Abstract
Objective:
Recent studies have shown that activation of the immune system, inflammatory cell infiltration, and activation of inflammatory mediators play an important role in the development of heart failure. The purpose of this study was to investigate whether cardiac function can be improved by regulating the balance of lymphocyte subsets and cytokines.
Methods:
Ninety-six patients with chronic heart failure (CHF) who were older than 60 years were randomly divided into two groups: CHF testing group (CHFT) received regular therapy and thymopentin (2 mg thymopentin per day, 15th as a course, three courses in total). CHF control group (CHFC) received regular therapy. Forty-five healthy individuals older than 60 years were used as normal controls. The ejection fraction of left ventricle (LVEF), inner diameter of left ventricular end-diastole (LVEDD), inner diameter of left ventricular end-systole (LVESD), plasma high sensitive C-reactive protein (hsCRP), plasma brain natriuretic peptide (BNP), 6-min walking distance (6MWT), Minnesota Living with Heart Failure Questionnaire (MLHFQ) assessment, lymphocyte subsets, and inflammatory cytokines were tested.
Results:
The levels of LVEF, 6MWT, CD 3+, CD4+T cells, natural killer cells, CD4+/CD8+ and IL-10 in CHFT were increased (p<0.01) compared with CHFC, while BNP, hsCRP, MLHFQ, CD8+, TNF-a, IL-1b, and TNF-a/IL-10 ratio in CHFT were decreased (p<0.01). LVEDD and LVESD were decreased, even though there was no significant difference between the two CHF groups.
Conclusion:
These data suggest that immune modulation therapy improve cardiac function and regulate cytokines and lymphocyte subsets in older patients with CHF.
Keywords: heart failure, thymopentin, therapy, BNP, TNF
Introduction
Chronic heart failure (CHF) is a final outcome of most cardiovascular diseases and has become a serious threat to cardiac patients. Compared with young adults, the elderly tend to have impaired nutritional and immune conditions, which may predict a poor outcome in patients with CHF (1). Neurohormonal mechanisms form the pathophysiological basis for the development of heart failure. Recent studies have shown that inflammatory cell infiltration and inflammatory mediator activation play an important role in HF progression (2, 3). T lymphocytes activation and infiltration in the left ventricle may induce cardiac fibrosis and hypertrophy (4–6). B lymphocyte cells also played a contributory role in the progression of CHF, partly by production of proinflammatory cytokines (7). Therefore, we launched the present study to investigate the effect of the immune modulatory therapy on older patients with CHF. Thymopentin, a five-peptide extracted from the thymus of animals (cattle or pigs), was chosen to be the immune modulator. It can specifically promote the differentiation and maturation of T cells and natural killer cells (NK) and enhance the function of T helper cells. It can achieve a two-way adjustment to the immune system by increasing the levels of intracellular cyclic adenosine monophosphate, elevating the activity of T cells, and regulating the proportions of T cell subsets.
Methods
Patient selection
Ninety-six hospitalized patients (54 males and 42 females) with primary CHF were enrolled from Beijing Anzhen Hospital, Department of Cardiology, 12th ward from October 2007 to February 2009. The patients were 60–78 (mean 70.3±7.5) years old with New York Heart Association (NYHA) functional class II–IV, left ventricular ejection fraction (LVEF) ≤40%. Patients were excluded based on the presence of a tumor, acute or chronic infection, immune system disease, recent major surgery or trauma (within 6 months), rheumatoid activity, acute cerebrovascular disease (within 6 months) and liver, kidney, or pulmonary insufficiency. Forty-five healthy individuals (22 male and 23 female) aged 60–80 (mean 68.8±7.7) years who went for a health check-up and then were screened by Physical Examine Center of Beijing Anzhen Hospital during the period of October 2007 to February 2009 were used as normal controls.
Study treatment
Patients enrolled were randomly divided into two groups: CHF treatment group (n=48) and CHF control group (n=48). There were no statistically significant differences of sex ratio, mean age, diagnosis, NYHA classification, drug treatment, or other basic indicators between the two groups (Table 1). CHF testing group (CHFT) received regular therapy and thymopentin, and the CHF control group (CHFC) received regular therapy and placebo (3 ml of saline). Thymopentin treatment method was as follows: 2 mg thymopentin (HeXin 1 mL: 1 mg, produced by Hainan Zhonghe Pharmaceutical Co., Ltd., Haikou, China) added to l mL saline for intramuscular injection, once per day for 15th (one course). Patients received three courses of treatment. At the end of each treatment course, injections of thymopentin were stopped for 15th. Then the next course would start for a total of 75th’ treatment. Some patients developed drowsiness but no other side effects were observed. Regular therapy was administered according to the Guidelines for Diagnosis and Treatment of Chronic Heart Failure in China in 2007, including diuretics, angiotensin-converting enzyme inhibitors, or angiotensin II receptor antagonist, b-adrenergic receptor antagonists, aldosterone receptor antagonist agents, and digitalis and other drug treatments.
