Abstract
Objective:
To evaluate the acute phase (pre- and in-hospital) antithrombotic management patterns (AMPs) and in-hospital outcomes for patients hospitalized with an acute coronary syndrome (ACS).
Methods:
In total, 1034 patients [514 patients with ST-segment elevation myocardial infarction (STEMI) and 520 with unstable angina/non-STEMI (UA/NSTEMI)] hospitalized for ACS within 24 h of symptom onset were included in this multicenter prospective registry study conducted at 34 hospitals across Turkey. Patient characteristics, index event description, pre- and in-hospital AMPs, and clinical outcomes were evaluated.
Results:
Majority (89.1%) of patients did not receive pre-hospital treatment. Overall 87.9% patients with STEMI and 55.6% patients with NSTEMI underwent percutaneous coronary intervention and dual antiplatelet therapy (DAPT) was based mainly on acetylsalicylic acid (ASA) and clopidogrel during hospitalization (99.8% and 98.2%, respectively). DAPT use at discharge was 98.4% and 86.8%, respectively. The percentage of patients with STEMI who received pre-hospital care, in-hospital cardiac catheterization, and pre and/or in-hospital triple antiplatelet therapy was higher than that of patients with UA/NSTEMI. In addition, higher rate of in-hospital hemorrhagic (2.3% vs. 0.8%) and cardiac ischemic (1.2% vs. 0.4% for MI and 1.6% vs. 0.8% for recurrent ischemia) complications and earlier induction of pre and/or in-hospital antiplatelet therapy and cardiac catheterization were also noted in patients with STEMI than in those with UA/NSTEMI.
Conclusion:
Our findings revealed in-hospital and at-discharge management to be mainly based on DAPT in patients with ACS. Interventional strategies were used in the majority of patients with STEMI, while the usage and timing of immediate pre-hospital ECG from symptom onset should be improved in these patients.
Keywords: acute coronary syndrome, antithrombotic management, pre-hospital care, in-hospital care, real-life setting, Turkey
Introduction
Acute coronary syndrome (ACS) is a clinical syndrome of distinct clinical entities with a common etiology that is acute plaque disruption or erosion causing an abrupt imbalance between myocardial oxygen supply and demand (1–3). Depending on the severity of occlusive coronary thrombi, it is characterized by a spectrum of symptomatic coronary artery disease that ranges from unstable angina (UA) to non-ST-segment elevation myocardial infarction (NSTEMI) and to STEMI (2, 4).
Because of the role of acute thrombotic occlusion of coronary arteries in the etiology of the disease, current medical thera- pies for patients with ACS focus on the coagulation cascade and platelet inhibition and evolve rapidly in recent years based on the data from new randomized controlled trials and subsequent changes in guidelines recommendations (1, 2, 5–8). However, despite progress in evidence-based treatments, a very high mortality rate at 5 years in all three sub-categories of ACS (19% for STEMI, 22% for NSTEMI, and 17% for UA patients) indicate the consequences of ACS to remain serious, and the management of ACS to remain as a clinical challenge (9–11).
Considering the possibility of a wide variability in the patterns of use of antithrombotic therapies along with the broad choice of dose and timings for their use for the in-hospital and at-discharge treatment of ACS, differences in clinical outcomes (ischemic and bleeding events), quality of life, and economic costs are quite likely (12). However, there is a shortage of evidence concerning the physicians’ practices in the use of antithrombotic drugs in different settings and countries as well as the benefits and risks of the many potential antithrombotic agent combinations used in a real-life setting and the interaction with the different invasive strategies (12).
Therefore, the present study, representing the Turkish arm of multinational cross-sectional EPICOR (long-term follow up of antithrombotic management patterns in patients with acute coro- nary syndrome) study (12), was designed to describe the acute phase (pre- and in-hospital) and long-term (post-discharge 24-month follow up) antithrombotic management patterns (AMPs) for patients hospitalized with an ACS (i.e., STEMI, UA/NSTEMI) and to evaluate the relationship between AMPs with in-hospital and post-discharge clinical outcomes, quality of life, and economic aspects in a “real-life” setting.
