with reduced EF. Since relevant data is scarce, we also evaluated the association of changes in serum sodium (sNa) at discharge with 1-year mortality. This study is expected to fill the above-mentioned gap related with hyponatremia and gain insight into clinical outcomes of Turkish patients hospitalized for HF.
Methods
We performed a retrospective chart review study among patients who were hospitalized because of worsening HF in 19 tertiary care cardiology clinics in Turkey. Adult patients (>18 years of age) were eligible to take part in the study if they had signs and symptoms of HF, were hospitalized for the treatment of worsening HF before April 2012, and had a left ventricular EF <45% as seen on echocardiogram at the time of hospital admission. The first 500 charts across the study hospitals were selected according to the index hospitalization admission date. There were no additional exclusion criteria. Patients were identified using the International Classification of Disease: ninth Revision Codes from the hospital database.
All variables, including patient history and detailed in-hospital drug history, were obtained from the patient treatment records and entered into the electronic case report forms (CRFs). Laboratory data, including sNa, potassium, creatinine, and BUN levels, were recorded on admission. Renal function [creatinine clearance (CrCl)] was calculated using the Cockroft–Gault formula. The discharge sNa (defined as the last sNa within 48 h before discharge) was also recorded in CRF. Missing information was noted as unknown. This study complied with the Declaration of Helsinki. The study protocol was approved by the Institutional Ethics Committee of the coordinating center.
Out of 500 patients, a total of 487 patients’ data were included in the analysis. Thirteen patients were excluded due to missing sNa values at the time of index admission. Hyponatremia was defined as sNa concentration <135 mEq/L. The patients were divided into two groups: hyponatremia (sNa<135 mEq/L) and normonatremia (sNa 135–145 mEq/L).
The primary objectives of this study were to compare 1-year all-cause mortality and re-hospitalization rates following index hospitalization for HF between patients with initial hyponatremia and normonatremia. All-cause mortality and re-hospitalization data at the follow up were extracted from patient treatment files or obtained from a family member or government agency by a phone call. In the absence of documented death, patients were presumed to be alive at the time of analysis. Further elucidation of cause of death was not performed because of the inability to obtain complete records from all patients.
Secondary objectives included clinical characteristics, the length of index hospitalization, and whether changes in sNa levels at the time of discharge affect clinical outcomes. To analyze the last secondary objective, the patients were further separated into four groups according to their sNa levels on admission and at discharge as corrected hyponatremia (initial sNa<135 mEq/L that increased to ≥135 mEq/L at discharge); persistent hyponatremia (sNa<135 mEq/L both at admission and discharge); hospital-acquired hyponatremia (initial sNa≥135 mEq/L that decreased <135 mEq/L at discharge); and normonatremia (sNa≥135 both at admission and discharge). Of the 483 patients included in the analysis, 416 patients’ (86%) admission and discharge sNa values were entered in CRFs. One-year all-cause mortality and re-hospitalization were determined in these patients.
Statistics
Statistical analyses were performed in accordance with the International Conference of Harmonization (ICH) E9 guidelines (14). Admission sNa values were categorized into two discrete groups: low sNa (Na<135 mmol/L) and normal sNa (Na≥135 mmol/L). Based on the EuroHeart Failure Survey study, the 1-year mortality rate among hospitalized HF patients was 29.5% and 18.9% for patients with low sodium levels (Na<135 mmol/L) and normal sodium level (Na≥135 mmol/L), respectively. Sample size per region of 500 patients allowed for detecting a significant difference in 1-year mortality rates between the low and normal sNa groups at a power of 0.80 and a two-sided alpha level of 0.05 (15). Patient characteristics and treatments were compared using the chi-square test for categorical variables and Mann–Whitney U test for continuous variables not normally distributed. The relation between sNa concentration and long-term outcomes was evaluated among patients with the post-discharge Cox-proportional hazards model. Kaplan–Meier survival curves were constructed to illustrate mortality. The long-rank test was used to assess differences between groups. Potential confounders were identified from patient comparisons conducted across predefined groups. Results were presented as hazard ratios with 95% confidence intervals. All data were analyzed using SPSS 21 software (SPSS Inc., Chicago, IL, USA). Values of p <0.05 were considered to be statistically significant.
