Abstract
Objective:
There are many factors related to high left atrial volume index (LAVI) and global left ventricular longitudinal peak systolic strain (GLS-%) decline in chronic kidney disease. The purpose of our study is to investigate the relation between the β-2 microglobulin (β-2µ) and GLS-% and LAVI in patients with chronic kidney disease not yet on dialysis.
Methods:
Our study was a non-randomized, controlled, prospective study. We included 87 consecutive patients with eGFR levels below 60 ml/min/m2 not on dialysis and 82 normal healthy individuals with complaints of atypical chest pain and negative stress tests as control group in our study. Patients with hospitalization related to dialysis or heart failure attacks within 3 months, active malignancy, malnutrition, pregnancy, and uncontrolled hypertension were excluded. Brachial pulse wave velocity (PWV), augmentation index, augmentation pressure and central hemodynamics, and PWV analysis were performed in order to assess the arterial stiffness and blood pressure. According to the distribution of data, Spearman and Pearson correlations and multiple linear regression were used to determine significant and independent factor associated with high LAVI and low GLS-%.
Results:
There were significant correlations between β-2µ with LAVI (r=0.313, p=0.004) and with GLS-% (r=–0.222, p=0.04). In multiple linear regression, the relationship between β-2µ with GLS-% [β=–0.037, 95% CI (–0.062, –0.013), p=0.004] and LAVI [β=4.522, 95% CI (2.806, 6.238), p<0.001] was independent of age, PWV, central and peripheral blood pressures, parathormone, CalciumXPhospor, Hgb levels, and eGFR.
Conclusion:
Increasing β-2µ levels were found to be associated with increased LAVI and decreased GLS-%. Additional experimental studies are needed to clarify these relationships.
Keywords: cardiac dysfunction, beta-2 microglobulin, chronic kidney disease, global longitudinal strain, left atrial volume index
Introduction
In normal physiologic circumstances, β-2 microglobulin (β-2µ) is a part of the major histocompatibility (MHC) complex in nucleated cells and works for the stabilization of the MHC complex. Additionally, there are several studies suggesting β-2µ’s proinflammatory properties (1) in various pathological conditions. β-2µ was an independent predictor for cardiovascular events and mortality in the general population (2–4) and in patients with chronic kidney disease (CKD) and/or end-stage renal disease (5–7). As a consequence of uremia-related oxidative stress and low-grade inflammation and β-2µ rise in inflammation, it can be argued that β-2µ may be associated with increased cardiovascular abnormality in patients with CKD (8).
Many studies investigating cardiac dysfunction associated with uremia were performed in dialysis patients. These patients commonly exhibited atherosclerotic and related increase in arterial stiffness (9), anemia (10), and hyperparathyroidism with rising CaXPhosphor product (11), which all can lead to the cardiac dysfunction. However, cardiomyopathy has been less intensively investigated in patients with stages 3–5 CKD. In non-dialysis patients, uremic solute such as β-2µ can be seen retentioned, and this related inflammatory effect may be considered to be responsible for uremia-associated cardiac dysfunction (1).
In detection of uremia-related asymptomatic cardiac dysfunction, there is some difficulty in measuring it. In this regard, new echocardiographic techniques such as speckle tracking echocardiography are proven to be superior in comparison with the clinical parameters, left ventricular filling pressures and left ventricular ejection fraction (12).
Since endocardial border localization, longitudinal myocardial fibers are more vulnerable for insufficiency of the microvascular system. Therefore, global longitudinal strain imaging techniques are more sensitive to demonstrating asymptomatic myocardial dysfunction as a result slowly progress harmful effect of pathological condition (13). In our study, we aimed to investigate the relation between the hidden cardiac dysfunction and β-2µ levels, conventional risk factors, such as increased arterial stiffness, low hemoglobin levels, and advanced age, in patients with CKD not yet on dialysis.
Method
Patient selection
This observational study was conducted at Antalya Training and Research Hospital. Our study consisted of 87 consecutive CKD stage 3–5 patients according to the MDRD (14) formula but not on dialysis and 82 normal healthy individuals with atypical chest pain and negative stress test as a control group. For CKD patients, exclusion criteria were as follows: hospitalization related to dialysis or heart failure attacks within 3 months, ejection fraction lower than 50%, active malignancy, malnutrition, pregnancy, and uncontrolled hypertension. All of the patients provided informed consent. This study was approved by the Antalya Training and Research Hospital.
