Abstract
Objective:
Subendocardial viability ratio (SEVR), defined as diastolic to systolic pressure-time integral ratio, is a useful tool reflecting the balance between coronary perfusion and arterial load. Suboptimal SEVR creating a supply–demand imbalance may limit favorable cardiac response to cardiac rehabilitation (CR). To explore this hypothesis, we designed a study to analyze the relationship between baseline SEVR and response to CR in patients with coronary artery disease (CAD).
Methods:
In this prospectively study, after baseline arterial tonometry, echocardiography, and cardiopulmonary exercise tests (CPETs), patients undergone 20 sessions of CR. Post-CR echocardiographic and CPET measurements were obtained for comparison.
Results:
Final study population was comprised of fifty subjects. Study population was divided into two subgroups by median SEVR value (1.45, interquartile range 0.38). Although both groups showed significant improvements in peak VO2, significant improvements in oxygen pulse (πO2) (from 16.1±3.4 to 19.1±4.8 mL O2.kg–1.beat–1; p<0.001) and stroke volume index (from 31±5 to 35±6 mL; p=0.008) were observed in only the patients in the above-median subgroup. The change in πO2 was also significantly higher in the above-median SEVR subgroup (2.9±3.3 vs. 0.5±2.4; p=0.007).
Conclusion:
Our study shows that baseline supply–demand imbalance may limit systolic improvement response to CR in patients with CAD.
Keywords: arterial tonometry, cardiac rehabilitation, coronary artery disease, exercise training, subendocardial viability ratio
Introduction
Ischemic heart disease constitutes a wide spectrum of syndromes caused by myocardial supply and demand imbalance. Coronary revascularization procedures focus only on central aspect of this critical equilibrium, but it is increasingly being re- cognized that peripheral factors are also crucially important for an optimal cardiovascular performance (1, 2). Exercise-based cardiac rehabilitation (CR) is a multifaceted intervention with favorable effects that extend beyond coronary vasculature (3, 4). It may have a comparable efficacy to coronary revascularization in improving symptom-free exercise tolerance, maximum exercise capacity, and survival, even in patients with angiographically documented stenosis amenable for intervention (4, 5). CR may show these beneficial effects not only by increasing supply via a healthier coronary endothelial function (6), but also by lowering demand via an improved mechanical efficiency (7, 8) and vascular load (9–11). On the other hand, baseline supply–demand imbalance may have a negative effect on CR success and may be frustrating by causing time and resource consumption.
Although it may be difficult to estimate myocardial supply–demand ratio precisely, many clinical methods were proposed for its evaluation. A practical reflection of this information resides in the aortic pressure curve. While the systolic part of the aortic pressure curve reflects afterload and the area under it represents a measure of myocardial oxygen consumption (12, 13), the diastolic difference between aortic and ventricular pressure curves is a surrogate for diastolic coronary blood supply (14, 15). Thus, subendocardial viability ratio (SEVR), which consists of a diastolic to systolic pressure-time integral ratio, is an index of myocardial oxygen supply and demand (Fig. 1) (16, 17). Aortic pressure curve also contains arterial stiffness and ventriculo-vascular interrogation data by means of wave reflections (18). Given that both systolic and diastolic part of aortic pressure wave can be affected by reflected waves; myocardial supply–demand ratio may critically be influenced by peripheral vascular system. Until recently, invasive measurements were needed to elucidate this important interaction, but it has now become possible to construct aortic pressure curve noninvasively with the help of applanation tonometry.
Figure 1.
