Abstract
Objective:
Carotid intima-media thickness (CIMT) is reliable marker of subclinical atherosclerosis and cardiovascular events. Until today, there was no study that investigated whether epicardial adipose tissue (EAT), which is a surrogate for lipid depot in a special visceral tissue or circulating lipids, is more important for CIMT and atherosclerotic plaque.
Methods:
Our study, having cross-sectional and prospective observational design, included 252 patients who were admitted to our outpatient clinic. EAT identified as an echo-free space under the pericardial layer on 2-dimensional echocardiography, was measured perpendicularly in front of the right ventricular free wall at end-systole.
Results:
EAT significantly correlated with CIMT (r=0.623, p<0.001). CIMT was significantly increased with rising EAT thickness (0.72±0.15 mm, 0.85±0.16 mm, and 0.95±0.12 mm in patients with EAT <5 mm, 5–7, and >7 mm, p<0.001, respectively). Multiple linear regression analysis revealed that age (Beta: 0.406, p<0.001), male gender (Beta: 0.244, p<0.001), and EAT (Beta: 0.450, p<0.001) as independent correlates of CIMT. Otherwise, in logistic regression analysis, only EAT (OR, 1.386; 95% CI, 1.203–1.597, p<0.001) and LDL cholesterol (OR, 1.013; 95% CI, 1.002–1.013, p=0.02) were independent predictors for presence of carotid plaque.
Conclusion:
Our study showed that EAT has a relationship with both CIMT and the presence of carotid plaque, but LDL is independently related to the plaque. This finding suggests that EAT thickness may be a risk factor and biomarker, playing an important role beginning from early stages of atherosclerosis, unlike LDL cholesterol, which appear to have a role in later stages of atherosclerosis.
Keywords: epicardial adipose tissue, carotid intima-media thickness, LDL cholesterol, atherosclerotic plaque, subclinical atherosclerosis
Introduction
Carotid intima-media thickness (CIMT) and atherosclerotic plaques are reliable markers of subclinical atherosclerosis and cardiovascular events (1). Traditional risk factors, such as age, diabetes, systolic blood pressure, high-density lipoprotein (HDL) cholesterol (low levels), and LDL cholesterol, are associated with CIMT according to cross-sectional studies (2). However, Rosvall et al. (3) showed that predictive power of baseline low-density lipoprotein (LDL) cholesterol attenuated with increased intima-media thickness and high CIMT progression rate. An individualized approach for detection of subclinical atherosclerosis can be achieved by using novel risk factors and biomarkers such as epicardial adipose tissue (EAT) (4).
Epicardial adipose tissue is a kind of visceral adipose tissue enclosed within the pericardial cavity. It has many protective roles, such as uptake of excess free fatty acids (5) and anti-inflammatory and anti-atherosclerotic effects, mainly mediated via adiponectin (4). In visceral obesity, EAT undergoes conformational and functional changes such that it begins to secrete pro-inflammatory and pro-atherogenic adipokines (e.g., interleukin-6, tumor necrosis factor a, adiponectin, leptin, and plasminogen activator inhibitor) (6). EAT itself is a marker for future cardiovascular events independent of CIMT/carotid plaque (7, 8). Incorporating EAT to models comprised of traditional risk factors may pave the way for a better understanding of factors affecting CIMT. Until today, there was no study that investigated whether epicardial adipose tissue (EAT), which is a surrogate for lipid depot in a special visceral tissue or circulating lipids, is more important for CIMT and atherosclerotic plaque. Therefore, our primary aim is to evaluate the associations of traditional risk factors, including circulating lipids and visceral adipose tissue, with CIMT and the plaque.
Methods
Patient population and study protocol
Our study, having cross-sectional and prospective observational design, included 252 patients who were admitted to our outpatient clinic of the Rize University, Department of Cardiology, Rize, Turkey. Patients with hypertension, diabetes mellitus, and hyperlipidemia were selected for the study. Any previous history of coronary artery disease (CAD), left ventricular systolic dysfunction, and moderate to severe valve disease were accepted as exclusion criteria. We also excluded any patient with symptoms suggestive of CAD, which was confirmed by relevant findings with exercise ECG and perfusion scan. By the exclusion criterion, we aimed to decrease the number of confounding factors and to provide homogeneity in our study.