Data on acute phase (pre- and in-hospital) AMPs and in-hospital cardiac, and hemorrhagic and functional outcomes in the Turkish cohort are presented in this paper.
Methods
Study population
EPICOR is a multinational, multicenter, observational, pros- pective, and longitudinal cohort study, which enrolled 10,568 consecutive patients surviving an ACS (4943 with STEMI, and 5625 with UA/NSTEMI) in a real-life setting between September 1, 2010, and March 31, 2011, from 555 hospitals in 20 countries including Argentina, Belgium, Brazil, Denmark, Finland, France, Germany, Greece, Italy, Luxembourg, Mexico, Netherlands, Norway, Poland, Romania, Slovenia, Spain, Turkey, UK, and Venezuela based on 4 pre-defined regions: Northern Europe (n=3782), Southern Europe (n=2337), Eastern Europe/Turkey (n=2380), and Latin America (n=2069) (12).
Representing Turkey arm of the EPICOR study, the present study was conducted between September 1, 2010 and February 28, 2011 with 1034 patients (514 patients with STEMI and 520 with UA/NSTEMI) from 34 hospitals [regional/community/rural hospital (n=3), non-university general hospital (n=6), university gene- ral hospital (n=24), and other type of hospital/clinic (n=1)] who were hospitalized for ACS within 24 h of symptom onset and who had a final diagnosis of UA, STEMI, or NSTEMI and survived to discharge. All 34 centers had coronary/intensive care unit, and 33 (97.1%) centers had Cath Lab Facilities of which 24/7 primary PCI Program was available in 32 (97.0%). Men and women aged 18 years or older were eligible for inclusion in the study if (i) they were hospitalized within 24 h of symptom onset of the index event for the first time and had a final (discharge) diagnosis of STEMI or UA/NSTEMI, (ii) they provided written informed consent at the time of hospital discharge, and (iii) they completed a Contact Order Form, in which they agreed to be contacted by telephone for regular follow-up interviews during the post-discharge phase. The occurrence of ACS in association with surgery, trauma, gastrointestinal bleeding, post-percutaneous coronary intervention (PCI) or during hospitalization for other reasons, presence of conditions that may limit the complete follow-up of the patient, previous enrollment in the EPICOR study or current participation in another clinical trial, and presence of severe comorbidities that may limit short-term (i.e., 6-month) life expectancy were the exclusion criteria of the study.
All patients underwent routine clinical assessments and receive the standard medical care, as determined by the treating physician with no experimental intervention or treatment as a consequence of their participation in the study. The study design and patient flow chart is shown in Figure 1.
Figure 1.
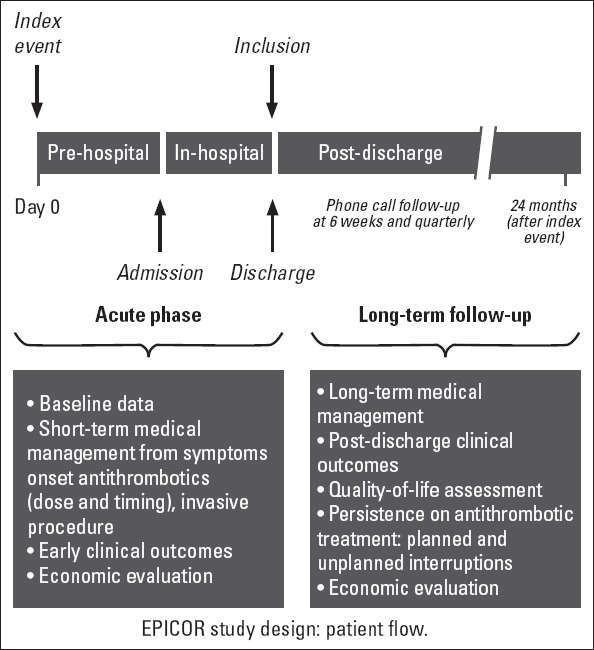
EPICOR study design: patient flow
Written informed consent was obtained from each subject following a detailed explanation of the objectives and protocol of the study which was conducted in accordance with the ethical principles that are consistent with the Declaration of Helsinki revision, International Conference on Harmonization Good Clinical Practice guideline, and the applicable legislation on non-interventional studies and approved by the Institutional Ethics Committee.