β-2µ assay
Serum β-2µ levels were measured with chemiluminescence (IMMULITE 2000 β-2 microglobulin, SIEMENS Henkestrasse 127 D-91052, Erlangen, Germany). Blood samples were obtained via venous access with 10-mL tubes with vacutainer systems. To eliminate lipid components, serum samples were ultracentrifuged at 18,000 g for 20 min. Obtained serums were kept at –80°C until used for the study. Samples were prediluted to 1:41 in β-2µ Sample Diluent according to the manufacturer’s instructions, and 5-mL samples were taken for the assay procedure.
Echocardiography
Echocardiography was performed with synchronized electrocardiography by two experienced cardiologists who were blinded to the clinical data with a commercially-available system (Philips iE33, Bothell, WA, USA) equipped with a broadband S5-1 transducer (frequency transmitted: 1.7 MHz; received: 3.4 MH). For global 2D strain analysis, a digital loop was acquired from apical 4-chamber, and apical-2 and apical-3 chamber views. Two-dimensional speckle tracking was performed using a semiautomatic algorithm. The mean frame rate for strain analysis was 65±10 frames/s. Analysis was feasible in 93% of the segments (2118 of 2278).
The maximal left atrial volume was measured from the apical four-chamber view by using the biplane area-length method in end-systole before mitral valve opening. Tissue Doppler-derived early mitral septal anulus velocity (e’) and early diastolic mitral pulse velocities (E) were derived.
Assessment of pulse wave velocity
Mobil-O-Graph® 24-hour pulse wave analysis monitor [I.E.M. Industrielle Entwicklung Medizintechnik und Vertriebsgesellschaft mbH Cockerillstraße Stolberg (Rheinland), Germany] was used for the measurements of arterial stiffness and central blood pressure analysis (15). Built-in combining algorithms in the device were used for central aortic and brachial blood pressure measurements and PWV analysis. Assessment of pulsatile hemodynamics: PWV was determined as a measure of arterial stiffness; augmentation index (Aug I), augmented pressure (Aug P) and central systolic blood pressure (C sys BP), and central diastolic blood pressures (C dia BP) were determined as measures of central hemodynamics.
Statistical analysis
Categorical variables were expressed as percentages. Values for continuous variables are given as mean±standard deviation or median (maximum–minimum). The means of continuous variables were compared using student’s t-test for independent samples. For univariate analysis, Spearman and Pearson correlation test was performed on dataset depending on the distribution of data. Multiple linear regression models were also used for to evaluate the independent association of echocardiographic results with β-2µ levels. Statistical analyses were conducted using SPSS 17.0 for Windows (SPSS Inc.). Statistical significance was set at two-sided p value <0.05.
Results
In comparison with the healthy control group, atherosclerotic, hypertensive, and diabetic nature of our patients could be seen (Tables 1 and 2). Echocardiographic examination showed higher left ventricular mass indexes, higher E/e’, higher LAVI, and lower GLS-% in patient group than did healthy controls (Table 2). β-2µ levels were higher in the patient group than in controls (Table 1).
Table 1.