The patients were evaluated in terms of age, demographical properties, and cardiovascular risk factors. Hypertension was described as the active use of antihypertensive drugs or confirmation of blood pressure more than 140/90 mm Hg. Diabetes mellitus (DM) was described as fasting plasma glucose levels over 126 mg/dL. Also, plasma glucose level over 200 mg/dL at any measurement or active use of antidiabetic treatment was accepted as DM. Dyslipidemia was defined as fasting blood total cholesterol >200 mg/dL, fasting blood low-density lipoprotein levels >130 mg/dL, or previously receiving anti-hyperlipidemic medication. Patients who were using tobacco products on admission to our hospital and those who quit smoking within the last year were considered smokers. The family history for CAD was defined as a history of CAD or sudden death in a first-degree relative before the age of 55 years for men and 65 years for women.
Informed consent was obtained from all patients prior to the study. This study was performed in accordance with the principles stated in the Declaration of Helsinki and approved by the local Ethics Committee.
Routine measurements
Blood samples were drawn by venipuncture to measure routine blood chemistry parameters after fasting for at least 8 hours. Glucose, creatinine, and lipid profile were determined by standard methods. Fasting blood glucose, serum creatinine, uric acid levels, total cholesterol, HDL cholesterol, and triglyceride levels were recorded (UniCel DxC 800, Beckman Coulter USA). The Friedewald’s formula was used for LDL cholesterol measurement (9). When triglyceride level exceeded 400 mg/dL, the direct measurement technique was used for LDL measurement (used for 14 patients). Serum C-reactive protein (CRP) was analyzed using a nephelometric technique (Beckman Coulter Immage 800; Fullerton, CA, USA; normal range 0–0.8 mg/dL).
Anthropometric measurements
Weight and height were measured while the subjects were in fasting state and wearing only their undergarments. Body mass index (BMI) was determined by the following formula: BMI=weight (kg)/height2 (m). Waist circumference (in centimeters; circumference between the lower rib margin and the iliac crest, midwaist) was measured while the subjects were standing with their heels together. These variables were used as linear variable in analyses, because their cut-off levels can change by time and population.
Echocardiography
Patients were imaged in the left lateral decubitus position by two experienced cardiologists using commercially available systems with a GE-Vingmed Vivid S5 (GE-Vingmed Ultrasound AS, Horten, Norway) according to echocardiography guidelines (10).
Evaluation of epicardial adipose tissue
Epicardial adipose tissue was assessed on the free wall of right ventricle from the parasternal long-axis view, using aortic annulus as an anatomic reference. We magnified each image for better visualization and precise measurement of EAT thickness and measured the thickest point of EAT in each cycle. The area of above the right ventricle to measure EAT thickness was preferred, because this area is known to have the thickest EAT layer. EAT was measured perpendicularly in front of the right ventricular free wall at end-systole (11, 12) and was identified as an echo-free space under the pericardial layer on two-dimensional echocardiography (Fig. 1). The average value comprising three cardiac cycles of each echocardiographical view was used for the statistical analysis. Inter-observer and intra-observer variability on epicardial fat thickness measurement was excellent: coefficients of correlation were 0.94 and 0.98, respectively.
Figure 1.
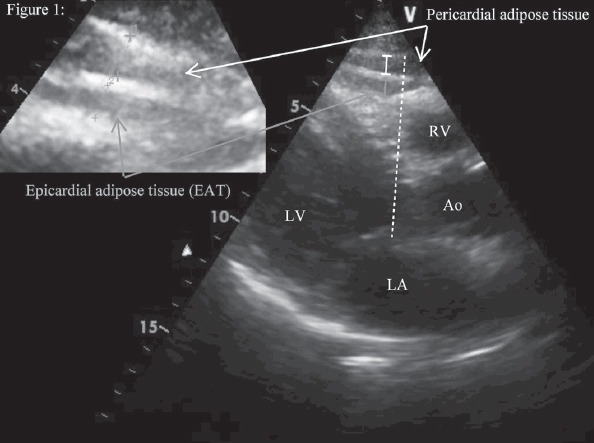
Evaluation of epicardial adipose tissue
EAT - identified as an echo-free space between the myocardium and visceral pericardium from the parasternal long-axis view on two-dimensional echocardiography, was measured perpendicularly in front of the right ventricular free wall at end-systole
Measurement of carotid intima-media thickness
Measurements were performed for the right and left carotid arteries (13). The patient was lying supine, the head directed away from the side of interest, and the neck slightly extended. The transducer was manipulated so that the near and far walls of the common carotid artery were parallel, and the lumen diameter was maximized in the longitudinal plane. The region 1 cm proximal to the carotid bifurcation was identified, and the CIMT of the far wall was evaluated as the distance between the lumen–intima interface and the media–adventitia interface. The CIMT was measured on the frozen frame of a suitable longitudinal image, with the image magnified to achieve a higher resolution of detail. The CIMT measurement was obtained from four contiguous sites at 1 mm intervals, and the average of all eight measurements was used for analyses. Ultrasonography was performed on all patients using a high-resolution ultrasonography scanner (VingMed Vivid 3, GE Medical System, Horten, Norway) with a 7.0 MHz linear array transducer.