Data collection
Data were collected in two phases including acute phase during which pre-hospital and in-hospital data collection occurred (through hospital discharge for the index ACS event that triggered recruitment), and follow-up phase (up to 2 years post discharge), during which information was obtained through telephone interviews. In this paper, data on pre- and in-hospital management and outcomes are presented.
During the acute phase, initial data collection was performed by the investigator and included patient characteristics [demographics, cardiovascular (CV) risk factors, medical history, and previous medication and healthcare consumption (cardiac interventions, medication, imaging, laboratory tests, procedures, and hospital stay) up to 3 months prior to index event date], index event description, pre-hospital management [date/time of symptom(s) onset, first-aid treatment and clinical management from symptoms onset until arrival at the hospital] and in-hospital management [diagnostic and therapeutic procedures for the index event and treatments (antithrombotic and others prescribed to treat the index event from symptoms onset) during the hospitalization for the index event and at discharge].
Data on pre- and in-hospital AMPs (the choice of antiplatelet and anticoagulant drugs, dosing, timing, combination with the different reperfusion, and invasive strategies during hospitali- zation) were recorded in hospitalized patients with STEMI vs. NSTEMI ACS in association with clinical outcomes [ischemic events (myocardial infarction, UA, ischemic stroke, and transient ischemic attack) and bleeding events] and healthcare resource consumption.
Study variables
The primary outcome variable was the rate of AMPs used for patients with STEMI and UA/NSTEMI during hospitalization and at discharge. Secondary pre-specified outcomes include the associations of the most frequent AMPs with the rates of clinical outcomes, and health resources consumption. Because of the non-interventional character of the study, there was no proactive safety data collection, with the exception of treatment associated bleeding events.
The definitions used for ACS
Criteria for STEMI diagnosis were the history of chest pain/discomfort and persistent ST-segment elevation (>30 min) of ≥0.1 mV in 2 or more contiguous electrocardiogram (ECG) leads or presumed new left bundle branch block (LBBB) on admission, and elevation of cardiac biomarkers (CK-MB, troponins) to at least 1 value above the 99th percentile of the upper reference limit.
Criteria for NSTEMI diagnosis included the history of chest pain/discomfort, lack of persistent ST segment elevation, LBBB or intraventricular conduction disturbances, and elevation of cardiac biomarkers (CK-MB, troponins) to at least 1 value above the 99th percentile of the upper reference limit.
Criteria for UA diagnosis included symptoms of angina at rest or on minimal exercise, transient ST-T changes, and no significant increase in biomarkers of necrosis but objective evidence of ischemia by non-invasive imaging or significant coronary stenosis at angiography.
Statistical analysis
For the multinational EPICOR study, the sample size was estimated at 10,600 patients, anticipating that 5 strategies would be employed by ≥10% population and ensuring an 80% power with a 2-sided type I error of 1% (corresponding to a Bonferoni correction for 5 comparisons) to detect a relative risk of at least 1.5 for the comparison of 2 of the groups, assuming a 2-year event rate of 10% for the primary event. Results of the descriptive analysis of the Turkish arm of the study of 1034 patients are presented here.
All statistical analysis was performed by means of the SAS statistical software system (version 9.2, SAS Institute, Inc, Cary, NC).
Results
Of the 1034 patients [mean standard deviation (SD) age 56.5 (11.2) years, 863 (83.5%) men] enrolled in the study, 520 patients (50.3%) were diagnosed with UA/NSTEMI and 514 (49.7%) diagnosed with STEMI. Sociodemographic and anthropometric characteristics of the patients by diagnosis are presented in Table 1.
Table 1.