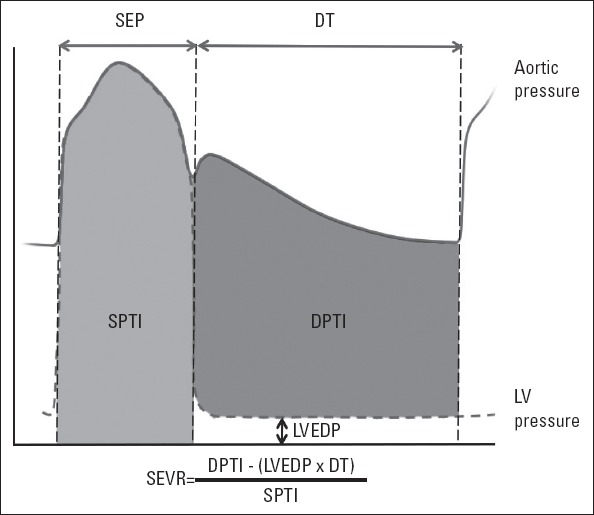
The key parameters for the calculation of subendocardial viability ratio (SEVR). SEVR is defined as diastolic pressure-time index (DPTI) divided by systolic pressure time index (SPTI). Diastolic pressure-time index is the area between aortic and left ventricular end-diastolic pressure (LVEDP) curves during diastolic time (DT), whereas systolic pressure-time index is the area under aortic pressure curve during systolic ejection period (SEP)
We hypothesized that a suboptimal supply–demand balance, which may be caused by negative macrovascular characteristics, may limit favorable cardiac response to CR. To explore this hypothesis, we designed a study to analyze the association bet- ween SEVR and response to CR in patients with coronary artery disease (CAD).
Methods
Patients
Study was executed at Hôpital Lariboisière, a tertiary center for CR. Consecutive outpatient CR referral requests were screened between November 2013 and May 2014. Patients with a history of recent (<2 months) hospital admission for an acute coronary syndrome and/or revascularization procedure were included. Patients with non-sinus rhythms, severe valvular di- sease, left main CAD, uninterpretable electrocardiograms with respect to ischemic changes were excluded. Also it was planned that the patients with an ischemic response in first cardiopulmonary exercise test to be excluded. Patients were under optimized, stable treatment, and medications were not withdrawn or changed for the study. All patients gave their informed consent. The study was approved by the Local Ethical Committee.
Study protocol
Blood chemistry analysis, transthoracic echocardiography, arterial tonometry were performed before exercise training program. Echocardiographic examination was performed immediately following arterial tonometry and both examinations were done at the same day within two hours before the first cardiopulmonary exercise test.
Arterial tonometry
A high-fidelity tonometer (SphygmoCor Px PWA System, AtCor Medical, West Ryde, Australia) was used to obtain pressure waveforms by applying sufficient pressure over the left radial artery. The device was repositioned until the strongest pulse signal is identified. After the calibration with manually measured brachial blood pressure, sequential pressure waveforms were acquired to obtain an averaged peripheral waveform. A corresponding central waveform was derived using dedicated software utilizing wave transfer function. Only measures with a quality index above 80%, which represents reproducibility of the waveform, were included in this study. Systolic and diastolic time integrals were defined as the area under the systolic and diastolic parts of aortic pressure curve, respectively (19, 20). Diastolic time integral was corrected for left ventricular (LV) diastolic pressure, which was estimated echocardiographically, as detailed elsewhere (21). SEVR was calculated as diastolic time integral divided by systolic time integral. Augmentation pressure (AP), was estimated by subtracting the pressure at the first peak shoulder of the aortic pulse wave from aortic systolic blood pressure. Augmentation index (AIx) was defined as AP divided by pulse pressure. AIx was corrected for an HR of 75 beats per minute (AIx@75) as defined previously (22).
Echocardiography
Two-dimensional images, flow and tissue Doppler recordings were obtained for all patients with use of a Doppler transthoracic echocardiograph with a 3.5-MHz transducer (GE Vivid I or 7, Horten, Norway). LV volumes were calculated by modified Simpson’s biplane method from apical four chamber and two chamber views. Doppler recordings were obtained in the apical 4-chamber view by positioning sample volume at the tips of the mitral leaflets. The sample volume was positioned at the medial mitral annulus on apical 4-chamber view to measure early dias- tolic tissue Doppler velocity (E’). LV diastolic pressure was estimated as mitral inflow E wave divided by mitral septal annular E’ wave (23). All echocardiographic and tonometric examinations were performed by the same investigator (E.A.).
Exercise test
A standard advice including abstaining from smoking, coffee, heavy meals have been given to patients before the procedure. Exercise test was performed on a bicycle ergometer while patients were wearing a mask covering their mouth and nose for the measurements of breathing gases. Ventilation (VE), oxygen consumption (VO2), and carbon dioxide production (VCO2) were measured continuously on a breath-by-breath basis with a dedicated spirometer (Oxycon Pro Jaeger (San Diego, CA, USA). ECG was monitored continuously along with periodic manual blood pressure measurements. The workload was controlled by an electronically braked bicycle ergometer system.