Results
The clinical characteristics of all patients and patients classified into two groups, hyponatremia and normonatremia, are shown in Table 1. Patients had a median age 65 years and were mostly men (70%). The majority of patients (61.4%) had coronary artery disease as the etiology of HF.
Table 1.
Patient characteristics
| Total n=487 | Hyponatremia n=143 | Normonatremia n=344 | P | |
|---|---|---|---|---|
| Age, years | 65 (18–92) | 64 (18–92) | 66 (23–91) | 0.594 |
| Men, % | 341 (70.0) | 99 (69.2) | 242 (70.3) | 0.974 |
| BMI, kg/m2* | 28.6±4.5 | 28.1±4.0 | 28.8±4.7 | 0.421 |
| Diabetes mellitus | 65 (13.3) | 25 (17.5) | 40 (11.6) | 0.107 |
| Smoking history | ||||
| Smoker | 44 (9.0) | 16 (11.2) | 28 (8.1) | 0.326 |
| Ex-smoker | 87 (17.9) | 20 (14.0) | 67 (19.5) | |
| Non-smoker | 114 (23.4) | 30 (21.0) | 84 (24.4) | |
| Unknown | 236 (48.5) | 76 (53.1) | 160 (46.5) | |
| Heart failure etiology | ||||
| Ischemic | 299 (61.4) | 88 (61.5) | 211 (61.3) | 0.114 |
| Non-ischemic | 151 (31.0) | 39 (27.3) | 112 (32.6) | |
| Unknown | 37 (7.6) | 16 (11.2) | 21 (6.1) | |
| NYHA class | ||||
| I | 7 (1.4) | 1 (0.7) | 6 (1.7) | 0.108 |
| II | 90 (18.5) | 25 (17.5) | 65 (18.9) | |
| III | 237 (48.7) | 66 (46.2) | 171 (49.7) | |
| IV | 113 (23.2) | 44 (30.8) | 69 (20.1) | |
| Unknown | 37 (7.6) | 5 (3.5) | 32 (9.3) | |
| LV EF, % | 30 (10–44) | 27 (10–44) | 30.0 (10–44) | 0.002 |
| SBP, mm Hg | 120 (80–230) | 114 (80–160) | 120 (80–230) | 0.003 |
| DBP, mm Hg | 73 (40–120) | 70 (40–92) | 80 (40–120) | 0.001 |
| SBP, ≤100 mm Hg | 53 (10.9) | 22 (15.4) | 31 (9.0) | 0.054 |
| SBP, ≥140 mm Hg | 75 (15.4) | 13 (9.1) | 62 (18.0) | 0.006 |
| Heart rate, bpm | 82 (40–152) | 84 (60–146) | 82 (40–152) | 0.165 |
| Sodiu, mEq/L | 138 (111–145) | 132 (111–134) | 139 (135 -145) | <0.001 |
| Potasium, mEq/L | 4.5±0.7 | 4.5±0.9 | 4.4±0.6 | 0.259 |
| Creatinine, mg/dL | 1.1 (0.9–7) | 1.22 (0.9–6) | 1.10 (0.9–7) | 0.001 |
| Creatinine, | 118 (24.2) | 48 (33.6) | 70 (20.3) | 0.003 |
| >1.5, mg/dL | ||||
| BUN, mg/dL | 26 (7–144) | 35 (10–144) | 25 (7–128) | <0.001 |
| CrCI, mL/min | 66.4 (10–230) | 54.7 (10–226) | 68.7 (22–230) | 0.018 |
BMI - body mass index; BUN - blood urea nitrogen; CrCl - creatinine clearance; DBP - diastolic blood pressure; LVEF - left ventricle ejection fraction; SBP - systolic blood pressure.