Baseline demographics and results
| Patients(n=87) | Control (n=82) | P | |
|---|---|---|---|
| Age, years | 63 (23–97) | 46.3±11.3 | <0.001 |
| Gender, male, % | 63.6 | 52.8 | <0.001 |
| DM, % | 52.3 | 21.2 | <0.001 |
| History of HT,% | 68.1 | 23.5 | <0.001 |
| eGFR, | 27.9 (8.0–53.6) | 87±10.3 | <0.001 |
| Etiology of CKD | |||
| DM or HT,% | 69.3 | ||
| Others, % | 30.7 | ||
| Laboratory parameters | |||
| β–2µ, ng/mL | 5.2±1.9 | 2.3±0.4 | <0.001 |
| Glucose, mg/dL | 102 (68–311) | 93±17.5 | <0.001 |
| BUN, mg/dL | 3 (16-94) | 12.9±19 | <0.001 |
| U, acid, mg/dL | 7.0±1.4 | 5.1±1.2 | <0.001 |
| T protein, g/dL | 7.2±0.5 | 7.22±1.5 | ns |
| Alb, g/dL | 3.9 (3.3–4.7) | 4.5±0.28 | <0.001 |
| TG, mg/dL | 162.1 (72.5) | 131±100 | ns |
| LDL, mg/dL | 127.9 (42.7) | 131±40 | ns |
| HDL, mg/dL | 42 (23–105) | 49±11 | 0.01 |
| CRP, mg/dL | 3.9±4.1 | 2.7±1.1 | <0.001 |
| iPTH, pg/mL | 115 (2.0–570) | 46±15 | 0.03 |
| P, mg/dL | 3.8±0.5 | 3.6±0.5 | ns |
| Hgb, g/dL | 12.2±1.5 | 12.2±1.3 | ns |
| CaXP, mg2/dL2 | 36.0±6.4 | 29.3±11.2 | <0.001 |
| Medications used | |||
| CC-B, % | 58 | 0.8 | – |
| β-B, % | 37.5 | 1.1 | – |
| RAS-B, % | 60.2 | 2.1 | – |
| Th, % | 30.7 | 2.0 | – |
| α-B, % | 17.2 | – | – |
| EPO, % | 25.0 | – | – |
| ViD-R, % | 63.6 | – | – |
| ASA for CAD, % | 14.8 | 0.3 | – |
| Furosemide, % | 8 | – | – |
α-B - alpha blocker; β-B - beta blocker; ASA - acetylsalicylic acid; BUN - blood urea nitrogen; CAD - coronary artery disease; CaXP - calcium XPhosphor; CC-B - Ca++ channel blocker; CRP - C-reactive protein; CKD - chronic kidney disease; DM - diabetes mellitus; eGFR - estimated glomerular filtration rate; EPO - erythropoietin replacement; HDL - high density lipoprotein; Hgb - hemoglobin; HT - hypertension; iPTH - intact parathormone; LDL - low density lipoprotein; P - phosphor; RAS-B - renin angiotensin system blocker; Th - thiazide; ViD-R - vitamin D replacement; ns - not significant; mL/min/1.73 m2
Table 2.
Comparison of arterial stiffness, blood pressures, and echocardiographic variables
| Patients (n=87) | Control (n=82) | P | |
|---|---|---|---|
| Arterial stiffness and blood pressures | |||
| PWV, m/sn | 9.3±1.7 | 6.3±1.6 | <0.001 |
| Sys BP, mm Hg | 140.2±20.6 | 119.3±9.7 | <0.001 |
| Dia BP, mm Hg | 85.2±11.2 | 79.4±9.5 | <0.001 |
| C sys BP, mm Hg | 126,6±21.8 | 111.4±9.8 | <0.001 |
| C dia BP, mm Hg | 87.8±11.4 | 85.7±9.3 | <0.001 |
| MAP, mm Hg | 111.4±14.2 | 97.6±8.8 | <0.001 |
| PP, mm Hg | 57.1±16.4 | 39.9±7.6 | <0.001 |
| Aug I, @%75 | 25.2±13.9 | 19.1±13.9 | 0.07 |
| Aug P, mm Hg | 7 (1–42) | 6.1±5.0 | 0.01 |
| Echocardiography | |||
| GLS, % | 17 (11–21) | 18.2±2.6 | <0.001 |
| LAVI, ml/m2 | 32.1±9.2 | 24.3±5.8 | <0.001 |
| E/e’ | 9.6±4.0 | 7.5±2.4 | 0.02 |
| EF, % | 69.7±7.8 | 70.5±5.8 | 0.087 |
| Sys Dia, mm | 25.6±5.4 | 25.4±3.6 | ns |
| Dia Dia, mm | 44.3±5.9 | 44.1±6.0 | ns |
| LVMI, g/m2 | 114.7±28.9 | 86.0±17.2 | <0.001 |
Aug - augmentation; BP - blood pressure; C - central; Dia - diastolic; EF - ejection fraction; I- index; LVMI - left ventricular mass index; MAP - mean arterial pressure; P - pressure; PP - pulse pressure; Sys - systolic
In CKD patients, mean β-2µ level was 5.2±1.9 ng/mL. There were no significant differences in terms of β-2µ levels between hypertensive diabetic patients (n=60) and other patients (n=27) (5.0±1.9 vs. 5.2±1.7) (Table 1).