Cycling rate was kept approximately at a rate of 60 cycling per minute with the help of a digital cyclometer. All patients were encouraged to exercise up to exhaustion (peak respiratory exchange ratio >1.1) (24). The peak oxygen pulse (πO2) was defined as peak VO2 divided by instantaneous heart rate. The percent predicted peak VO2 was calculated as peak VO2 divided by maximal predicted peak VO2 according to the values reported by Wasserman et al. (25). Ventilatory threshold was measured by classical methods (26). The peak circulatory power was defined as peak VO2 x peak systolic blood pressure and was expressed in mL.mm Hg.min–1.kg–1. Exercise tests were performed before and after completion of rehabilitation program on the same machine.
Cardiac rehabilitation
Patients underwent 2–3 training sessions per week for 7–10 weeks until a total of 20 sessions were completed. Each session was composed of an endurance training part with bicycle exercise and a resistance training part with gymnastics and low weightlifting. The bicycle exercise was executed at an intensity level corresponding to the ventilatory threshold determined at the initial exercise test (assessed by heart rate). Patients who accomplished their assigned intensity level were allowed to gradually increase their work rate and duration. The cycling duration was started from 20 min and progressively increased to 45 min, whereas gymnastics took 30 min. Blood pressure and heart rate were monitored by measurements at rest, during cycling and recovery.
Statistical analysis
Baseline characteristics were summarized using standard descriptive statistics. Baseline comparisons were made using independent t test, Fisher exact tests for dichotomous data, or chi-square tests for categorical data. Paired sample t test was used to compare baseline and final cardiopulmonary exercise test variables. Continuous variables were analyzed by Shapiro-Wilk test for normality assumption in both groups and normally distributed continuous data were analyzed by independent samples t test. Pearson correlation test was used to explore the relationship between SEVR and the change in peak VO2, predicted percent of peak VO2, πO2, and circulatory power. Partial correlation test was used to correct these relationships for potential confounders (age, systolic and dias- tolic blood pressure, ejection fraction, CCr, and BNP). All analyses were computed using Statistical Package for Social Sciences software (SPSS Version 22; IBM Corporation, Armonk, New York, USA).
Results
A total of 76 patients were screened during study period, five patients were excluded because the presence of atrial fibrillation. Of seventy-one consecutive outpatient subjects enrolled, twenty-one patients did not adhere rehabilitation program and quitted at some point before completing 20 exercise sessions. Thus, final study population comprised of fifty patients. There were no electrocardiographically positive ischemic tests or procedure related adverse events during study.
Tonometric measurements
Mean baseline SEVR was 1.45±0.29 (range, 0.82–2.19; interquartile range 0.38). The patients were divided into two subgroups with respect to basal median SEVR value (1.445) (Group I, below the median and Group II above the median value). Baseline characteristics of the patients were summarized in Table 1. No significant differences have been observed in these demographic and clinical characteristics, except body weight. There were no differences between subgroups with respect to baseline AP (11.2±7.5 vs. 8.8±4.7 mm Hg, respectively; p=0.192), AIx (29±11 vs. 24±11, respectively; p=0.110), and AIx@75 (22±11 vs. 21±11 mm Hg, respectively; p=0.650).
Table 1.