The “±” values are means+SD. Results presented as median, minimum, and maximum values for continuous variables with not-normally distributed and numbers (percentages) for categorical variables. They were compared using the chi-square test for categorical variables and Mann–Whitney U test for continuous variables not-normally distributed
The frequency of hyponatremia on hospital admission was 29%. As expected, sNa was lower in patients with hyponatremia (132 mEq/L vs. 139 mEq/L, respectively, p<0.001). Age, gender, diabetes, and smoking history were comparable between the two groups. Patients with hyponatremia were more likely to have more severe symptoms [New York Heart Association (NYHA) Functional Class IV; 30.8% vs. 20.1%, p<0.001] and lower blood pressure (BP) (p=0.003 for systolic BP and p=0.001 for diastolic BP) on admission. In hyponatremic patients, serum creatinine and BUN levels were significantly higher (p=0.001 and p<0.001, respectively) and CrCl and left ventricle EF were significantly lower (p=0.018 and p=0.002) than patients with normonatremia.
In-hospital medications were compared between the two groups (Table 2). The use of angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and beta-blockers were similar. The use of intravenous diuretics, inotropic drugs (dopamine and dobutamine), and spiranolactone were more common in patients with hyponatremia (p=0.004, p=0.001, p=0.022 and p=0.036, respectively).
Table 2.
In-hospital management according to presence or absence of hyponatremia
| Total n=487 (%) | Hyponatremia n=143 (%) | Normonatremia n=344 (%) | P | |
|---|---|---|---|---|
| ACE inhibitors | 46.8 | 51.7 | 44.8 | 0.164 |
| ARBs | 10.7 | 9.1 | 11.3 | 0.522 |
| Beta blockers | 56.7 | 61.5 | 54.7 | 0.192 |
| Antiarrhythmics | 7.6 | 7.7 | 7.6 | 0.546 |
| Digitalis | 20.5 | 23.1 | 19.5 | 0.390 |
| IV furosemide | 46.4 | 56.6 | 42.2 | 0.004 |
| HCTZ | 18.3 | 23.1 | 16.3 | 0.094 |
| Spiranolactone | 24.2 | 30.8 | 21.5 | 0.036 |
| Dopamine | 15.0 | 23.8 | 11.3 | 0.001 |
| Dobutamine | 5.1 | 9.1 | 3.5 | 0.022 |
| Levosimendan | 4.3 | 4.2 | 4.4 | 0.577 |
| Insulin | 10.5 | 15.4 | 8.4 | 0.033 |
| ASA | 60.8 | 65.7 | 58.7 | 0.155 |
| Warfarin | 14.2 | 14.7 | 14.0 | 0.887 |
ACE - angiotensin converting enzyme; ARB - angiotensin receptor blocker; ASA - asetilsalyclic acid; HCTZ - hydrochlorothiazide; IV - intravenous. Values are given as percentages. The chi-square test was used
According to admission and discharge sNa levels, 61 (14.7%) patients had corrected hyponatremia, 59 (14.2%) patients had persistent hyponatremia, 77 (18.5%) patients had hospital-acquired hyponatremia, and 219 (52.6%) patients had normonatremia.
Outcomes
The median duration of follow-up was 330 days. Overall, 29 patients (6%) died during the follow-up period. Hyponatremia was associated with higher 1-year mortality compared with patients without hyponatremia (14% vs. 2.6% respectively, p<0.001). Kaplan–Meier survival curves for the patients with hyponatremia and normonatremia are shown in Figure 1. The length of hospital stay was significantly longer in patients with hyponatremia (12.8±24.9 days vs. 9.3±24.7 days, p=0.017). The rehospitalization rate because of HF was 46.9% in patients with hyponatremia and 33.7% in those with normonatremia (p=0.005).
Figure 1.