A carotid intima-media thickness lower than 0.9 mm was considered normal; a thickness higher than 0.9 mm but lower than 1.3 was considered increased; a thickness higher than 1.3 mm was defined as a plaque (14). We also checked 1.5 mm cut-off of CIMT, which is the last definition in the ESC guideline, but similar results were achieved (15).
Statistical analysis
Continuous variables were given as mean±SD; categorical variables were defined as percentages. Data were tested for normal distribution using the Kolmogorov-Smirnov test. Only CRP was non-normally distributed. It is given as median (range). We performed logarithmical transformation to normalize CRP and presented this form additionally. Spearman’s rank correlation coefficient was used to analyze the relationship between the variables. Mean values were compared by ANOVA followed by the Tukey’s HSD test among different groups. Multiple logistic and linear regression analyses were used to assess multivariate relations among CIMT and carotid plaque, EAT, LDL, HDL, and various variables. In these analyses, a stepwise method for all independent variables were used, including age, gender, waist circumference, body mass index, diabetes, hypertension, dyslipidemia, family history of CAD, smoking status, lipids, EAT, alanine aminotransferase, gamma-glutamyl transferase, CRP, leukocytes (including monocyte), bilirubins, uric acid, creatinine, glucose, and hemoglobin levels. After first pre-elimination, linear and logistic regression analyses with enter method were repeated for independent variables, which were included if they were significantly different in the univariate analyses. The last elimination analysis was repeated with stepwise method for the previous significant determined variables.
Statistical significance was defined as p<0.05. All tests of significance were two-tailed. The SPSS statistical software (SPSS for windows, version 15.0, Inc., Chicago, IL, USA) was used for all statistical calculations.
Results
Clinical characteristics of the patients are presented in Table 1. Our study included 252 patients (mean age: 46±8 years) with a male preponderance (71%). Correlations of EAT, LDL, HDL, and CIMT to other study parameters is detailed in Table 2.
Table 1.
Baseline demographics and clinical characteristics of study population
| Variables | Patients (n=252) |
|---|---|
| Age, years | 46±8 |
| Gender, male | 71% |
| BMI, kg/m2 | 30.4±4.7 |
| Waist circumference, cm | 101±11 |
| Hypertension | 65% |
| Diabetes mellitus | 8% |
| Smoking status | 20% |
| Dyslipidemia | 35% |
| Family history of CAD | 45% |
| Glucose, mg/dL | 103±31 |
| Creatinine, mg/dL | 0.84±0.16 |
| Uric acid, mg/dL | 5.3±1.5 |
| T. Bilirubin, mg/dL | 0.81±0.45 |
| Total cholesterol, mg/dL | 216±41 |
| LDL-cholesterol, mg/dL | 136±36 |
| HDL-cholesterol, mg/dL | 45±12 |
| Triglycerides, mg/dL | 176±121 |
| CRP, mg/dL, median/range | 0.32 (3.96) |
| CRP, log-transformed | -0.46±0.33 |
| ALT, U/L | 28±20 |
| GGT activity, U/L | 32±22 |
| Leukocytes, 103/mm3 | 7.6±1.8 |
| Hemoglobin, g/dL | 14.5±1.5 |
| Platelets, 103/mm3 | 280±64 |
| CIMT, mm, mean | 0.81±0.17 |
| Presence of carotid plaque | 18% |
| EAT, mm | 5.8±2.5 |
ALT - alanine aminotransferase; BMI - body mass index; CAD - coronary artery disease; CIMT - carotid intima-media thickness; CRP - C-reactive protein; EAT - epicardial adipose tissue thickness; GGT - gamma-glutamyl transferase; HDL - high density lipoprotein; LDL - low density lipoprotein. Continuous variables were given as mean±SD; categorical variables were defined as percentages
Table 2.