In CKD patients, there was a statistically significant correlation between β-2µ levels and eGFR (r=–0.716 p<0.001). In patients taking RAS blocker±thiazide diuretics (median taken to maximal dose ratio for each agent =75%), the β-2µ level was found to be significantly lower (4.8 vs. 5.9 ng/mL, p=0.007), and in patients taking beta blockers (median taken to maximal dose ratio for each agent =50%), β-2µ level was found to be significantly higher (5.8 vs. 4.9 ng/mL, p=0.031). eGFR measurements indicated that patients taking RAS blockers have higher eGFR levels than patients not taking it (30.8 vs. 24.3 mL/min/m2, p=0.034).
β-2µ was correlated to CaXPhosphor product (r=0.371, p<0.001) and spot urine micrototal protein /creatinine ratio (r=0.321, p=0.004). We could find a significant positive correlation between the parathormone and β-2µ levels (r=0.258, p=0.01).
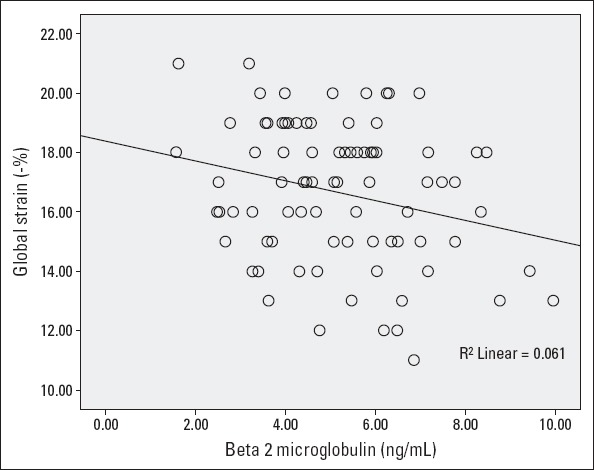
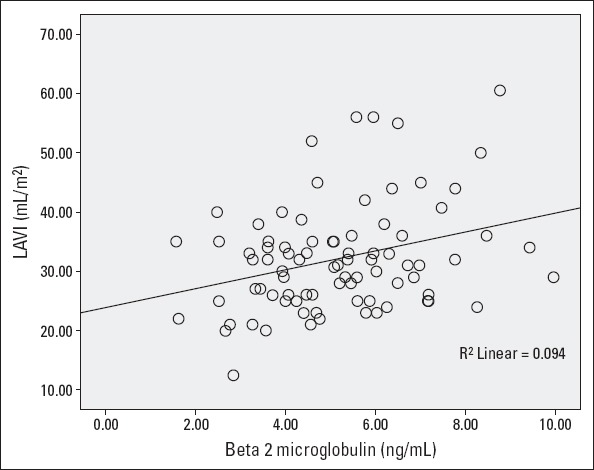
There was a strong correlation between parameters β-2µ with GLS-% (Fig. 1) and LAVI (Fig. 2). There was a statistically significant correlation between the GLS-% and systolic blood pressure (r=–0.227, p=0.036), pulse pressure (r=–0.231, p=0.03), and mean arterial pressure (r=–0.224, p=0.04). The correlation between the GLS-% and PWV was statistically not significant (r=–0.190, p=0.09). Multiple linear regression (method=stepwise) analysis showed only β-2µ was independently associated with the GLS-% after adjusting for confounding factors [β=–0.037, 95% CI (–0.062, –0.013), p=0.004].
Figure 1.
LAVI and β-2µ relation
Figure 2.
GLS-% and β-2µ relation
No significant relation was found between LAVI and PWV (r=0.107, p=0.351). Conversely, PWV and E/e’ was significantly correlated (r=0.372, p=0.001). β-2µ and LAVI was significantly correlated (r=0.313, p=0.004). Multiple linear regression analysis showed β-2µ [β=2.793 (1.355–4.231), p<0.001], hgb [β=–1.854 (–3.028, –0.680), p=0.012], diuretic use [β=8.340 (1.819–14.861), p=0.036] were associated with LAVI after adjusting for confounding factors (method=stepwise) (Table 3).
Table 3.