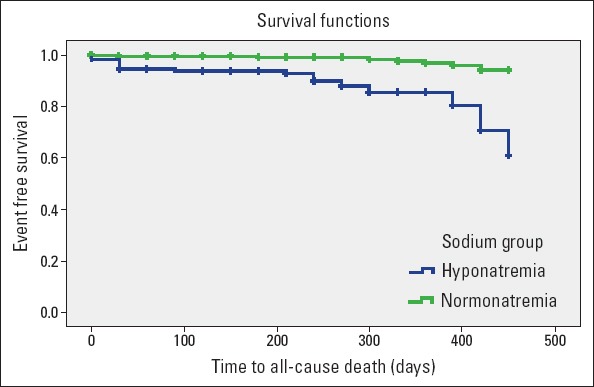
Kaplan–Meier survival curves for patients with hyponatremia and without hyponatremia
The predictors of mortality in the whole group were hyponatremia [hazard ratio (HR) 5.38; 95% confidence interval (CI), 2.45–11.8, p<0.001] and left ventricle EF [HR 1.075; 95% CI, 1.02–1.13, p=0.006].
In a logistic regression model using normonatremic patients as the reference, hyponatremia was significantly associated with 1-year all-cause mortality unadjusted HR 6.71; 95% CI, 2.96–15.15; p<0.001. Hyponatremia was independently associated with 1-year all-cause mortality adjusted HR 4.762; 95% CI, 1.94–11.76; p=0.001 even after adjusting for left ventricle EF, systolic BP, and serum creatinine.
Results of 1-year all-cause mortality stratified by groups according to the change in sNa levels at discharge are presented in Table 3. Patients with normonatremia had the lowest 1-year all-cause mortality, whereas those with persistent hyponatremia had the highest (2.5% vs. 20%, p<0.001). All-cause mortality rates between persistent hyponatremia vs. hospital-acquired hyponatremia and corrected hyponatremia vs. normonatremia groups were significantly different (p=0.014 and p=0.012, respectively). Kaplan–Meier survival curves for the four groups are shown in Figure 2. Similarly, rehospitalization rate was highest in the persistent hyponatremia group and lowest in the normonatremia group (49.2% vs. 29.2%, p=0.005). Patients with corrected hyponatremia at discharge had lower mortality and rehospitalization rates than patients with persistent hyponatremia (11.3% vs. 20% for mortality and 37.7% vs. 49.2% for rehospitalization), though this did not reach statistical significance.
Table 3.
Clinical outcomes in groups according to change in sNa levels at discharge
| Corrected hyponatremia n=61 | Persistent hyponatremia n=59 | Hospital-acquired hyponatremia n=77 | Normonatremia n=219 | P | |
|---|---|---|---|---|---|
| 1-year mortality | 6 (11.3) | 10 (20) | 3 (4.3) | 5 (2.5) | <.001 |
| Rehospitalization | 23 (37.7) | 29 (49.2) | 30 (39%) | 64 (29.2) | 0.028 |
Values are given as numbers (%). The chi-square test was used
Figure 2.
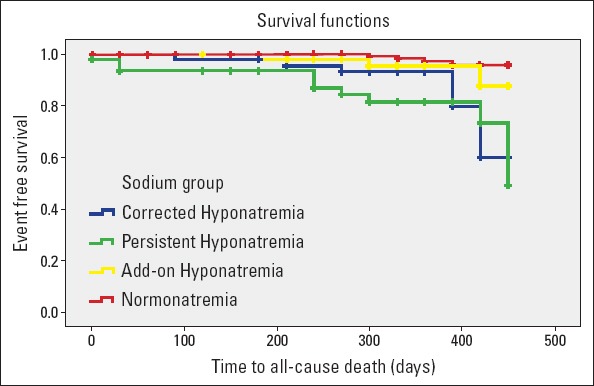
Kaplan–Meier survival curves for patients stratified into four groups according to changes in sNa levels from baseline to discharge
Discussion
The results of this retrospective observational study performed in Turkish patients corroborate with previous findings from various countries, showing that patients with HF and hyponatremia have increased mortality and rehospitalization rates compared with normonatremic patients (2, 5, 12, 16). Hence, it can be said that the poor prognostic impact of hyponatremia on clinical outcomes in HF is conserved in Turkish patients. The patients with hyponatremia have worse left ventricular systolic function and lower kidney function. Additionally while the patients with persistent hyponatremia have the worst clinical outcomes, in-hospital correction of hyponatremia on admission may improve rehospitalization and mortality rates. Similar to other studies (2, 6, 16, 17), patients with hyponatremia had prolonged hospital stays, which will likely increase the economic burden of the disease.