Correlations of epicardial adipose tissue and serum lipids with study parameters
| EAT, mm | LDL, mg/dL | HDL, mg/dL | CIMT, mm, mean | |||||
|---|---|---|---|---|---|---|---|---|
| Parameters | R | P† | R | P† | R | P† | R | P† |
| Age, years | 0.259 | <0.001 | 0.283 | <0.001 | 0.168 | 0.001 | 0.467 | <0.001 |
| BMI, kg/m2 | 0.229 | <0.001 | 0.068 | 0.174 | 0.018 | 0.713 | 0.106 | 0.031 |
| Waist circumference, cm | 0.539 | <0.001 | -0.035 | 0.536 | -0.184 | 0.001 | 0.358 | <0.001 |
| Glucose, mg/dL | 0.016 | 0.888 | 0.044 | 0.382 | 0.029 | 0.556 | 0.135 | 0.007 |
| Creatinine, mg/dL | 0.178 | 0.007 | -0.010 | 0.845 | -0.290 | <0.001 | 0.176 | 0.001 |
| T. Bilirubin, mg/dL | -0.075 | 0.262 | -0.051 | 0.340 | 0.104 | 0.045 | -0.079 | 0.130 |
| Uric acid, mg/dL | 0.298 | <0.001 | 0.125 | 0.015 | -0.288 | <0.001 | 0.284 | 0.001 |
| Total cholesterol, mg/dL | 0.101 | 0.121 | 0.843 | <0.001 | 0.155 | 0.002 | 0.087 | 0.083 |
| LDL-cholesterol, mg/dL | 0.099 | 0.132 | – | – | 0.177 | <0.001 | 0.138 | 0.007 |
| HDL-cholesterol, mg/dL | -0.132 | 0.043 | 0.154 | 0.002 | – | – | -0.095 | 0.060 |
| Triglycerides, mg/dL | 0.133 | 0.041 | -0.093 | 0.062 | -0.482 | <0.001 | 0.059 | 0.241 |
| LDL/HDL ratio | 0.150 | 0.022 | – | – | – | – | 0.163 | 0.001 |
| TK/HDL ratio | 0.181 | 0.005 | – | – | – | – | 0.135 | 0.007 |
| CRP, mg/dL | 0.322 | <0.001 | 0.191 | <0.001 | -0.032 | 0.546 | 0.234 | <0.001 |
| CRP, log-transformed | 0.349 | <0.001 | 0.189 | <0.001 | -0.021 | 0.697 | 0.215 | <0.001 |
| ALT, U/L | 0.150 | 0.021 | 0.090 | 0.079 | -0.188 | <0.001 | 0.065 | 0.198 |
| GGT activity, U/L | 0.198 | 0.003 | 0.058 | 0.275 | -0.300 | <0.001 | 0.158 | 0.003 |
| Leukocytes, /mm3 | 0.057 | 0.388 | -0.010 | 0.851 | -0.232 | <0.001 | 0.035 | 0.498 |
| Monocyte, /mm3 | 0.119 | 0.070 | -0.027 | 0.595 | -0.197 | <0.001 | 0.129 | 0.012 |
| Hemoglobin, g/dL | 0.110 | 0.094 | -0.027 | 0.607 | -0.326 | <0.001 | 0.167 | 0.001 |
| Platelets, 103/mm3 | 0.096 | 0.148 | 0.051 | 0.323 | 0.059 | 0.244 | -0.057 | 0.269 |
| EAT, mm | – | – | 0.623 | <0.001 | ||||
| CIMT, mm, mean | 0.623 | <0.001 | 0.138 | 0.007 | -0.095 | 0.060 | – | – |
| Right, mm | 0.612 | <0.001 | 0.133 | 0.010 | -0.090 | 0.073 | – | – |
| Left, mm | 0.630 | <0.001 | 0.122 | 0.017 | -0.106 | 0.035 | – | – |
ALT - alanine aminotransferase; BMI - body mass index; CIMT - carotid intima-media thickness; CRP - C-reactive protein; EAT - epicardial adipose tissue thickness; GGT - gamma-glutamyl transferase; HDL - high density lipoprotein; LDL - low density lipoprotein, TK - total cholesterol.
Pearson&Spearmen tests were used to analyze the relationship between EAT and study variables where appropriate
Epicardial adipose tissue significantly correlated to CIMT (r=0.623, p<0.001), as well as age, BMI, waist circumference, creatinine, uric acid, and CRP. CIMT correlated to age, waist circumference, uric acid, CRP, and EAT (Table 2). CIMT was significantly increased with rising EAT thickness (0.72±0.15 mm, 0.85±0.16 mm, and 0.95±0.12 mm in patients with EAT <5, 5–7, and >7 mm, p<0.001, respectively) (Table 3).
Table 3.