Baseline characteristics, medical history and chronic medication and healthcare use
| STEMI (n=514) | UA/NSTEMI (n=520) | |
|---|---|---|
| Patient characteristics | ||
| Age, years | ||
| n | 514 | 520 |
| Mean (SD) | 55.1 (11.65) | 57.9 (10.57) |
| Gender, n (%) | ||
| Men | 460 (89.5) | 403 (77.5) |
| Women | 54 (10.5) | 117 (22.5) |
| Race, n (%) | ||
| Caucasian | 487 (94.7) | 488 (93.8) |
| Unknown or other | 27 (5.3) | 32 (6.1) |
| Educational level, n (%) | ||
| No formal education | 34 (6.6) | 53 (10.2) |
| Primary | 249 (48.4) | 248 (47.7) |
| Secondary | 116 (22.6) | 100 (19.2) |
| University | 69 (13.4) | 59 (11.3) |
| Unknown | 46 (8.9) | 60 (11.5) |
| Weight, kg | ||
| n | 494 | 488 |
| Mean (SD) | 77.7 (12.10) | 78.3 (13.00) |
| Height, cm | ||
| n | 491 | 486 |
| Mean (SD) | 170.2 (7.14) | 170.3 (7.58) |
| BMI, kg/m2 | ||
| n | 491 | 485 |
| Mean (SD) | 26.8 (3.72) | 27.0 (4.31) |
| Medical history | ||
| Presence of CV risk factors, n (%) | 312 (60.7) | 384 (73.8) |
| Hypertension | 194 (37.7) | 303 (58.3) |
| Hypercholesterolemia | 114 (22.2) | 175 (33.7) |
| Diabetes mellitus | 96 (18.7) | 123 (23.7) |
| Family history of CAD | 162 (31.5) | 156 (30.0) |
| Current smoking | 282 (54.9) | 191 (36.7) |
| Obesity, BMI >30 kg/m2 | 91 (17.7) | 92 (17.7) |
| Previous CVD, n (%) | 107 (20.8) | 228 (43.8) |
| Myocardial infarction | 49 (9.5) | 101 (19.4) |
| Prior PCI | 47 (9.1) | 127 (24.4) |
| Prior CABG | 9 (1.8) | 74 (14.2) |
| Coronary angiography for CAD | 73 (14.2) | 161 (31.0) |
| Chronic angina | 21 (4.1) | 55 (10.6) |
| Heart failure | 8 (1.6) | 14 (2.7) |
| Atrial fibrillation | 3 (0.6) | 2 (0.4) |
| TIA/Stroke | 13 (2.5) | 12 (2.3) |
| Peripheral vascular disease | 5 (1.0) | 10 (1.9) |
| Previous non-CVD, n (%) | 46 (8.9) | 52 (10.0) |
| Chronic renal failure | 3 (0.6) | 13 (2.5) |
| COPD or other chronic lung disease | 23 (4.5) | 14 (2.7) |
| Chronic anemia | 4 (0.8) | 7 (1.3) |
| Cancer (in the last 10 years) | 5 (1.0) | 6 (1.2) |
| Severe liver disease | 2 (0.4) | 0 (0.0) |
| Oesophageal varices | 0 (0.0) | 0 (0.0) |
| Major surgery, n (%)1 | 3 (0.6) | 2 (0.4) |
| Major bleeding events, n (%)2 | 0 (0.0) | 1 (0.2) |
| Chronic medication use3 | ||
| Antiplatelets, n (%) | 111 (21.6) | 253 (48.7) |
| Anticoagulants, n (%) | 3 (0.6) | 4 (0.8) |
| Chronic healthcare use3 | ||
| GP/Family physician consultation | ||
| n (%) | 5 (1.0) | 11 (2.1) |
| Median (min–max) | 1.0 (1.0–1.0) | 1.0 (1.0–4.0) |
| Cardiologist consultation | 26 (5.1) | 42 (8.1) |
| Other specialist consultation | 2 (0.4) | 7 (1.3) |
| Emergency room visit | ||
| n (%) | 8 (1.6) | 21 (4.0) |
| Median (min–max) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
| Hospitalization | ||
| n (%) | 10 (2.0) | 21 (4.0) |
| Median (min–max) | 5.0 (1.0–10.0) | 3.0 (1.0–6.0) |
BMI - body mass index; CABG - coronary-artery bypass grafting; CAD - coronary artery disease; COPD - chronic obstructive pulmonary disease; CV - cardiovascular; CVD - cardiovascular disease; ER - emergency room; GP - general practitioner; Min - minimum; Max - maximum; NSTEMI - non-ST-segment elevation myocardial infarction; PCI - percutaneous coronary intervention; SD - standard deviation; STEMI - ST-segment elevation myocardial infarction; TIA - transient ischemic attack; UA - unstable angina 1in the 6 months prior to index event, 2gastrointestinal bleeding, not related to a medical/surgical procedure and required no medical intervention, 3in the 3 months previous to the index ACS