In this study, we found that the indicators of more severe HF, such as lower EF, lower BP, and impaired renal function were more common in patients with hyponatremia. Although the relation between lower left ventricular EF and hyponatremia was not significant in some studies (2, 12), the findings of others support the significant relation (13, 18) as this study demonstrates. Lower systolic BP, another indicator of poor prognosis in HF, was more prevalent in hyponatremic patients. When related literature was reviewed, this appears to be an almost unanimous finding (5, 6, 10, 12, 13, 17). Unsurprisingly, this increased the need for intravenous inotropes, which was shown in other studies as well. The levels of serum creatinine, BUN, and CrCl indicate worse renal function in hyponatremic patients in our study cohort. Similarly, serum creatinine levels were found to be higher in most of the previous studies (6, 12, 17, 18).
Hyponatremic patients stayed in the hospital for a longer time than patients with normal sNa levels. Other studies comparing length of hospital stay between hypo- and normonatremic patients have reported similar findings (2, 4, 6, 19). Neither previous studies nor our study have analyzed the independent impact of sNa levels on the length of hospital stay; however, it is possible that this is, in part, due to more advanced stages of HF observed in hyponatremic patients. Longer hospital stays are expected to increase the economic burden of the disease irrespective of the reason.
Because it is a poor prognostic indicator and measure of quality of life and cost, rehospitalization is usually included either alone or belonging to a composite clinical end point in studies examining various aspects of HF. We found that patients with low sNa levels experienced higher rehospitalization rates than normonatremic patients in our cohort. In other studies, when examined as a composite end-point usually with mortality, it was observed that rehospitalization incidence was increased in hyponatremic patients (2, 4, 12, 13). However, this relation does not always exist when it is examined as an individual end-point. In the Optimize HF registry, the rehospitalization rate was high in hyponatremics but was independent of sNa levels. Likewise, RICA investigators reported that after Cox regression adjustment, it was the co-morbidities rather than hyponatremia that affected readmission rates (16). On the other hand, in ambulatory patients with HFrEF, hyponatremia was found to be an independent predictor of hospitalization (10).
In most studies, hyponatremia was found to be an independent predictor of mortality in HF patients (2, 5, 12). In our cohort, there was a strong and independent association between hyponatremia and mortality. However, the causality of this association is still questioned. Chawla et al. (20) demonstrated that the nature of the underlying illness, rather than the severity of hyponatremia, best explain mortality associated with hyponatremia. More recently, regarding rehospitalization, RICA investigators stated that comorbidities not hyponatremia were independent predictors of mortality (16). We accept that there might be an interaction between hyponatremia, comorbidities, and mortality, but because the current available data overwhelmingly indicates an independent association, this study supports that hyponatremia increases mortality rates in HF patients.