The distributions of study parameters according to EAT subgroups
| Variables | EAT (<5 mm) (n=119) | EAT (5–7 mm) (n=70) | EAT (>7 mm) (n=63) | P* |
|---|---|---|---|---|
| Age, years | 46±8 | 50±8a | 50±9a | 0.009 |
| Gender, male | 50% | 63% | 63% | 0.094 |
| BMI, kg/m2 | 30.7±5.0 | 30.1±3.4 | 33.7±4.3a,b | <0.001 |
| WC, cm | 96.7±10.3 | 100.4±7.9a | 110.7±9.0a,b | <0.001 |
| Hypertension | 82% | 84% | 87% | 0.709 |
| Diabetes mellitus | 8% | 1% | 2% | 0.019 |
| Smoking status | 22% | 15% | 24% | 0.367 |
| Dyslipidemia | 27% | 35% | 40% | 0.160 |
| Family history of CAD | 67% | 44% | 53% | 0.081 |
| Glucose, mg/dL | 101±19 | 100±16 | 99±14 | 0.818 |
| Creatinine, mg/dL | 0.81±0.22 | 0.84±0.13 | 0.80±0.10 | 0.537 |
| Uric acid, mg/dL | 4.8±1.4 | 5.3±1.4 | 5.7±1.4a | <0.001 |
| T. Bilirubin, mg/dL | 0.95±0.59 | 0.80±0.42 | 0.77±0.32 | 0.051 |
| Total cholesterol, mg/dL | 215±39 | 219±38 | 227±41 | 0.158 |
| LDL-cholesterol, mg/dL | 137±31 | 140±35 | 145±35 | 0.302 |
| HDL-cholesterol, mg/dL | 47±14 | 47±11 | 45±9 | 0.430 |
| Triglycerides, mg/dL | 157±81 | 159±116 | 184±107 | 0.211 |
| LDL/HDL ratio | 3.09±1.02 | 3.06±0.84 | 3.33±0.95 | 0.211 |
| TK/HDL ratio | 4.83±1.36 | 4.87±1.25 | 5.22±1.22 | 0.159 |
| CRP, mg/dL | 0.41±0.41 | 0.47±0.43 | 0.67±0.57a,b | 0.001 |
| CRP, log-transformed | -0.53±0.33 | -0.46±0.32 | -0.27±0.30a,b | <0.001 |
| ALT, U/L | 26±17 | 27±19 | 29±19 | 0.490 |
| GGT activity, U/L | 30±22 | 29±19 | 34±16 | 0.433 |
| Leukocytes, 103/mm3 | 7.4±2.1 | 7.3±1.4 | 7.5±1.7 | 0.896 |
| Hemoglobin, g/dL | 14.0±1.8 | 14.5±1.3 | 14.4±1.5 | 0.157 |
| Platelets, 103/mm3 | 279±57 | 278±62 | 292±66 | 0.368 |
| CIMT, mm, mean | 0.72±0.15 | 0.85±0.16a | 0.95±0.12a,b | <0.001 |
| PCP | 7% | 19% | 41% | <0.001 |
| Medications | ||||
| Acetylsalicylic acid | 15% | 20% | 11% | 0.625 |
| Antilipemic agents | 9% | 5% | 13% | 0.135 |
| Antihypertensive drugs | 50% | 60% | 67% | 0.895 |
| Antidiabetic drugs | 6% | 3% | 2% | 0.456 |
ALT - alanine aminotransferase; BMI - body mass index; CAD - coronary artery disease; CIMT - carotid intima-media thickness; CRP - C-reactive protein; GGT - Gamma-glutamyl transferase; HDL-C - high-density lipoprotein cholesterol; LDL - Low-density lipoprotein cholesterol; PCP - presence of carotid plaque; TK - total cholesterol, waist circumference.
Mean values were compared by analysis of variance (ANOVA) followed by the Tukey’s HSD test among different EAT groups.
When compared with EAT <5 mm, P<0.05.
When compared with EAT 5–7 mm, P<0.05
Multiple linear regression analysis revealed age (Beta: 0.406, p<0.001), male gender (Beta: 0.244, p<0.001), and EAT (Beta: 0.450, p<0.001) as independent correlates of CIMT. Otherwise, in logistic regression analysis, only EAT (OR, 1.386; 95% CI, 1.203–1.597, p<0.001) and LDL (OR, 1.013; 95% CI, 1.002–1.013, p=0.02) were independent predictors for presence of carotid plaque (Table 4).
Table 4.