Table 1.
Baseline data for CHFC and CHFT
| CHFC | CHFT | |
|---|---|---|
| Number | 48 | 48 |
| Sex ratio (male/female) | 2.75 | 2.80 |
| Average age | 69.6±7.8 | 70.9±7.5 |
| Diagnosis | ||
| Ischemic cardiomyopathy, n (%) | 36 (75.0%) | 40 (83.3%) |
| Dilated cardiomyopathy, n (%) | 5 (10.4%) | 4 (8.3%) |
| Rheumatic heart disease, n (%) | 3 (6.3%) | 2 (4.2%) |
| Hypertensive heart disease, n (%) | 4 (8.3%) | 2 54.2%) |
| Heart failure duration, year | 6.8±7.6 | 5.5±5.9 |
| NYHA functional class | ||
| II, n (%) | 14 (29.1%) | 13 (27.1%) |
| III, n (%) | 26 (54.2%) | 28 (58.3%) |
| IV, n (%) | 8 (16.7%) | 7 (14.6%) |
| LVEF (%) | 38.5±11.7 | 34.8±9.1 |
| Medication use, n (%) | ||
| Diuretics | 45 (93.8%) | 43 (89.6%) |
| ACEI or ARB | 39 (81.3%) | 39 (81.3%) |
| Beta-blocker | 33 (68.8%) | 37 (77.0%) |
| Aldosterone antagonist | 45 (93.8%) | 43 (89.6%) |
| Digitalis | 22 (45.8%) | 19 (39.6%) |
Unpaired t-test was used to determine the statistical significance of differences. There were no statistically significant differences in sex ratio, mean age, diagnosis, NYHA classification, drug treatment, and other basic indicators between the two groups. ACEI - angiotensin converting enzyme inhibitor; ARB - angiotensin receptor blockers; CHF - chronic heart failure; CHFC - CHF control group; CHFT - CHF testing group; LVEF - ejection fraction of left ventricle; NYHA - New York Heart Association
The ejection fraction of left ventricle (LVEF), the inner diameter of left ventricular end-diastole (LVEDD), the inner diameter of left ventricular end-systole (LVESD), plasma high sensitive C-reactive protein (hsCRP), plasma brain natriuretic peptide (BNP), 6-min walking distance (6MWT), Minnesota Living with Heart Failure Questionnaire (MLHFQ) assessment, T lymphocyte subsets (CD3+, CD4+, CD8+), B lymphocytes (CD19+), natural killer lymphocytes (CD56+CD16+), inflammatory cytokines including tumor necrosis factor-a (TNF-a), interleukin-1-b (IL-1b) and the anti-inflammatory cytokine IL-10 were tested at the following time points: before therapy, after the first course of treatment (15th), and after the third course of treatment (75th).
LVEF (Simpson’s method), LVEDD, and LVESD were detected by echocardiography using HP2500 and the detection combined M-mode and 2 dimensional echocardiograms. Lymphocyte subsets were analyzed by flow cytometry determination (FACSCalibur, BD Biosystems, San Jose, CA, USA). hsCRP and BNP were detected by an enzyme-linked immunoassay (ELISA) kit (BIOSIS, USA). Serum TNF-a and IL-1b were measured by radioimmunoassay (Technology Development Center of People’s Liberation Army General Hospital, kit number: 20090220). IL-10 was detected by ELISA kit (Wellscan MK3, Finland). All protocols were performed according to the manufacturers’ instructions.
6MWT was detected by Bittner programs. During the testing process, heart rate and rhythm were measured by remote monitoring ECG. Blood pressure was measured before and after the walk. All patients completed the walk test, while seven patients (three patients from CHFT group and four patients from CHFC group) developed symptoms of chest tightness, palpitations, and shortness of breath. The heart rates of six patients (three patients from CHFT group and three patients from CHFC group) were higher than 100 beats/min (mean 102–125 beats/min) when the walk was completed, but without chest pain or severe arrhythmia.