Cardiac dysfunction parameters and its relations
| GLS | LAVI | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate% | Univariate | Multivariate% | |||||
| r | P | Beta (%95CI)& | P | r | P | Beta (%95CI)& | P | |
| Age | -0.116$ | 0.290 | 0.184 | 0.097 | 0.139$ | 0.209 | 0.192 | 0.076 |
| eGFR | 0.124$ | 0.213 | -0.002 | 0.984 | -0.139$ | 0.211 | 0.333 (0.074–0.593) | 0.013 |
| β-2µ | -0.247# | 0.021 | -0.037 (-0.062, -0.013) | 0.004 | 0.313# | 0.004 | 2.793 (1.355–4.231) | <0.001 |
| Hgb | 0.092# | 0.405 | 0.027 | 0.813 | -0.325# | 0.003 | -1.854 (-3.028, -0.680) | 0.012 |
| iPTH | -0.115# | 0.308 | -0.064 | 0.567 | -0.004# | 0.975 | -0.105 | 0.356 |
| CaXPh | -0.075# | 0.498 | 0.024 | 0833 | -0.009# | 0.939 | -0.154 | 0.200 |
| Diuretic | -1.078%,* | 0.227 | -0.065 | 0.561 | 7.763%,* | 0.030 | 8.340(1.819-14.861) | 0.036 |
| PWV | -0.190$ | 0.092 | -0.073 | 0.553 | 0.107$ | 0.351 | 0.126 | 0.231 |
| Sys BP | -0.227$ | 0.036 | -0.146 | 0.204 | 0.052$ | 0.641 | -0.132 | 0.241 |
| DiaBP | -0.133$ | 0.225 | 0.106 | 0.481 | 0.003$ | 0.979 | -0.126 | 0.243 |
| C sys BP | -0.267$ | 0.015 | 0.139 | 0.495 | 0.135$ | 0.236 | -0.122 | 0.289 |
| C dia BP | -0.142$ | 0.205 | 0.090 | 0.559 | -0.019$ | 0.871 | -0.120 | 0.129 |
| MAP | -0.220$ | 0.050 | 0.264 | 0.447 | 0.077$ | 0.498 | -0.115 | 0.111 |
| PP | -0.231$ | 0.031 | -0.146 | 0.339 | 0.112$ | 0.322 | -0.080 | 0.487 |
| Aug I | 0.045$ | 0.693 | 0.094 | 0.419 | -0.561$ | 0.598 | -0.092 | 0.387 |
| Aug P | 0.038$ | 0.737 | 0.132 | 0.271 | 0.070$ | 0.545 | -0.089 | 0.862 |
Pearson correlation;
Spearman correlation;
Linear regression;
CI denoted if it was only statistically significant;
Beta; Aug I - augmentation index;
Aug P - augmentation pressure; BP - blood pressure; C - central; Dia - diastolic; eGFR - estimated glomerular filtration rate; Hgb - hemoglobin; iPTH - intact parathormone; MAP - mean arterial pressure; PP - pulse pressure; Sys - systolic
No statistically significant correlation could be found between eGFR and GLS-% (r=0.124, p=0.2).
A significant (r=–0.327, p<0.001) correlation was found between GLS-% and LAVI and modest correlation between LAVI and E/e’ (r=–0.221, p=0.006)
Pearson correlation analysis showed significant associations between β-2µ and pulse pressure (r=0.220, p=0.04), central systolic blood pressures (r=0.267, p=0.01), and no association could be found between PWV and β-2µ.
Discussion
In our study, we evaluated conventional risk factors for cardiac damage in CKD. According to previous studies, arterial stiffness parameters (PWV), central hemodynamic parameters (16), blood pressures (17), hemoglobin (18) and PTH (19) levels and cardiac dysfunction are well known, and we have also examined these parameters. In multivariate analysis including these confounding factors, β-2µ has continued to remain as an independent parameter to predict GLS-% and the LAVI.
In a study conducted by Sedighi et al. (20), the relationship between serum β-2µ levels and cardiac dysfunction as determined by conventional echocardiography was detected. However, in this study, arterial stiffness parameters were not used and possible effects on cardiac dysfunction were excluded in the analysis.