A more controversial issue related to sNa level and clinical outcomes in HF is how changes in sNa levels during the course of hospitalization affect clinical outcomes. HF patients with persistent hyponatremia, i.e., present both on admission and at discharge, have the highest mortality and rehospitalization rates compared with all-time normonatremics, and these differences were significant in this study. This is concordant with previously reported studies focusing on the same issue (2, 3, 21). Therefore, it seems reasonable to improve the clinical outcomes by correcting hyponatremia. There is a scarcity of studies addressing this issue with many contradictory results. In a report from OPTIME-CHF, study patients with corrected hyponatremia (>135 mEq/L) at discharge had a lower death rate compared with patients with persistent hyponatremia; however, it was not significant (2). In a study that included only hyponatremic patients, Madan et al. (21) reported that increase in sNA levels significantly decreased mortality rates. A substudy of ESCAPE Trial examining the prognostic value of persistent hyponatremia also looked for the effect of correcting this electrolyte abnormality on clinical outcomes. Compared with persistent hyponatremics, corrected hyponatremia improved neither mortality nor rehospitalization rates (3). However, when mortality and rehospitalization were considered as a composite end point, correcting low sNa levels significantly decreased adverse outcomes. A study from Korea showed that compared with the normonatremics, correcting hyponatremia does not improve clinical outcomes (22). Although they did not report any comparison between persistent and corrected hyponatremia, in their article, they depicted a better Kaplan–Meier survival curve for corrected hyponatremia. In our study, correction in sNa during the course of hospitalization tends to be associated with better outcomes than the persistent hyponatremia; however, this difference was not statistically significant. It can be briefly said that very limited data related with the correction of low sNa levels, and its clinical consequences is not conclusive. None of the studies reported measures taken to increase sNa levels. This uncertainty makes the issue even more complex. Therefore, it is obvious that in order to resolve this issue, a well-designed prospective randomized controlled trial is needed.
Study limitations
The present study inherits all the limitations of retrospective studies. Data were dependent on the accuracy of documentation and abstraction by centers that participated. We did not collect information regarding the dose of diuretics and nonpharmacological treatments, such as ICD and CRT. Because of the observational nature of the study, we are unable to provide insights into the mechanisms underlying the association between hyponatremia, mortality, and rehospitalization. In additiona, our study did not specifically address the importance of correcting electrolyte disorders. Despite covariate adjustment, other measured and unmeasured factors might have influenced outcomes. Although we adjusted for multiple covariates during the index admission, we did not adjust for changes in these parameters during the follow-up.
Conclusion
Hyponatremia is common in Turkish patients hospitalized for worsening HF. Low sNa levels in this population were an independent and strong risk factor for increased mortality and re-hospitalization. While patients with persistent hyponatremia during the course of their hospitalization had the worst outcomes, it remains unknown if correcting this electrolyte abnormality might increase event-free survival in HF patients. Prospective confirmation of our findings in a larger population is needed.
Acknowledgements:
This study could not have been carried out without the cooperation and support of the investigators and sub-investigators mentioned below.
Abdurrahman Oğuzhan, Ahmet Temizhan, Cağdaş Ozdol, Cevat Kırma, Hüseyin Şenocak, Ibrahim Sarı, K. Hakan Kültürsay, Mahmut Şahin, Mehmet Aksoy, Mustafa Demirtaş, Nizamettin Toprak, Sema Güneri, Ahmet Ekmekci, Ahmet Seyfettin Gürbüz, Bahri Akdeniz, Baktash Morrad, Benil NesiI Şahin, Bilgehan Karadağ, Emre Ozcalik, Kaan Okyay, M. Hakan Taş, Meral Kayıkcıoğlu, Murat Sünbül, Mustafa Inc, Nihat Polat, Suzan Polat, Ufuk Özgül, and Ufuk Yıldırım.
We thank them for allowing us to obtain the data. Epikriz Company provided us consultancy in statistical analyses.
Burçak Kılıçkıran Avcı, Murathan Küçük, Haldun Müderrisoğlu, Mehmet Eren, Merih Kutlu, Mehmet Birhan Yılmaz, Yüksel Çavuşoğlu, Zeki Öngen. The relation between serum sodium levels and clinical outcomes in Turkish patients hospitalized for heart failure: a multicenter observational study. Anatol J Cardiol 2015; 15 (Suppl 1): 33 [OP 114]
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Funding: This study was supported by Abdi İbrahim Otsuka. All aspects of the study design, data collection, analysis, and manuscript preparation were performed by the authors. The sponsor did not have access to the data but did review the manuscript before submission. All decisions regarding the final form of the manuscript were entirely at the discretion of the authors, who take full responsibility for its content.