MLHFQ was used to assess the quality of life in different groups and was measured at the following time points: before therapy, after the first course of treatment (15th), and after the third course of treatment (75th). MLHFQ included 21 items to evaluate the typical heart failure symptoms, signs, emotions, work, and lifestyle. All items used five class Likert scores, with a total score ranging from 0 to 105 (no loss of function to the largest degree of loss of function). Thus, the higher the score the worse the quality of the patient’s life. Assessment methods were as follows: after evaluation, staff fully explained the questionnaire and the subjects completed the self-evaluation questionnaires independently. If subjects could not complete the questionnaire independently because of lack of education, disease, or other reasons, evaluators would ask the questions and record the responses one by one based on the questionnaire.
Ethics statement
We have obtained appropriate institutional review board approval and have followed the principles outlined in the Declaration of Helsinki for all human experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Statistical analyses
All data are expressed as mean±SD. Shapiro–Wilk was used for normality tests. One way ANOVA, unpaired t-test, and paired t-test were used to determine the statistical significance of differences. All data were evaluated statistically using SPSS 13.0 (SPSS Inc., Chicago, IL, USA) software. Statistical significance was considered when p<0.05.
Results
Effect of immune modulation therapy on cardiac function in older patients with CHF
The levels of LVEDD and LVESD were significantly increased (p<0.01), and the levels of LVEF and 6MWT were markedly decreased in the CHFT and CHFC groups (p<0.01) compared with the normal control group before therapy. No difference was observed between CHFT and CHFC groups (Table 2).
Table 2.
Comparison of the indicators among CHFC, CHFT, and the healthy control (HC) groups before treatment
| HC Group (n=45) | CHFC Group (n=48) | CHFT Group (n=48) | |
|---|---|---|---|
| LVEF, % | 62.6±10.5 | 36.0±6.9** | 34.9±7.8** |
| LVEDD, mm | 48.0±5.0 | 60.3±9.4** | 63.2±6.6** |
| LVESD, mm | 32.4±4.2 | 52.0±5.3* | 54.7±6.9* |
| 6MWT, m | 572.0±56.0 | 323.0±44.8** | 322.0±37.5** |
| MLHFQ, score | 14.3±10.4 | 49.0±22.5** | 47.5±18.8** |
| BNP, pg/mL | 209.0±263.5 | 4331.5±3578.6** | 4869.4±3665.8** |
| hsCRP, mg/L | 2.3±1.5 | 13.2±15.4** | 15.8±15.2** |
| CD3+, % | 67.2±12.5 | 56.2±11.3** | 54.4±10.9** |
| CD3+/CD4+, % | 46.2±7.5 | 22.5±9.6** | 21.5±10.6** |
| CD3+/CD8+, % | 20.2±6.4 | 35.1±9.2** | 33.0±8.8** |
| CD4+/CD8+ | 2.3±0.6 | 0.6±0.9** | 0.6±0.8** |
| CD56+16+, % | 20.1±12.4 | 9.6±12.9** | 11.2±11.5** |
| CD19+, % | 16.3±7.8 | 12.2±6.8 | 12.6±5.7 |
| TNF-α, ng/mL | 0.813±0.104 | 1.009±0.079** | 0.989±0.094** |
| IL-1β, ng/mL | 0.173±0.041 | 0.228±0.044** | 0.218±0.027** |
| IL-10, pg/mL | 188.45±15.15 | 176.01±4.98** | 175.57±8.93** |
| TNF-α/IL-10 | 4.314±0.36 | 5.732±0.33** | 5.633±0.28** |
Data are expressed as mean±SD or %. Shapiro–Wilk was used for normality tests, and the variables are subjected (or approximatively subjected) to normal distribution. One-way ANOVA was used to determine the statistical significance of differences. Compared with HC group,
P<0.05,
P<0.01.
BNP - plasma brain natriuretic peptide; CHF - chronic heart failure; CHFC - CHF control group; CHFT - CHF testing group; hsCRP - plasma high sensitive C-reactive protein; IL-1β - interleukin-1-β; LVEDD - the inner diameter of left ventricular end-diastole; LVEF - ejection fraction of left ventricle; LVESD - the inner diameter of left ventricular end-systole; MLHFQ - Minnesota Living with Heart Failure Questionnaire assessment; TNF-α - tumor necrosis factor-α; 6MWT - 6-min walking distance
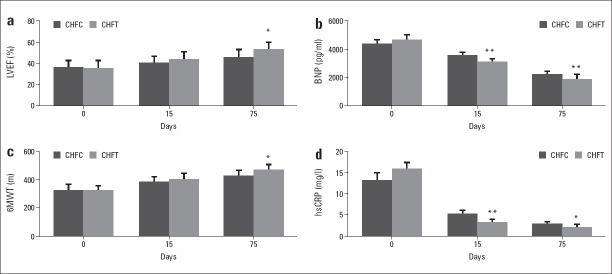
At 15th, compared with CHFC, the levels of LVEF and 6MWT in CHFT tended to be increased, while the levels of LVEDD, LVESD, and MLHFQ in CHFT were decreased, although no significant differences were observed between the two groups (Fig. 1, Table 3).