In contrast, Saijo et al. (21) reported that there was no significant correlation between β-2µ and PWV. However, these studies excluded patients with chronic renal failure. Our study population was relatively small and covers patients with chronic renal failure and provides information about the contribution to the development of atherosclerosis of uremic solutes retention in addition to conventional risk factors. Diabetes mellitus is one of the confounding factors, and 69% of our patients were diabetic. In our opinion, this may explain the difference between the two studies.
In a study conducted by Kals et al. (22), there was a positive correlation between β-2µ and PWV; however, in this study, chronic renal failure patients were not represented and β-2µ levels were much lower compared to those in our patient groups. On the contrary of by renal retention and inflammatory related of our patients, in this study, the source of β-2µ maybe only low grade inflammation. Additionally, these studies only reflect patients with peripheral arterial disease. Another study conducted by Raikou et al. (23) included hemodialysis patients, and this is different from our population. Nevertheless, they could not find any association between β-2µ and PWV. Also, in this study, significant correlation was found between β-2µ and PP, in agreement with our study. In our study, high β-2µ, which is an uremic toxin levels, were determined due to chronic renal failure and related inflammatory status. Additionally, our population is small, and our assessment of the relationship between arterial stiffness and β-2µ is difficult.
In patients with chronic renal failure, the relationship between β-2µ with cardiac dysfunction is well known. In hemodialysis patients, positive fluid balance, which may affect cardiac function significantly, may contribute significantly to myocardial dysfunction (24). Therefore, in this patient group, it appears difficult to establish an independent relationship between uremic solutes retention and myocardial dysfunction. In a study with non-dialysis patients (25) using conventional echocardiographic methods for the evaluation of left ventricular function, the negative correlation between left ventricular ejection fraction and β-2µ was found. In this study, myocardial infarction, which is a well-known cause of left ventricular systolic dysfunction, was not excluded. Left ventricular diameters have also been observed to be significantly greater. This finding underlying ischemic or non-ischemic dilated cardiomyopathy was not disputed to reflect. In this study, patients with left ventricular dilatation, which can be identified by conventional echocardiographic methods and indicate primary cardiac dysfunction, were excluded. In our study, a strong relation between GLS-% and β-2µ was demonstrated. eGFR values of our patients have fairly narrow range, and thus, we could not detect similar relationship between GLS-% and eGFR.
In cases of renal dysfunction, renin-angiotensin activity is increased. Increased RAS activation leads to an increase in low-grade inflammation (26). This situation can be reversed by blocking RAS (27). Increased inflammatory activity is associated with high β-2µ levels (28). Furthermore the rising levels of β-2µ increase vascular injury via upregulation of IL-1 and TNF-alpha expression (29). In our study, β-2µ levels were lower in patients treated with RAS blockers. This has been interpreted as due to the anti-inflammatory effect of RAS blockade.
As a result of left ventricular systolic and diastolic dysfunction, elevated left ventricular filling pressure may be seen. In echocardiographic estimation of left ventricular end-diastolic pressures, LAVI and E/e’ are very useful and well validated (30). We demonstrated a high correlation between LAVI and β-2µ. In our patients, between GLS-% and LAVI, LAVI and E/e’ are higher than the control group. This is considered as a marker of silent myocardial dysfunction. Similarly in previous studies, negative correlation between GLS-% with LAVI and E/e’ was found. Mean E/e’ level of our patients was found to be increased, but there was not any correlation between E/e’ and β-2µ levels. Some studies claimed E/e’ value reflects the state of the left ventricle instantaneous pressure change and influenced by the arterial blood pressure and current volume of patients (31). For these reasons, in our patients group, E/e’ may be difficult to reflect the value of silent myocardial dysfunction. LAVI is also a parameter that reflects the strength of chronic sudden changes in left ventricular filling pressure. Therefore, the ability to show the myocardial dysfunction is higher than E/e’. In our study, the positive correlation between β-2µ and LAVI and the negative correlation between LAVI and GLS-% are considered as the indicator.
Study limitations
Our study has only a small number of patients.
Conclusion
In our study, we showed that β-2µ, a uremic toxin, is negatively associated with GLS-% and positively associated with LAVI. In predialysis CKD, there is no association between PWV and β-2µ. In our opinion, interventional studies may be confirmed relation between that β-2µ and asymptomatic cardiac damage.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.Y., B.Y.; Design – A.Y.; Supervision – A.Y., B.Y.; Materials – B.Y.; Data collection &/or processing – A.Y., S.K.; Analysis and/or interpretation – A.Y., B.Y.; Literature search – A.Y.; Writing – A.Y., B.Y., S.K.; Critical review – A.Y.