Authorship contributions: Concept – B.K.A., Z.Ö.; Design – M.Küçük., H.M., M.E., M.Kutlu., M.B.Y., B.K.A., Y.Ç., Z.Ö.; Supervision – M.Küçük., H.M., M.Kutlu., M.B.Y., Y.Ç., Z.Ö., B.K.A.; Materials – N.A.; Data collection &/or processing – M.Küçük., H.M., M.E., M.Kutlu., M.B.Y., B.K.A., Y.Ç., Z.Ö.; Analysis &/or interpretation – B.K.A., Z.Ö.; Literature search – M.Küçük., H.M., M. Kutlu., M.B.Y., Y.Ç., Z.Ö., B.K.A.; Writing – B.K.A., Z.Ö.; Critical review – M.Küçük., H.M., M. Kutlu., M.B.Y., Y.Ç., Z.Ö., B.K.A.
References
- 1.De Luca L, Kelin L, Udelson JE, Orlandi C, Sardella G, Fedele F, et al. Hyponatremia in patients with heart failure. Am J Cardiol. 2005;96:19L–23L. doi: 10.1016/j.amjcard.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 2.Klein L, O’Connor CM, Leimberger JD, Gattis-stough W, Pifia IL, Felker GM, et al. OPTIME – CHF Investigators. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure. Results from the outcome of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure (OPTIME-CHF) study. Circulation. 2005;111:2454–60. doi: 10.1161/01.CIR.0000165065.82609.3D. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiade M, Rossi JS, Cotts W, Shin DD, Helkamp AS, Pifia IL, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE trial. Arch Intern Med. 2007;167:1998–2005. doi: 10.1001/archinte.167.18.1998. [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O’Connor CM, et al. OPTIMIZE-HF Investigators and Coordinators. Relationship between admission serum sodium concentration and clinical outcomes in patients with hospitalized for heart failure:an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–8. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 5.Rusinaru D, Tribouilloy C, Berry C, Richards AM, Whalley GA, Earle N, et al. Relationship of serum sodium concentration to mortality in a wide spectrum of heart failure patients with preserved and with reduced ejection fraction:an individual patient data meta-analysis:Meta-Analysis Global Group in Chronic heart failure (MAGGIC) Eur J Heart Fail. 2012;14:1139–46. doi: 10.1093/eurjhf/hfs099. [DOI] [PubMed] [Google Scholar]
- 6.Sato N, Gherorghiade M, Kajimoto K, Munakata R, Minami Y, Mizuno M, et al. ATTEND Investigators. Hyponatremia and in-hospital mortality in patients admitted for heart failure (from the ATTEND registry) Am J Cardiol. 2013;111:1019–25. doi: 10.1016/j.amjcard.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Hamaguchi S, Kinugawa S, Tsuchihashi-Makaya M, Matsushima S, Sakakibara M, Ishimori N, et al. Hyponatremia is an independent predictor of adverse clinical outcomes in hospitalized patients due to worsening heart failure. J Cardiol. 2014;63:182–8. doi: 10.1016/j.jjcc.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Kearney MT, Fox KA, Lee AJ, Prescott RJ, Shah AM, Batin PD, et al. Predicting death due to progressive heart failure in patients with mild-to-moderate chronic heart failure. J Am Coll Cardiol. 2002;40:1801–8. doi: 10.1016/s0735-1097(02)02490-7. [DOI] [PubMed] [Google Scholar]
- 9.Gankam-Kengne F, Ayers C, Khera A, de Lemos J, Maalouf NM. Mild hyponatremia is associated with an increased risk of death in an ambulatory setting. Kidney Int. 2013;83:700–6. doi: 10.1038/ki.2012.459. [DOI] [PubMed] [Google Scholar]