Baseline characteristics*
| GROUP I (n=25) | GROUP II (n=25) | P | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, years | 54 (47, 65) | 57 (47, 68) | 0.405 |
| Male | 23 (92) | 22 (88) | 1.000‡ |
| White | 25 (100) | 24 (96) | 1.000‡ |
| Height, m | 1.73 (1.69, 1.79) | 1.73 (1.67, 1.76) | 0.135 |
| Weight, kg | 84 (79, 91) | 74 (65, 85) | 0.008** |
| Medical history | |||
| Hypertension | 10 (40) | 5 (20) | 0.217 |
| Dyslipidemia | 25 (100) | 25 (100) | 1.000‡ |
| Diabetes | 6 (24) | 5 (20) | 1.000 |
| Tobacco use | 16 (64) | 14 (56) | 0.773 |
| Prior MI | 23 (92) | 19 (76) | 0.247‡ |
| Prior CABG | 2 (8) | 4 (16) | 0.667‡ |
| NYHA functional class | |||
| I | 12 (48) | 14 (56) | 0.865§ |
| II | 9 (36) | 5 (20) | |
| III | 4 (16) | 6 (24) | |
| Clinical measurements | |||
| Systolic blood pressure, | 117 (106, 125) | 117 (106, 121) | 0.975 |
| mm Hg | |||
| Diastolic blood pressure, | 71 (62, 80) | 72 (68, 78) | 0.660 |
| mm Hg | |||
| LVEF, % | 49 (38, 62) | 53 (43, 64) | 0.323 |
| Hemoglobin, g.dL–1 | 14 (12, 15) | 14 (13, 14) | 0.954 |
| CCr, mL.min–1 | 93 (70, 122) | 91 (74, 110) | 0.756 |
| BNP, pg.mL–1 | 114 (50, 275) | 73 (37, 208) | 0.245 |
| Number of diseased vessels† | |||
| 1 | 12 (48) | 11 (44) | 0.738§ |
| 2 | 7 (28) | 7 (28) | |
| 3 | 6 (24) | 7 (28) | |
| Treatment | |||
| ACE-I/ARB | 22 (88) | 21(84) | 1.000‡ |
| Beta-blockers | 22 (88) | 24 (96) | 0.609‡ |
| Diuretics | 2 (8) | 8 (32) | 0.074‡ |
| Aldosterone blocker | 6 (24) | 6 (24) | 1.000‡ |
| Statins | 25 (100) | 25 (100) | 1.000‡ |
| Nitrates | 1 (4) | 1 (4) | 1.000‡ |
Values are median (25th, 75th percentiles) or n (%).
Independent t test was used for comparison unless stated.
P<0.01;
Fischer’s exact test;
Chi-square test;
The number of coronary arteries with >50% luminal stenosis on coronary angiography.
ACE-I- angiotensin-converting enzyme inhibitors; ARB- angiotensin receptor blocker; BNP - B-type natriuretic peptide; CABG -coronary artery by-pass grafting; CCr - creatinine clearance (Cockcroft-Gault formula); LVEF- left ventricular ejection fraction; MI- myocardial infarction; NYHA- New York Heart Association
Echocardiographic measurements
Echocardiographic measurements were summarized in Table 2. The patients in the Group II showed significant improvements in stroke volume index (from 31±5 to 35±5 mL; p=0.008) whereas the patients in Group I showed no improvement in any of echocardiographic parameters. Between these subgroups there were no significant differences with respect to the changes in LVEF or stroke volume index.
Table 2.
Echocardiographic parameters before and after cardiac rehabilitation
| Group I (n=25) | Group II (n=25) | P for Δ comparison‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Δ* | P† | Before | After | Δ* | P† | ||
| LVEF, % | 49±12 | 50±13 | 1±6 | 0.665 | 53±14 | 55±15 | 2±5 | 0.123 | 0.550 |
| LVEDVI, mL.m–2 | 61±20 | 65±21 | 3±13 | 0.253 | 65±26 | 68±23 | 3±12 | 0.198 | 0.981 |
| LVESVI, mL.m–2 | 32±18 | 33±17 | 1±7 | 0.562 | 33±26 | 32±22 | 0±8 | 0.906 | 0.629 |
| SVI, mL | 29±8 | 31±9 | 2±7 | 0.154 | 31±5 | 35±6 | 3±6 | 0.008** | 0.557 |
∆ indicates the difference between post-cardiac rehabilitation minus pre-cardiac rehabilitation values in each group;
P<0.01;
***P<0.001;
Paired sample t test;
Independent t test
LVEDVI - left ventricular end-diastolic volume index; LVEF - left ventricular ejection fraction; LVESVI - left ventricular end-systolic volume index; SVI - stroke volume index
Cardiopulmonary exercise test-based measurements
These parameters were summarized in Table 3. The patients in the Group II showed significant improvements in peak VO2 (from 19.4±5.2 to 22.9±6.7 mL.kg–1.min–1; p<0.001), percent of predicted peak VO2 (from 72%±18% to 87%±25%; p=0.001), πO2 (from 16.1±3.4 to 19.1±4.8 mL O2.kg–1.beat–1; p<0.001), and circulatory power (from 3262±1353 to 3923±1474 mL.mm Hg.min–1.kg–1; p=0.004). The patients in the Group I also showed increases in peak VO2 (from 21.3±7.0 to 23.5±7.6 mL.kg–1.min–1; p<0.001), percent of predicted peak VO2 (from 78%±21% to 87%±27%; p=0.002), and circulatory power (from 3601±1455 to 4156±1560 mL.mm Hg.min–1.kg–1; p=0.001), but not in πO2 (from 17.5±4.7 to 18.1±4.2 mL O2.kg–1.beat–1; p=0.252). Between these subgroups there were no significant diffe- rences with respect to the changes in percent of predicted peak VO2 and circulatory power. Nevertheless, the change in πO2 was significantly higher in the Group II (2.9±3.3 vs. 0.5±2.4; p=0.007) (Table 3).