Figure 1.
Effect of immune modulation therapy on LVEF, 6MWT, BNP, and hsCRP in older patients with CHF
Compared with CHFC group, *P<0.05, **P<0.01. BNP - plasma brain natriuretic peptide; CHF - chronic heart failure; CHFC - CHF control group; CHFT - CHF testing group; hsCRP - plasma high sensitive C-reactive protein; LVEF - ejection fraction of left ventricle; 6MWT - 6-min walking distance
Table 3.
Comparison of the indicators among different groups at 15th and 75th
| At 15th | At 75th | |||
|---|---|---|---|---|
| CHFC (n=48) | CHFT (n=48) | CHF (n=48)C | CHFT (n=48) | |
| LVEF, % | 40.0±6.9 | 43.3±7.8 | 45.2±7.8 | 52.8±7.3* |
| LVEDD, mm | 60.1±6.4 | 58.8±7.5 | 57.4±8.4 | 54.8±6.0 |
| LVESD, mm | 50.8±8.2 | 48.5±6.7 | 46.8±9.6 | 42.1±7.9 |
| 6MWT, m | 382±39.8 | 401±47.8 | 425±44.5 | 466±42.0* |
| MLHFQ, score | 35.6±18.2 | 32.4±20.5 | 29.8±16.3 | 23.0±15.9* |
| BNP, pg/mL | 3535.5±267.6 | 3070.4±265.8** | 2166.2±278.4 | 1845.5±365.6** |
| hsCRP, mg/L | 5.2±0.5 | 3.1±0.4** | 2.8±0.3 | 2.0±0.3* |
| CD3+, % | 59.8±10.2 | 68.4±13.1* | 65.2±14.3 | 75.2±15.9** |
| CD3+/CD4+, % | 31.5±8.6 | 45.5±11.2** | 35.8±9.4 | 52.7±13.6** |
| CD3+/CD8+, % | 27.1±8.3 | 22.8±7.4* | 26.8±9.6 | 20.3±8.2* |
| CD4+/CD8+ | 1.1±0.3 | 2.1±0.4* | 1.1±0.3 | 2.3±0.34* |
| CD56+16+, % | 14.2±3.2 | 18.2±2.7* | 13.6±2.2 | 19.2±3.5* |
| CD19+, % | 14.3±2.9 | 19.5±3.8* | 14.2±3.7 | 19.9±4.8* |
| TNF-α, ng/mL | 0.944±0.106 | 0.882±0.096** | 0.929±0.101 | 0.838±0.100** |
| IL-1β, ng/mL | 0.216±0.068 | 0.194±0.068* | 0.208±0.074 | 0.181±0.032** |
| IL-10, pg/mL | 178.86±23.93 | 182.4±23.85** | 179.41±23.82 | 184.01±28.53** |
| TNF-α/IL-10 | 5.278±0.66 | 4.836±0.53 | 5.178±0.38 | 4.554±0.69** |
Data are expressed as mean±SD or %. Shapiro–Wilk was used for normality tests, and the variables are subjected (or approximatively subjected) to normal distribution. Unpaired t-test was used to determine the statistical significance of differences. Compared with CHFC group,
P<0.05,
P<0.01.
BNP - plasma brain natriuretic peptide; CHF - chronic heart failure; CHFC - CHF control group; CHFT - CHF testing group; hsCRP - plasma high sensitive C-reactive protein; IL-1β - interleukin-1-β; LVEDD - the inner diameter of left ventricular end-diastole; LVEF - ejection fraction of left ventricle; LVESD - the inner diameter of left ventricular end-systole; MLHFQ - Minnesota Living with Heart Failure Questionnaire assessment; TNF-α - tumor necrosis factor-α; 6MWT - 6-min walking distance
At 75th, compared with CHFC, the levels of LVEF and 6MWT were significantly increased (p<0.05), and the levels of MLHFQ were decreased in CHFT (p<0.05). LVEDD and LVESD were decreased but without a significant difference between the two CHF groups (Fig. 1, Table 3).