Linear and logistic regression analyses were used for prediction of CIMT and presence of carotid plaque
| Linear regression analysis | Dependent variable: Carotid intima-media thickness | |||||
|---|---|---|---|---|---|---|
| Independent variables | *P | Beta (Standardized) | Beta±SE (Unstandardized coefficients) | †P | Beta (Standardized) | Beta±SE (Unstandardized coefficients) |
| Age, years | <0.001 | 0.476 | 0.010±0.001 | <0.001 | 0.406 | 0.008±0.001 |
| Gender, male | <0.001 | 0.429 | 0.155±0.027 | <0.001 | 0.244 | 0.085±0.016 |
| Family history of CAD | 0.136 | 0.096 | 0.035±0.023 | |||
| HDL-C, mg/dL | 0.323 | 0.070 | 0.001±0.001 | |||
| LDL, mg/dL | 0.009 | 0.161 | 0.001±0.000 | |||
| LDL/HDL ratio | 0.294 | 0.068 | 0.012±0.011 | |||
| TK/HDL ratio | 0.691 | 0.026 | 0.003±0.008 | |||
| EAT, mm | <0.001 | 0.323 | 0.028±0.005 | <0.001 | 0.450 | 0.031±0.003 |
| Constant | 0.125 | -0.132±0.086 | 0.737 | 0.191±0.045 | ||
| Adjusted R2 | 0.571 | 0.534 | ||||
| Logistic regression analysis | Dependent variable: Presence of carotid plaque | |||||
| Independent variables | *P | Wald | OR (95% CI) | †P | Wald | OR (95% CI) |
| Age, years | 0.075 | 3.2 | 1.073 (0.993–1.159) | |||
| Gender, male | 0.066 | 3.4 | 6.147 (0.890–42.5) | |||
| Family history of CAD | 0.141 | 2.2 | 2.791 (0.711–10.9) | |||
| HDL-C, mg/dL | 0.491 | 0.5 | 1.024 (0.958–1.094) | |||
| LDL, mg/dL | 0.002 | 9.1 | 1.034 (1.012–1.057) | 0.020 | 5.4 | 1.013 (1.002–1.023) |
| LDL/HDL ratio | 0.068 | 3.3 | 1.699 (0.963–2.988) | |||
| TK/HDL ratio | 0.111 | 2.5 | 1.396 (0.926–2.104) | |||
| EAT, mm | 0.002 | 9.7 | 1.634 (1.199–2.226) | <0.001 | 20.4 | 1.386 (1.203–1.597) |
| Constant | <0.001 | 18.5 | <0.001 | 31 | ||
| Adjusted R2 | 0.455 | 0.203 | ||||
CAD - coronary artery disease; EAT - epicardial adipose tissue thickness; HDL - high density lipoprotein; LDL - low density lipoprotein; SE - standard error. Linear and logistic regression analyses with enter method were used for all relevant independent variables that were included if they were significantly different in the univariate analyses*. In addition, the analysis was repeated after a pre-elimination with stepwise method for the independent variables†
We showed that there is no increase in CIMT in spite of increasing LDL concentrations in patients with normal EAT levels (<5 mm) compared with the patients in other EAT groups (Fig. 2). Otherwise, LDL concentrations had a significant effect on presence of carotid plaque in all EAT groups (Fig. 3).
Figure 2.
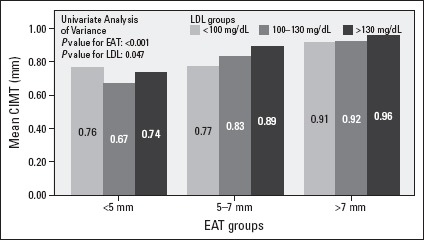
The changes in CIMT with increasing LDL concentrations among EAT groups
There is no increase in CIMT in spite of increasing LDL concentrations in patients with normal EAT levels (<5 mm) compared with the patients in other EAT groups
Figure 3.
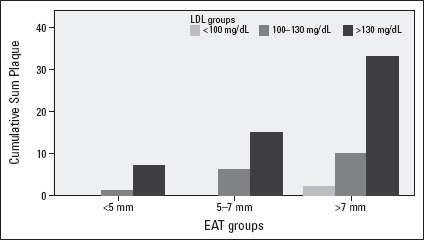
The effect on presence of carotid plaque of LDL concentrations among EAT groups
LDL concentrations had significantly effect on presence of carotid plaque in all EAT groups
Discussion
We revealed that EAT, compared with the other risk factors, had a stronger association with CIMT. Our best of knowledge, this study is first to investigate whether EAT, which is a surrogate for lipid deposition in a special visceral tissue, or circulating lipids is more important for CIMT and atherosclerotic plaque. Age, male gender, and EAT are independent variables for predicting CIMT in our middle-aged, obese, mainly hypertensive study population. Likewise, carotid plaque was only predicted by LDL cholesterol and EAT. In addition, we revealed that the relation between LDL and CIMT was more pronounced if EAT was increased (>5 mm). Indeed, there was no increase in CIMT in patients with normal EAT measuremets (<5 mm) irrespective of their LDL levels. In contrast, LDL concentrations had significant association with the presence of carotid plaque in all EAT groups.