References
- 1.Miyata T, Hori O, Zhang JH, Yan SD, Ferran L, Lida Y, et al. The receptor for advanced glycation end products (RAGE) is a central mediator of the interaction of AGE- beta(2)microglobulin with human mononuclear phagocytes via an oxidant-sensitive pathway—implications for the pathogenesis of dialysis related amyloidosis. J Clin Invest. 1996;98:1088–94. doi: 10.1172/JCI118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinkai S, Chaves PHM, Fujiwara Y, Watanabe S, Shibata H, Yoshida H, et al. Beta(2)-microglobulin for risk stratification of total mortality in the elderly population—comparison with cystatin C and C-reactive protein. Arch Int Med. 2008;168:200–6. doi: 10.1001/archinternmed.2007.64. [DOI] [PubMed] [Google Scholar]
- 3.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59:653–62. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prentice R, Paczesny S, Aragaki A, Amon LM, Chen L, Pitteri SJ, et al. Novel proteins associated with risk for coronary heart disease or stroke among postmenopausal women identified by in-depth plasma proteome profiling. Genome Med. 2010;2:48. doi: 10.1186/gm169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuno S, Ishimura E, Kohno K, Fujino-Katoh Y, Maeno Y, Yamakawa T, et al. Serum beta(2)-microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol Dial Transplant. 2009;24:571–7. doi: 10.1093/ndt/gfn521. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AK, Rocco MV, Yan GF, Levin NW, Greene T, Agodoa L, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–55. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 7.Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, Lemke HD, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82:1297–303. doi: 10.1038/ki.2012.301. [DOI] [PubMed] [Google Scholar]
- 8.Amighi J, Hoke M, Mlekusch W, Schlager O, Exner M, Haumer M, et al. Beta 2 microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke. 2011;42:1826–33. doi: 10.1161/STROKEAHA.110.600312. [DOI] [PubMed] [Google Scholar]
- 9.Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: comparison with intra-aortic catheter measurements. Blood Press Monit. 2013;18:173–6. doi: 10.1097/MBP.0b013e3283614168. [DOI] [PubMed] [Google Scholar]
- 10.Foley RN, Parfrey PS, Morgan J, Barré PE, Campbell P, Cartier P, et al. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2000;58:1325–35. doi: 10.1046/j.1523-1755.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 11.Foley R. Clinical epidemiology of cardiac disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin Dial. 2003;16:111–7. doi: 10.1046/j.1525-139x.2003.160271.x. [DOI] [PubMed] [Google Scholar]
- 12.Hestenes SM, Halvorsen PS, Skulstad H, Remme EW, Espinoza A, Hyler S, et al. Advantages of strain echocardiography in assessment of myocardial function in severe sepsis: An Experimental Study Crit Care Med. 2014;42:432–40. doi: 10.1097/CCM.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 13.Enomoto M, Ishizu T, Seo Y, Yamamoto M, Suzuki H, Shimano H, et al. Subendocardial systolic dysfunction in asymptomatic normotensive diabetic patients. 2015;79:1749–55. doi: 10.1253/circj.CJ-15-0012. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Inter Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hans-Josef F, Sebastian J. R, Gert K, Kremser C, Seidner B, Esterhammer R, et al. Comparison of an oscillometric method with cardiac magnetic resonance for the analysis of aortic pulse wave velocity. Plos One. 2015;10:e0116862. doi: 10.1371/journal.pone.0116862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnasamy R, Hawley CM, Stanton T, Pascoe EM, Campbell KL, Rossi M, et al. Left ventricular global longitudinal strain is associated with cardiovascular risk factors and arterial stiffness in chronic kidney disease. BMC Nephrol. 2015;16:106. doi: 10.1186/s12882-015-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szelényi Z, Fazakas Á, Szénási G, Tegze N, Fekete B, Molvarec A, et al. The mechanism of reduced longitudinal left ventricular systolic function in hypertensive patients with normal ejection fraction. J Hypertens. 2015;33:1962–9. doi: 10.1097/HJH.0000000000000624. [DOI] [PubMed] [Google Scholar]