At 75th, compared with before treatment, the levels of LVEF and 6MWT were significantly increased (p<0.05), and the levels of LVEDD, LVESD (p<0.05), and MLHFQ (p<0.01) were decreased in CHFT. While in CHFC, after three courses treatment, the levels of LVEF and 6MWT were significantly increased (p<0.05), and the level of MLHFQ (p<0.01) was decreased (Table 4).
Table 4.
Comparison of the indicators of CHFC and CHFT before and after three courses treatment (75th)
| CHFC (n=48) | CHFT (n=48) | |||
|---|---|---|---|---|
| Before treatment | After 75th | Before treatment | After 75th | |
| LVEF, % | 36.0±6.9 | 45.2±7.8* | 34.9±7.8 | 52.8±7.3* |
| LVEDD, mm | 60.3±9.4 | 57.4±8.4 | 63.2±6.6 | 54.8±6.0* |
| LVESD, mm | 52.0±5.3 | 46.8±9.6 | 54.7±6.9 | 42.1±7.9* |
| 6MWT, m | 323.0±44.8 | 425±44.5* | 322.0±37.5 | 466±42.0* |
| MLHFQ, score | 49.0±22.5 | 29.8±16.3** | 47.5±18.8 | 23.0±15.9** |
| BNP, pg/mL | 4331.5±3578.6 | 2166.2±278.4** | 4869.4±3665.8 | 1845.5±365.6** |
| hsCRP, mg/L | 13.2±15.4 | 2.8±0.3** | 15.8±15.2 | 2.0±0.3** |
| CD3+, % | 56.2±11.3 | 65.2±14.3 | 54.4±10.9 | 75.2±15.9** |
| CD3+/CD4+, % | 22.5±9.6 | 35.8±9.4* | 21.5±10.6 | 52.7±13.6** |
| CD3+/CD8+, % | 35.1±9.2 | 26.8±9.6 | 33.0±8.8 | 20.3±8.2** |
| CD4+/CD8+ | 0.6±0.9 | 1.1±0.3 | 0.6±0.8 | 2.3±0.34* |
| CD56+16+, % | 9.6±12.9 | 13.6±2.2 | 11.2±11.5 | 19.2±3.5* |
| CD19+, % | 12.2±6.8 | 14.2±3.7 | 12.6±5.7 | 19.9±4.8* |
| TNF-α, ng/mL | 1.009±0.079 | 0.929±0.101 | 0.989±0.094 | 0.838±0.100* |
| IL-1β, ng/mL | 0.228±0.044 | 0.208±0.074 | 0.218±0.027 | 0.181±0.032 |
| IL-10, pg/mL | 176.01±4.98 | 179.41±23.82 | 175.57±8.93 | 184.01±28.53 |
| TNF-α/IL-10 | 5.732±0.33 | 5.178±0.38 | 5.633±0.28 | 4.554±0.69* |
Data are expressed as mean±SD or %. Shapiro–Wilk was used for normality tests, and the variables are subjected (or approximatively subjected) to normal distribution. Paired t-test was used to determine the statistical significance of differences. Compared with before treatment,
P<0.05,
P<0.01.
BNP - plasma brain natriuretic peptide; CHF - chronic heart failure; CHFT - CHF testing group; hsCRP - plasma high sensitive C-reactive protein; IL-1β - interleukin-1-β; LVEDD - the inner diameter of left ventricular end-diastole; LVEF - ejection fraction of left ventricle; LVESD - the inner diameter of left ventricular end-systole; MLHFQ - Minnesota Living with Heart Failure Questionnaire assessment; TNF-α, tumor necrosis factor-α; 6MWT - 6-min walking distance
Effect of immune modulation therapy on BNP and hsCRP in older patients with CHF
Before therapy, compared with normal control group, the plasma levels of BNP (p<0.01) and hsCRP (p<0.01) were significantly increased in CHFT and CHFC, but no significant difference was observed between CHFT and CHFC (Table 2).
At 15th and 75th, compared with CHFC, the plasma levels of BNP (p<0.01) and hsCRP (p<0.01) in CHFT were significantly decreased (Fig. 1, Tables 2–3).
At 75th, compared with before treatment, the levels of BNP and hsCRP were significantly decreased both in CHFC and CHFT (p<0.01) (Table 4).
Effect of immune modulation therapy on lymphocyte subsets in older patients with CHF
Before therapy, compared with the normal control group, the levels of CD8+ T cells were significantly increased (p<0.01), and the levels of CD3+ T cells (p<0.01), CD4+ T cells (p<0.01), NK cells (p<0.01) and CD4+/CD8+ (p<0.01) were markedly decreased in CHFT and CHFC, while no difference was observed between CHFT and CHFC (Table 2).