Table 3.
Cardiopulmonary exercise test parameters before and after cardiac rehabilitation
| Group I (n=25) | Group II (n=25) | P for Δ comparison‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Δ* | P† | Before | After | Δ* | P† | ||
| Peak VO2, mL.kg–1.min–1 | 21.3±7.0 | 23.5±7.6 | 2.2 | 0.001*** | 19.4±5.2 | 22.9±6.7 | 3.5 | <0.001*** | 0.144 |
| % of predicted peak VO2, % | 78%±21% | 87%±27% | 9% | 0.002** | 72%±18% | 87%±25% | 15% | 0.001*** | 0.242 |
| Oxygen pulse (πO2), mL O2.kg-1.beat-1 | 17.5±4.7 | 18.1±4.2 | 0.5±2.4 | 0.252 | 16.1±3.4 | 19.1±4.8 | 2.9±3.3 | <0.001*** | 0.007** |
| Circulatory power, mL.min.mm Hg.kg–1 | 3601±1455 | 4156±1560 | 555 | 0.001*** | 3262±1353 | 3923±1474 | 661 | 0.004** | 0.680 |
∆ indicates the difference between post-cardiac rehabilitation minus pre-cardiac rehabilitation values in each group.
P<0.01;
P≤0.001;
Paired sample t test;
Independent t test; Peak VO2- Maximal oxygen consumption
When patients were analyzed as a whole group, significant correlations were found between baseline SEVR and the change in peak VO2 (r=0.370, p=0.008), predicted percent of peak VO2 (r=0.340, p=0.016), πO2 (r=0.396, p=0.004) (Fig. 2), but not with circulatory power (r=0.225, p=0.115) and the changes in any of echocardiographic parameters, including the change in LVEF (r=–0.017, p=0.909) and SVI (r=0.069, p=0.635). When these correlations were corrected for potential confoun- ders peak VO2 (r=0.272, p=0.070), circulatory power (r=0.140, p=0.358) and predicted percent of peak VO2 (r=0.264, p=0.080) lost their significances, bt πO2 (r=0.392, p=0.008) remained significant.
Figure 2.
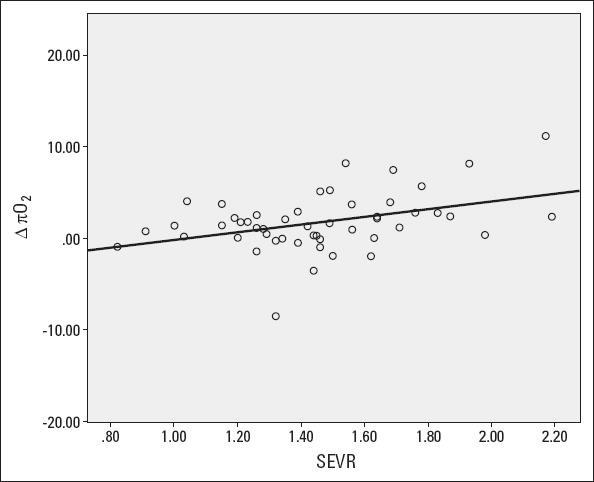
Relation between baseline SEVR and the change in πO2 after cardiac rehabilitation. SEVR - subendocardial viability ratio, πO2=peak oxygen pulse
Discussion
Our study, for the first time, shows that baseline LV supply–demand relationship may affect the response to CR in terms of improvement in systolic function. Our results indicate that the patients with an unfavorable initial SEVR value may not show improvements in resting and exercise-induced contractile function, as assessed by resting stroke volume index and peak πO2, respectively. Of these, peak πO2 predominantly reflects peak LV stroke volume during exercise; thus, it is more sensitive to changes in myocardial systolic function compared with those in peak VO2, which may be improved by the effects of exercise training on many extracardiac parameters (such as vascular, pulmonary, muscular, autonomic, and inflammatory factors) (3, 27, 28). It is also supporting that only the group with a better SEVR showed an increase in stroke volume index, which can be regarded as the resting counterpart of πO2.