- 18.Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884–90. doi: 10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 19.Amann K, Neusüss R, Ritz E, Irzyniec T, Wiest G, Mall G. Changes of vascular architecture independent of blood pressure in experimental uremia. Am J Hypertens. 1995;8:409–17. doi: 10.1016/0895-7061(94)00248-a. [DOI] [PubMed] [Google Scholar]
- 20.Sedighi O, Abediankenari S, Omranifar B. Association between plasma Beta-2 microglobulin level and cardiac performance in patients with chronic kidney disease. Nephrourol Mon. 2014;7:e23563. doi: 10.5812/numonthly.23563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saijo Y, Utsugi M, Yoshioka E, Horikawa N, Sato T, Gong Y, et al. Relationship of beta2-microglobulin to arterial stiffness in Japanese subjects. Hypertens Res. 2005;28:505–11. doi: 10.1291/hypres.28.505. [DOI] [PubMed] [Google Scholar]
- 22.Kals J, Zagura M, Serg M, Kampus P, Zilmer K, Unt E, et al. β2-microglobulin, a novel biomarker of peripheral arterial disease, independently predicts aortic stiffness in these patients. Scand J Clin Lab Invest. 2011;71:257–63. doi: 10.3109/00365513.2011.558108. [DOI] [PubMed] [Google Scholar]
- 23.Raikou VD, Tentolouris N, Kyriaki Evaggelatou A, Tzanatou H. β2-Microglobulin, pulse pressure and metabolic alterations in hemodialysis patients. Nephron Clin Pract. 2011;117:237–45. doi: 10.1159/000320193. [DOI] [PubMed] [Google Scholar]
- 24.Shotan A, Dacca S, Shochat M, Kazatsker M, Blondheim DS, Meisel S. Fluid overload contributing to heart failure. Nephrol Dial Transplant. 2005;20(Suppl 7):24–7. doi: 10.1093/ndt/gfh1103. [DOI] [PubMed] [Google Scholar]
- 25.Hüting J, Kramer W, Schütterle G, Wizemann V. Analysis of left-ventricular changes associated with chronic hemodialysis. A noninvasive follow-up study. Nephron. 1988;49:284–90. doi: 10.1159/000185077. [DOI] [PubMed] [Google Scholar]
- 26.Becher UM, Endtmann C, Tiyerili V, Nickenig G, Werner N. Endothelial damage and regeneration: the role of the renin-angiotensin- aldosterone system. Curr Hypertens Rep. 2011;13:86–92. doi: 10.1007/s11906-010-0171-x. [DOI] [PubMed] [Google Scholar]
- 27.Dessì M, Piras A, Madeddu C, Cadeddu C, Deidda M, Massa E, et al. Long-term protective effects of the angiotensin receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress and myocardial dysfunction. Exp Ther Med. 2011;2:1003–9. doi: 10.3892/etm.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brevetti G, Piscione F, Cirillo P, Galasso G, Schiano V, Barbato E, et al. In concomitant coronary and peripheral arterial disease, inflammation of the affected limbs predicts coronary artery endothelial dysfunction. Atherosclerosis. 2008;201:440–6. doi: 10.1016/j.atherosclerosis.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Wilson AM, Kimura E, Harada RK, Nair N, Narasimhan B, Meng XY, et al. Beta2-microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation. 2007;116:1396–403. doi: 10.1161/CIRCULATIONAHA.106.683722. [DOI] [PubMed] [Google Scholar]
- 30.Özer N, Okutucu S, Kepez A, Aksoy H, Deveci OS, Atalar E, et al. Diagnostic accuracy and clinical utility of echocardiographic indices for detecting left ventricular diastolic dysfunction in patients with coronary artery disease and normal ejection fraction. Anadolu Kardiyol Derg. 2011;11:666–73. doi: 10.5152/akd.2011.186. [DOI] [PubMed] [Google Scholar]
- 31.Huynh QL, Kalam K, Iannaccone A, Negishi K, Thomas L, Marwick TH. Functional and anatomic responses of the left atrium to change in estimated left ventricular filling pressure. J Am Soc Echocardiogr. 2015 Sep 3; doi: 10.1016/j.echo.2015.07.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