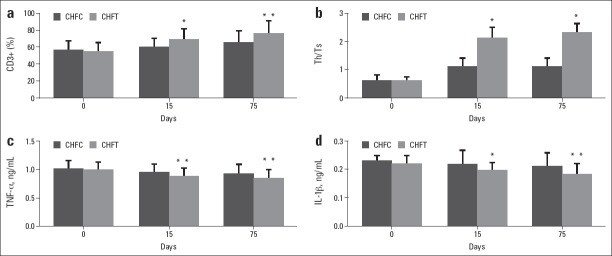
At 15th, compared with CHFC, the levels of CD3+ T cells (p<0.05), CD4+ T cells (p<0.01), CD19+ B cells (p<0.05), and NK cells (p<0.05) in CHFT were markedly increased in CHFT, while the levels of CD8+ T cells in CHFT were decreased, but no significant difference was observed between the two groups (Table 3).
At 75th, compared with CHFC, the levels of CD3+ T cells (p<0.01), CD4+ T cells (p<0.01), CD19+ B cells (p<0.05), NK cells (p<0.05), and CD4+/CD8+ (p<0.05) in CHFT were increased, while the levels of CD8+ T cells were decreased in CHFT (p<0.05) (Fig. 2, Table 3).
Figure 2.
Effect of immune modulation therapy on lymphocyte subsets and inflammatory/anti-inflammatory cytokines in older patients with CHF
Compared with CHFC group, *P<0.05, **P<0.01. CHF - chronic heart failure; CHFC - CHF control group; CHFT - CHF testing group; IL-1b - interleukin-1-b; TNF-a - tumor necrosis factor-a
At 75th, compared with before treatment, the levels of CD3+ T cells (p<0.01), CD4+ T cells (p<0.01), CD19+ B cells (p<0.05), NK cells (p<0.05), and CD4+/CD8+ (p<0.05) were increased, while the levels of CD8+ T cells (p<0.01) were decreased in CHFT. As for CHFC, after three courses treatment, the level of CD4+ T cells was increased (p<0.05), while the other lymphocyte subsets were not changed (Table 4).
Effect of immune modulation therapy on inflammatory/anti-inflammatory cytokines in older patients with CHF
Before therapy, compared with the normal control group, the levels of TNF-a (p<0.01), IL-1b (p<0.01), and TNF-a/IL-10 ratio (p<0.01) were significantly increased, and the levels of IL-10 (p<0.01) were decreased in CHFT and CHFC. No difference between CHFT and CHFC was observed (Table 2).
At 15th, compared with CHFC, the levels of IL-10 (p<0.01) were significantly increased, while the levels of TNF-a (p<0.01) and IL-1b (p<0.05) were significantly decreased in CHFT. The TNF-a/IL-10 ratio was decreased but was not significant (Fig. 2, Table 3).
At 75th, compared with CHFC, the levels of IL-10 (p<0.01) in the patients of CHFT were increased, while the levels of TNF-a (p<0.01), IL-1b (p<0.01), and TNF-a/IL-10 ratios (p<0.01) were decreased in CHFT (Fig. 2, Table 3).
At 75th, compared with before treatment, the levels of TNF-a (p<0.05) and TNF-a/IL-10 ratios (p<0.05) were decreased in CHFT. While in CHFC, these inflammatory cytokines were not changed after three courses treatment (Table 4).
Discussion
In the present study, we investigated the effect of immunomodulation therapy on CHF. Neurohumoral mechanisms and ventricular remodeling theory forms an important physiological basis for CHF; however, recent studies have shown that immune activation (8) and excessive production of inflammatory cytokines (9) play an important role in the development of heart failure. Thus, the progression and deterioration of CHF may occur when the dynamic balance of the immune system is altered and abnormally activated immune lymphocytes over-express inflammatory cytokines.