Carotid intima-media thickness is an already established risk marker for subclinical atherosclerosis (1). Increases in CIMT thickness or carotid plaque burden are associated with increased atherosclerotic coronary artery disease event risk (16). CIMT and carotid plaque reflect different stages of atherosclerotic process. Although CIMT represents tunica media smooth cell hypertrophy due to high sheer stress (17), carotid plaque is a typical lesion for atherosclerosis. In the ARIC study, diabetes, current smoking, pulse pressure, and white blood cell count associated with CIMT in positive direction, but HDL cholesterol and triglyceride levels negatively correlated with CIMT (18). The recently completed The Malmö Diet and Cancer Study revealed that age, LDL-cholesterol, systolic blood pressure, and smoking were significantly associated with increased progression rate of CIMT measured at common carotid artery, while HDL-cholesterol showed a negative association (3). Carotid plaque had been shown to be associated with total cholesterol, systolic blood pressure, and smoking (17). In our study, presence of the etiologic factors in progression of CIMT and carotid plaque suggested that study population characteristics are suitable with literature to compare study results. Demographic, anthropomorphic, and laboratory parameters like LDL cholesterol may only enable rough estimation of CIMT as well as coronary artery disease risk (3, 16). Rosvall et al. (3) showed that predictive power of baseline LDL cholesterol was attenuated with increased intima-media thickness and high CIMT progression rate, and the correlation between LDL and CIMT is not linear. This finding may be related to the poor effect of LDL on CIMT, unlike the plaque. Our study findings also support this situation in which CIMT is more related to visceral lipid depot than circulatory lipids. We thought more precision is needed about what factors are responsible for progression of subclinical atherosclerosis. EAT may be an answer because it reflects individual’s cumulative risk over his/her lifespan (19, 20). Epicardial adipose tissue mainly has local effects on coronary vessels (21) and is directly associated with coronary artery disease development (22). However, EAT also has systemic proatherogenic effects probably mediated via its proinflammatory properties and release of adipokines, including adiponectin (21, 23–25). We did not able to measure specific adipokines levels in our study, but higher CRP levels in patients with increased EAT suggested that EAT is a possible inflammatory link between EAT and CIMT. Previous studies demonstrated that EAT has strong and independent association with CIMT (19, 20, 26, 27).
Epicardial adipose tissue measurement adds little time to routine echocardiographic examination (28) and creates a huge opportunity for more appropriate selection of patients. If the patient has increased EAT thickness, he or she should be an appropriate candidate for further CIMT testing irrespective of LDL cholesterol levels. Because by the CIMT and carotid plaque measurement, almost 10% of the patients could be reclassified above what is provided by traditional risk factor calculation. This approach may also lessen the number of ever growing non-invasive imaging exams (29).
Study limitations
The cross-sectional nature of our study is a major limitation. Risk factors change within a given time window may be a more reliable marker for estimating the patient’s subclinical atherosclerosis status. We did not evaluate incremental values of EAT measurement over CIMT/carotid plaque visualization in our study. A large randomized prospective study is certainly needed with which comparative or incremental value of CIMT/carotid plaque and EAT measurements for future cardiovascular events will be determined.
We did not provide data about visceral adipose tissue other than EAT, which can be an important contributor to our study findings. Our patients showed higher waist circumference and triglyceride levels when stratified by EAT. Based on this finding, we thought study patients had an increased visceral adipose tissue. But we prefer EAT as a surrogate for lipid depot in visceral adipose tissue.
Conclusion
Our study showed that EAT has a relationship to both CIMT and carotid plaque but LDL is only independently related to carotid plaque. Therefore, we thought that EAT may have the probable role in the initiation and progression of atherosclerosis by providing continuous pro-atherogenic and pro-inflammatory stimulus. Prevention strategies aiming at decreasing EAT thickness may be a priority in an individual patient.
Our study suggested a possible role of EAT as a novel cardiovascular risk predictor. We can only speculate that EAT as in coronary calcium scoring can provide incremental information about patients for whom primary prevention strategies will more strongly be implemented.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – S.A.K.; Design – S.A.K., O.B.; Supervision – M.Ç.; Materials – M.E.D.; Data collection &/or processing – M.E.D., T.E.; Analysis &/or interpretation – S.A.K.; Literature search – S.A.K.; Writing – T.K.A., M.R.K.; Critical review – M.R.K., E.P.O.; Other – O.B.