SEVR, formerly known as Buckberg ratio (16), consists of diastolic to systolic pressure-time integral ratio, derived from the pressures measured in the aorta and LV. Systolic pressure time integral is reported to be a reliable index of myocardial oxygen consumption for LV afterload (12, 13). Diastolic pressure time integral, on the other hand, takes into account the following three critical factors affecting coronary flow: (1) coronary artery diastolic pressure (14), which is equal to aortic diastolic pressure in patients with unobstructed coronary arteries; (2) the gradient in diastole between coronary arteries pressure and LV pressure; and (3) the duration of diastole (15, 29). Therefore, SEVR estimates the balance between cardiac blood flow supply and demand.
Although the main aim of CR is increase exercise capacity as a whole, reflected by the improvement in by peak VO2, and both groups showed an improvement in peak VO2 in our study, patients with a better baseline SEVR showed a better improvement, though statistically insignificant. Our study was not po- wered enough to elucidate whether a better systolic response to CR translates into a greater peak VO2 improvement; it serves as a hypothesis generator, calling for larger studies.
Our study also reminds that coronary patency is only one of the several dimensions of optimal myocardial supply–demand relationship, which may be imbalanced enough to hamper positive response to CR despite the absence of a critical stenosis (17, 30–34). It has been shown that a lower SEVR ratio may limit coronary dilatation capacity even in patients with patent coronary arteries (20). SEVR can be negatively influenced by cent- ral and peripheral vasculature via increased wave reflections and large systolic-diastolic pressure undulations caused by increased macrovascular stiffness, decreased compliance, increased peripheral resistance (16, 18, 34, 35). Although, we did not find any difference in wave reflection parameters between SEVR subgroups, this may be explained by the limited statistical power of the study. Since cellular regeneration processes are highly intertwined with myocardial energetics (36), the failure to improve myocardial systolic function with CR due to a worse supply–demand ratio is highly conceivable.
Lastly, although our study is predominantly a mechanistic one, its results may have a practical message by proposing that some measures may be needed to be undertaken before CR to optimize baseline supply–demand ratio. These may include reducing afterload by decreasing peripheral vascular resistance and wave reflections (e.g., peripheral vasodilators) or augmenting diastolic blood flow by increasing duration of the diastole (e.g., ivabradine). Theoretically, the overall gain from CR may be increased with these priming measures. Further studies are needed for the clarification of these propositions.
Study limitations
The main limitation of our study is its limited size. Larger studies are needed for clarifying whether a suboptimal myocardial supply–demand lowers maximum benefit from CR in terms of peak VO2 improvement. Confounding effects of medications may not be eliminated because they were not withdrawn in the study, even if these medications are usually used in coronary heart disease patients. A second tonometric test after completion of the program may have shown the change in arterial mechanics parameters, which may be of some practical value. We failed measure pulse wave velocity, which may have contributed the article by adding more specific vascular stiffness data on top of vascular reflection parameters. Lastly, SEVR may not directly represent supply–demand relationship of LV. Although systolic part includes aortic systolic pressure and ejection duration as determinants of myocardial oxygen demand, it does not contain other ventricular parameters (such as stroke volume, ventricular mass and shape), which can also influence myocardial energy requirements. Also, diastolic pressure-time index does not take epicardial and microvascular resistance into account, which may overestimate coronary flow, especially in patients with significant coronary stenoses.
Conclusion
Our study shows that baseline supply–demand imbalance, as measured by SEVR, may limit systolic improvement response to CR in patients with CAD. Further studies are needed to elucidate whether this limitation lowers maximum achievable peak VO2 improvement with CR. Furthermore, the measures optimizing baseline supply–demand ratio and their effects on CR results need to be clarified.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – All authors; Design – All authors; Supervision – All authors; Materials – All authors; Data collection &/or processing – All authors; Analysis &/or interpretation – All authors; Literature search – All authors; Writing – All authors; Critical review – All authors.