- 10.Bavishi C, Ather S, Bambhroliya A, Jneid H, Virani SS, Bozkurt B, et al. Prognostic significance of hyponatremia among ambulatory patients with heart failure and preserved and reduced ejection fractions. Am J Cardiol. 2014;113:1834–8. doi: 10.1016/j.amjcard.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Corona G, Giuliani C, Parenti G, Norello D, Verbalis JG, Forti G, et al. Moderate hyponatremia is associated with increased risk of mortality:evidence from a meta-analysis. PLoS One (serial online) 2013;8:e80451. doi: 10.1371/journal.pone.0080451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapłon-Cieślicka A, Ozierański K, Balsam P, Tymińska A, Peller M, Galas M, et al. Clinical characteristics and 1-year outcome of hyponatremic patients hospitalized for heart failure. Pol Arch Med Wewn. 2015;125:120–31. doi: 10.20452/pamw.2701. [DOI] [PubMed] [Google Scholar]
- 13.Balling L, Schou M, Videbæk L, Hildebrandt P, Wiggers H, Gustafsson F. Danish Heart Failure Clinics Network. Prevalence and prognostic significance of hyponatraemia in outpatients with chronic heart failure. Eur J Heart Fail. 2011;13:968–73. doi: 10.1093/eurjhf/hfr086. [DOI] [PubMed] [Google Scholar]
- 14.ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med. 1999;18:1905–42. [PubMed] [Google Scholar]
- 15.Harjola VP, Follath F, Nieminen MS, Brutsaert D, Dickstein K, Drexler H, et al. Characteristics, outcomes, and predictors of mortality at 3 months and 1 year in patients hospitalized for acute heart failure. Eur J Heart Fail. 2010;12:239–48. doi: 10.1093/eurjhf/hfq002. [DOI] [PubMed] [Google Scholar]
- 16.Arévalo Lorido JC, Carretero Gómez J, Formiga F, Montero Pérez-Barquero M, Trullás Vila JC, Aramburu Bodas O, et al. RICA Investigators. Hyponatremia as predictor of worse outcome in real world patients admitted with acute heart failure. Cardiol J. 2013;20:506–12. doi: 10.5603/CJ.2013.0136. [DOI] [PubMed] [Google Scholar]
- 17.Shorr AF, Tabak YP, Johannes RS, Gupta V, Saltzberg MT, Costanzo MR. Burden of sodium abnormalities in patients hospitalized for heart failure. Congest Heart Fail. 2011;17:1–7. doi: 10.1111/j.1751-7133.2010.00206.x. [DOI] [PubMed] [Google Scholar]
- 18.Sharma AK, Vegh EM, Kandala J, Orencole M, Januszkiewicz L, Bose A, et al. Usefulness of hyponatremia as a predictor for adverse events in patients with heart failure receiving cardiac resynchronization therapy. Am J Cardiol. 2014;114:83–7. doi: 10.1016/j.amjcard.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Shchekochikhin DY, Schrier RW, Lindenfeld J, Price LL, Jaber BL, Madias NE. Outcome differences in community- versus hospital-acquired hyponatremia in patients with a diagnosis of heart failure. Circ Heart Fail. 2013;6:379–86. doi: 10.1161/CIRCHEARTFAILURE.112.000106. [DOI] [PubMed] [Google Scholar]
- 20.Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD. Mortality and serum sodium:do patients die from or with hyponatremia? Clin J Am Soc Nephrol. 2011;6:960–5. doi: 10.2215/CJN.10101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madan VD, Novak E, Rich MW. Impact of change in serum sodium concentration on mortality in patients hospitalized with heart failure and hyponatremia. Circ Heart Fail. 2011;4:637–43. doi: 10.1161/CIRCHEARTFAILURE.111.961011. [DOI] [PubMed] [Google Scholar]
- 22.Lee SE, Choi DJ, Yoon CH, Oh IY, Jeon ES, Kim JJ, et al. KorHF Registry. Improvement of hyponatraemia during hospitalisation for acute heart failure is not associated with improvement of prognosis:an analysis from the Korean Heart Failure (KorHF) registry. Heart. 2012;98:1798–804. doi: 10.1136/heartjnl-2012-302334. [DOI] [PubMed] [Google Scholar]