From Ahmet Ünver’s collections
References
- 1.Bauer M, Caviezel S, Teynor A, Erbel R, Mahabadi AA, Schmidt-Trucksass A. Carotid intima-media thickness as a biomarker of subclinical atherosclerosis. Swiss Med Wkly. 2012;142:w13705. doi: 10.4414/smw.2012.13705. [DOI] [PubMed] [Google Scholar]
- 2.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations:Associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134:250–6. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 3.Rosvall M, Persson M, Ostling G, Nilsson PM, Melander O, Hedblad B, et al. Risk factors for the progression of carotid intima-media thickness over a 16-year follow-up period:The Malmo Diet and Cancer Study. Atherosclerosis. 2015;239:615–21. doi: 10.1016/j.atherosclerosis.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease:Basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3:e000582. doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iacobellis G, Bianco AC. Epicardial adipose tissue:Emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab. 2011;22:450–7. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–70. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 7.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–60. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden:The Framingham heart study. Eur Heart J. 2009;30:850–6. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography:A new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–10. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 12.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome:A new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–8. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 13.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd 4th and 5th watching the risk symposia, at the 13th 15th and 20th european stroke conferences, Mannheim, Germany 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–6. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.2003 European Society of Hypertension-European society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–53. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension:The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34:2159–219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 16.Weber LA, Cheezum MK, Reese JM, Lane AB, Haley RD, Lutz MW, et al. Cardiovascular imaging for the primary prevention of atherosclerotic cardiovascular disease events. Curr Cardiovasc Imaging Rep. 2015;8:36. doi: 10.1007/s12410-015-9351-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herder M, Johnsen SH, Arntzen KA, Mathiesen EB. Risk factors for progression of carotid intima-media thickness and total plaque area:A 13-year follow-up study:The Tromso study. Stroke. 2012;43:1818–23. doi: 10.1161/STROKEAHA.111.646596. [DOI] [PubMed] [Google Scholar]
- 18.Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, et al. Risk factors for progression of common carotid atherosclerosis:The atherosclerosis risk in communities study, 1987-1998. Am J Epidemiol. 2002;155:38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Cabrera-Rego JO, Iacobellis G, Castillo-Herrera JA, Valiente-Mustelier J, Gandarilla-Sarmientos JC, Marin-Julia SM, et al. Epicardial fat thickness correlates with carotid intima-media thickness, arterial stiffness, and cardiac geometry in children and adolescents. Pediatr Cardiol. 2014;35:450–6. doi: 10.1007/s00246-013-0799-9. [DOI] [PubMed] [Google Scholar]
- 20.Nelson MR, Mookadam F, Thota V, Emani U, Al Harthi M, Lester SJ, et al. Epicardial fat:An additional measurement for subclinical atherosclerosis and cardiovascular risk stratification? J Am Soc Echocardiogr. 2011;24:339–45. doi: 10.1016/j.echo.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT. Epicardial adipose tissue:Far more than a fat depot. Cardiovasc Diagn Ther. 2014;4:416–29. doi: 10.3978/j.issn.2223-3652.2014.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu FZ, Chou KJ, Huang YL, Wu MT. The relation of location-specific epicardial adipose tissue thickness and obstructive coronary artery disease:Systemic review and meta-analysis of observational studies. BMC Cardiovasc Disord. 2014;14:62. doi: 10.1186/1471-2261-14-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katsiki N, Mikhailidis DP, Wierzbicki AS. Epicardial fat and vascular risk:A narrative review. Curr Opin Cardiol. 2013;28:458–63. doi: 10.1097/HCO.0b013e3283605fba. [DOI] [PubMed] [Google Scholar]
- 24.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–71. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 25.Gaborit B, Abdesselam I, Dutour A. Epicardial fat:More than just an “epi” phenomenon? Horm Metab Res. 2013;45:991–1001. doi: 10.1055/s-0033-1358669. [DOI] [PubMed] [Google Scholar]
- 26.Boyraz M, Pirgon O, Akyol B, Dundar B, Çekmez F, Eren N. Importance of epicardial adipose tissue thickness measurement in obese adolescents, its relationship with carotid intima-media thickness, and echocardiographic findings. Eur Rev Med Pharmacol Sci. 2013;17:3309–17. [PubMed] [Google Scholar]
- 27.Kocaman SA, Durakoğlugil ME, Cetin M, Erdoğan T, Ergül E, Canga A. The independent relationship of epicardial adipose tissue with carotid intima-media thickness and endothelial functions:The association of pulse wave velocity with the active facilitated arterial conduction concept. Blood Press Monit. 2013;18:85–93. doi: 10.1097/MBP.0b013e32835ebbb5. [DOI] [PubMed] [Google Scholar]
- 28.Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat:Definition, measurements and systematic review of main outcomes. Arq Bras Cardiol. 2013;101:e18–28. doi: 10.5935/abc.20130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virnig BA, Shippee ND, O’Donnell B, Zeglin J, Parashuram S Trends in the use of echocardiography 2007 to 2011:Data points #20. Data points publication series. Rockville (MD): 2011. [Google Scholar]