From Prof. Dr. Cumhur Ertekin’s collections
References
- 1.Benetos A, Thomas F, Joly L, Blacher J, Pannier B, Labat C, et al. Pulse pressure amplification a mechanical biomarker of cardiovascular risk. J Am Coll Cardiol. 2010;55:1032–7. doi: 10.1016/j.jacc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 2.Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989;80:78–86. doi: 10.1161/01.cir.80.1.78. [DOI] [PubMed] [Google Scholar]
- 3.Leon AS, Franklin BA, Costa F, Balady GJ, Berra KA, Stewart KJ, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease:an American Heart Association scientific statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity), in collaboration with the American association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2005;111:369–76. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 4.Hambrecht R, Walther C, Möbius-Winkler S, Gielen S, Linke A, Conradi K, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease. Circulation. 2004;109:1371–8. doi: 10.1161/01.CIR.0000121360.31954.1F. [DOI] [PubMed] [Google Scholar]
- 5.Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2016;1:CD001800. doi: 10.1002/14651858.CD001800.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hambrecht R, Wolff A, Gielen S, Linke A, Hofer J, Erbs S, et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–60. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 7.Giannuzzi P, Temporelli PL, Corra U, Gattone M, Giordano A, Tavazzi I. Attenuation of unfavorable remodeling by exercise training in postinfarction patients with left ventricular dysfunction:results of the Exercise in Left Ventricular Dysfunction (ELVD) trial. Circulation. 1997;96:1790–7. doi: 10.1161/01.cir.96.6.1790. [DOI] [PubMed] [Google Scholar]
- 8.Yu CM, Li LS, Lam MF, Siu DC, Miu RK, Lau CP. Effect of a cardiac rehabilitation program on left ventricular diastolic function and its relationship to exercise capacity in patients with coronary heart disease:experience from a randomized, controlled study. Am Heart J. 2004;147:e24. doi: 10.1016/j.ahj.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol. 1994;266:H693–701. doi: 10.1152/ajpheart.1994.266.2.H693. [DOI] [PubMed] [Google Scholar]
- 10.Pal S, Radavelli-Bagatini S, Ho S. Potential benefits of exercise on blood pressure and vascular function. J Am Soc Hypertens. 2013;7:494–506. doi: 10.1016/j.jash.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure;a randomized trial. JAMA. 2000;283:3095–101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 12.Sarnoff SJ, Braunwald E, Welch GH, Jr, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol. 1958;192:148–56. doi: 10.1152/ajplegacy.1957.192.1.148. [DOI] [PubMed] [Google Scholar]
- 13.Gutterman DD, Cowley AW., Jr Relating cardiac performance with oxygen consumption:historical observations continue to spawn scientific discovery. Am J Physiol Heart Circ Physiol. 2006;291:H2555–6. doi: 10.1152/classicessays.00044.2006. [DOI] [PubMed] [Google Scholar]
- 14.Merkus D, Kajiya F, Vink H, Vergroesen I, Dankelman J, Goto M, et al. Prolonged diastolic time fraction protects myocardial perfusion when coronary blood flow is reduced. Circulation. 1999;100:75–81. doi: 10.1161/01.cir.100.1.75. [DOI] [PubMed] [Google Scholar]
- 15.Fokkema DS, VanTeeffelen JW, Dekker S, Vergroesen I, Reitsma JB, Spaan JA. Diastolic time fraction as a determinant of subendocardial perfusion. Am J Physiol Heart Circ Physiol. 2005;288:H2450–6. doi: 10.1152/ajpheart.00790.2004. [DOI] [PubMed] [Google Scholar]
- 16.Buckberg GD, Fixler DE, Archie JP, Hoffman JI. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res. 1972;30:67–81. doi: 10.1161/01.res.30.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman JI, Buckberg GD. Pathophysiology of subendocardial ischaemia. Br Med J. 1975;1:76–9. doi: 10.1136/bmj.1.5949.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man:relationship to pressure wave forms. Circulation. 1980;62:105–16. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 19.Chemla D, Nitenberg A, Teboul JL, Richard C, Monnet X, le Clesiau H, et al. Subendocardial viability index is related to the diastolic/systolic time ratio and left ventricular filling pressure, not to aortic pressure:an invasive study in resting humans. Clin Exp Pharmacol Physiol. 2009;36:413–8. doi: 10.1111/j.1440-1681.2008.05084.x. [DOI] [PubMed] [Google Scholar]