T cells, especially CD4+ T cells, were shown a modulating effect on myocardial fibrosis and cardiac remodeling in heart failure progression (4, 6). Meanwhile, a recent report (7) found that B cells probably mediated hypertrophy and collagen deposition in myocardium and contributed to deterioration of left ventricular function in the progression of HF by means of the influence on pro-inflammatory cytokines production, immunoglobulin G deposition, and apoptosis. The present study revealed that plasma percentage of CD3+ and CD4+ T cells, NK cells, and the CD4+/CD8+ ratio in the CHF group were significantly decreased, while CD8+ T cells were increased compared to the healthy control group. Then, at the end of the first and third thymopentin treatment courses (Table 3), compared with CHFC, the percentage of CD4+ T cell, B cells, and NK cells were increased, while CD8+ T cells were decreased in CHFT. As for the comparison of before and after treatment in CHFT (Table 4), the similar changed trend of lymphocyte subsets was observed. It seemed to be difficultly explained that the CD4+ T cells, which mediate the pathological mechanism of CHF, decreased in CHF patients. We supposed such a change of lymphocyte subsets that before therapy may be a transient and negative feedback modulation to the development of myocardial fibrosis and remodeling. After thymopentin treatment, the percentage of CD4+ T cell and B cell were increased. We are uncertain whether the raised percentage of these subsets may result in the evolution of cardiac remodeling and whether such a change trend may be temporary. However, we noticed the modulatory effect of thymopentin on the percentage or number of lymphocyte subsets. A longer term of observation may possibly reveal a different change of lymphocyte subsets.
As far as the role of inflammatory cytokines in CHF is concerned, as shown in this research, the serum levels of Th1-type inflammatory cytokines, including TNF-a and IL-1b, were significantly increased, while the level of IL-10, a Th2-type anti-inflammatory cytokine, was decreased in CHFT and CHFC compared with the normal control group (Table 2). These results suggest a Th1/Th2 ratio imbalance in CHF patients, which is consistent with Ma et al. (10) findings. More recently, Nevers et al. (5) also reported a remarkable upregulation of Th1 signature cytokines and pointed out the contribution of TNF-a to cardiac dysfunction. The increase of TNF-a and IL-1b may have a negative inotropic effect on the heart because of the activation of nitric oxide synthase and increasing the production of nitric oxide, which may cause a decline of myocardial contractility. In addition, TNF-a and IL-1b may stimulate cardiac hypertrophy, promote myocardial necrosis, induce cardiomyocyte apoptosis, and myocardial remodeling, as well as enhance expression of IL-6 that has a negative inotropic effect (11, 12). Moreover, the decreased IL-10 may also weaken the protective effect of anti-inflammatory cytokines on myocardium. Obviously, the disequilibrium of inflammatory/anti-inflammatory cytokines may bring a negative influence on CHF patients’ hearts. However, thymopentin treatment ameliorated the disordered status. At 15th and 75th of treatment, the levels of plasma TNF-a and IL-1b in CHFT were significantly decreased, while the levels of IL-10 were significantly increased compared with CHFC (Table 3). Thus, we also observed in CHFT a reduced TNF-a/IL-10 ratio, which was reported by literatures to be an important indicator for measuring immune function. These findings suggest that thymopentin can ameliorate the maladjusted equilibrium of inflammatory/anti-inflammatory cytokines and maintain immune system stability.
As for the comparison of cardiac function, the results showed that LVEF in CHFT group was remarkably increased and the MLHFQ score was significantly decreased. LVEDD and LVESD in CHFT also had a tendency to decrease after treatment, although no significant difference was observed between groups (Table 3). One cause of this finding may due to that ventricular compliance be able to be hardly affected by immune regulation therapy because of left ventricular enlargement, myocardial interstitial fibrosis, and ventricular remodeling of CHF patients. Meanwhile, compared with before treatment, patients in CHFT also demonstrated the remarkably improved indicators of heart function (Table 4). What’s more, as shown in our study, BNP and hsCRP were markedly decreased after treatment, both of which predict the severity of heart failure (13–16). As a whole, the heart function of CHF patients after thymopentin treatment was observably improved, and we believed that this amelioration might result from, in part at least, the modulatory effects of thymopentin mentioned above on cytokines.
Study limitations
Given to time limited, the modulatory effects of thymopentin on percentage of lymphocyte subsets in a relative long-term in the development of CHF remain unclear. The present study does not allow us to evaluate the effect of immune therapy on the incidence of major adverse cardiovascular events of the included patients. In addition, the conclusion would be more convincing if the myocardium pathological changes were presented in this study. All concerns mentioned above may need further research.
Conclusion
In summary, the use of thymopentin as an immunomodulator in older patients with CHF may modulate the number of lymphocyte subsets and disequilibrium of inflammatory/anti-inflammatory cytokines. This effect would help recover cardiac function and increase activity tolerance to improve the quality of life of CHF patients. Immune regulation therapy may provide a new direction for clinical treatment strategies for CHF patients. However, the change of percentage of lymphocyte subsets in the development of CHF remains unclear. Whether immunomodulators can reduce the clinical endpoints and mortality of CHF patients will require further study.
Acknowledgement
This study was supported by the Beijing Municipal Health Bureau Health Foundation of China (Grant No. 06–06).
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.

From Handan Yörükçü’s collections
References
- 1.Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb. 2016 Jan 18; doi: 10.5551/jat.31526. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Zhou Y, Meng L, Lu X, Ou N, Li X. Inflammatory mediators in Chinese patients with congestive heart failure. J Clin Pharmacol. 2009;49:591–9. doi: 10.1177/0091270009333265. [DOI] [PubMed] [Google Scholar]
- 3.Doehner W, von Haehling S, Anker SD, Lainscak M. Neurohormonal activation and inflammation in chronic cardiopulmonary disease:a brief systematic review. Wien Klin Wochenschr. 2009;121:293–6. doi: 10.1007/s00508-009-1194-7. [DOI] [PubMed] [Google Scholar]
- 4.Laroumanie F, Douin-Echinard V, Pozzo J, Lairez O, Tortosa F, Vinel C, et al. CD4+T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation. 2014;129:2111–24. doi: 10.1161/CIRCULATIONAHA.113.007101. [DOI] [PubMed] [Google Scholar]
- 5.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velazquez F, Aronovitz M, et al. Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure. Circ Heart Fail. 2015;8:776–87. doi: 10.1161/CIRCHEARTFAILURE.115.002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos G, Hofmann U, Frantz S. Myocardial fibrosis seen through the lenses of T-cell biology. J Mol Cell Cardiol. 2016;92:41–5. doi: 10.1016/j.yjmcc.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Cordero-Reyes AM, Youker KA, Trevino AR, Celis R, Hamilton DJ, Flores-Arredondo JH, et al. Full expression of cardiomyopathy is partly dependent on B-Cells:A pathway that involves cytokine activation, immunoglobulin deposition, and activation of apoptosis. J Am Heart Assoc. 2016;5:e002484. doi: 10.1161/JAHA.115.002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niethammer M, Sieber M, von Haehling S, Anker SD, Munzel T, Horstick G, et al. Inflammatory pathways in patients with heart failure and preserved ejection fraction. Int J Cardiol. 2008;129:111–7. doi: 10.1016/j.ijcard.2007.05.061. [DOI] [PubMed] [Google Scholar]
- 9.Goonewardena SN, Stein AB, Tsuchida RE, Rattan R, Shah D, Hummel SL. Monocyte subsets and inflammatory cytokines in acute decompensated heart failure. J Card Fail. 2015 Dec 17; doi: 10.1016/j.cardfail.2015.12.014. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma HX, You ZL, Wang RG. Effect of total flavones from Cuscuta chinensis on expression of Th type-1/Th type-2 cytokines, serum P and PR in abortion rats model. Zhong Yao Cai. 2008;31:1201–4. [PubMed] [Google Scholar]
- 11.Candia AM, Villacorta H, Jr, Mesquita ET. Immune-inflammatory activation in heart failure. Arq Bras Cardiol. 2007;89:183–90. doi: 10.1590/s0066-782x2007001500009. [DOI] [PubMed] [Google Scholar]
- 12.Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp (Warsz) 2009;57:165–76. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto T, Takase H, Toriyama T, Sugiura T, Sato K, Ueda R, et al. Circulating concentrations of cardiac proteins indicate the severity of congestive heart failure. Heart. 2003;89:1303–7. doi: 10.1136/heart.89.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berton G, Cordiano R, Palmieri R, Pianca S, Pagliara V, Palatini P. C-reactive protein in acute myocardial infarction:association with heart failure. Am Heart J. 2003;145:1094–101. doi: 10.1016/S0002-8703(03)00098-X. [DOI] [PubMed] [Google Scholar]
- 15.Araujo JP, Lourenco P, Azevedo A, Frioes F, Rocha-Goncalves F, Ferreira A, et al. Prognostic value of high-sensitivity C-reactive protein in heart failure:a systematic review. J Card Fail. 2009;15:256–66. doi: 10.1016/j.cardfail.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 16.KoçM Bozkurt A, Acartürk E, Şahin DY, Ünal I. Usefulness of N-terminal pro-B-type natriuretic peptide increase with exercise for predicting cardiovascular mortality in patients with heart failure. Am J Cardiol. 2008;101:1157–62. doi: 10.1016/j.amjcard.2007.11.070. [DOI] [PubMed] [Google Scholar]