- 20.Tsiachris D, Tsioufis C, Syrseloudis D, Roussos D, Tatsis I, Dimitriadis K, et al. Subendocardial viability ratio as an index of impaired coronary flow reserve in hypertensives without significant coronary artery stenoses. J Hum Hypertens. 2012;26:64–70. doi: 10.1038/jhh.2010.127. [DOI] [PubMed] [Google Scholar]
- 21.Aslanger E, Assous B, Bihry N, Beauvais F, Logeart D, Cohen-Solal A. Effects of exercise on postexercise ventricular-arterial coupling and pulsatile efficiency in patients with systolic dysfunction. Eur J Clin Invest. 2015;45:1042–51. doi: 10.1111/eci.12504. [DOI] [PubMed] [Google Scholar]
- 22.Aslanger E, Assous B, Bihry N, Beauvais F, Logeart D, Cohen-Solal A. Effects of cardiopulmonary exercise rehabilitation on left ventricular mechanical efficiency and ventricular-arterial coupling in patients with systolic heart failure. J Am Heart Assoc. 2015;4:e002084. doi: 10.1161/JAHA.115.002084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures. A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 24.Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, et al. American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology;Council on Epidemiology and Prevention;Council on Peripheral Vascular Disease;Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s Guide to cardiopulmonary exercise testing in adults:a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman K, Hansen J, Sue D, Whipp B. Normal values. In: Wasserman K, Hansen J, Sue D, et al., editors. Priniciples of exercise testing and interpretation. Philadelphia: Lea and Fibiger; 1987. pp. 72–85. [Google Scholar]
- 26.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986;60:2020–7. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 27.Legallois D, Belin A, Nesterov SV, Milliez P, Parienti JJ, Knuuti J, et al. Cardiac rehabilitation improves coronary endothelial function in patients with heart failure due to dilated cardiomyopathy:A positron emission tomography study. Eur J Prev Cardiol. 2016;23:129–36. doi: 10.1177/2047487314565739. [DOI] [PubMed] [Google Scholar]
- 28.Lavie CJ, Milani RV, Mehra MR. Peak exercise oxygen pulse and prognosis in chronic heart failure. Am J Cardiol. 2004;93:588–93. doi: 10.1016/j.amjcard.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Ferro G, Duilio C, Spinelli L, Liucci GA, Mazza F, Indolfi C. Relation between diastolic perfusion time and coronary artery stenosis during stress-induced myocardial ischemia. Circulation. 1995;92:342–7. doi: 10.1161/01.cir.92.3.342. [DOI] [PubMed] [Google Scholar]
- 30.Salvi P, Revera M, Faini A, Giuliano A, Gregorini F, Agostoni P, et al. Changes in subendocardial viability ratio with acute high-altitude exposure and protective role of acetazolamide. Hypertension. 2013;61:793–9. doi: 10.1161/HYPERTENSIONAHA.111.00707. [DOI] [PubMed] [Google Scholar]
- 31.Brazier J, Cooper N, Buckberg G. The adequacy of subendocardial oxygen delivery:the interaction of determinants of flow, arterial oxygen content and myocardial oxygen need. Circulation. 1974;49:968–77. doi: 10.1161/01.cir.49.5.968. [DOI] [PubMed] [Google Scholar]
- 32.Barnard RJ, MacAlpin R, Kattus AA, Buckberg GD. Ischemic response to sudden strenuous exercise in healthy men. Circulation. 1973;48:936–42. doi: 10.1161/01.cir.48.5.936. [DOI] [PubMed] [Google Scholar]
- 33.Griggs DM, Jr, Chen CC. Coronary hemodynamics and regional myocardial metabolism in experimental aortic insufficiency. J Clin Invest. 1974;53:1599–606. doi: 10.1172/JCI107710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Manipulation of ascending aortic pressure and flow wave reflections with the Valsalva maneuver:relationship to input impedance. Circulation. 1981;63:122–32. doi: 10.1161/01.cir.63.1.122. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women:the Framingham Heart Study. Hypertension. 2004;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 36.Page BJ, Banas MD, Suzuki G, Weil BR, Young RF, Fallavollita JA, et al. Revascularization of chronic hibernating myocardium stimulates myocyte proliferation and partially reverses chronic adaptations to ischemia. J Am Coll Cardiol. 2015;65:684–97. doi: 10.1016/j.jacc.2014.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