Medical history
Overall, 696 patients (67.3%) had CV risk factors with slightly higher rate noted in patients with UA/NSTEMI than in those with STEMI (73.8% vs. 60.7%, respectively). Hypertension (48.1%) was the most common CV risk factor identified in the overall study population (37.7% in STEMI and 58.3% in UA/NSTEMI groups). Overall, only 32.4% patients (43.8% patients in UA/NSTEMI group and 20.8% in STEMI group) had a history of previous CV disease. A list of all CV risk factors and CV and non-CV morbidities is given in Table 1.
Chronic medication and healthcare use in the 3 months previous to the index ACS
Overall 35.2% patients were receiving antiplatelet therapy [acetylsalicylic acid (ASA) in 33.8%]. The percentage of patients with a diagnosis of UA/NSTEMI receiving antiplatelet therapy was higher than than that with a diagnosis of STEMI (Table 1). Less than one percent of patients with UA/NSTEMI (n=3) and STEMI (n=4) were receiving anticoagulant therapy (Table 1).
Low rates were noted in terms of consulting a general practitioner/family physician [16 (1.5%)], cardiologist [68 (6.6%)], or other specialist [9 (0.9%)], and emergency room visit [29 (2.8%)] or hospitalization [32 (3.1%)] in all patients. Similar percentages were obtained in patients with STEMI and UA/NSTEMI (Table 1).
Index event information and pre-hospital care (medication and/or ECG)
The majority of patients [921 (89.1%)] did not receive pre-hospital care after symptoms onset. Of those patients who received pre-hospital care, more had a STEMI diagnosis [75 (14.6%)] rather than a UA/NSTEMI diagnosis [38 (7.3%)]. Albeit data are available only in 29 patients, the median (min–max) time from symptom onset of the index event to first medical attention was 1.0 (0.17–16.00) h. With the available data from 100 patients, the median (min–max) number of hours from symptom onset to first medical attention or pre-hospital ECG was 1.0 (0.02–20.45) h.
Drug treatment was started in 2.9% total patients before hospitalization [in 4.5% STEMI patients with ASA (4.5%), clopidogrel (0.1%), unfractioned heparin (UFH, 0.4%), and low molecular weight heparin (LMWH, 1.9%) and in 1.3% UA/NSTEMI patients with ASA (1.2%), LMWH (0.6%), and fondaparinux (0.2%)]. Pre-hospital fibrinolysis was given in none of the patients. Conside- ring antiplatelet therapy, none of the patients received ticlopidine, prasugrel, or any glycoprotein (GP) IIb/IIIa inhibitors other than tirofiban. Considering anticoagulant therapy, none of the patients received bivalirudin, warfarin/acenocoumarol, or dabigatran in the pre-hospital period.
The median (min–max) time from symptom onset to ECG was 2.25 (0.0–720.8) h in total (n=926) and found to be longer in UA/NSTEMI (Table 2). Only 8.7% patients received their first ECG in the pre-hospital setting. ECG was abnormal for ischemia in 78.0% patients (in 46.9% ischemic abnormality was new/presumed new) with a higher percentage of patients with a STEMI diagnosis having an ECG abnormal for ischemia (Table 2). Detailed information by diagnosis about the index event is presented in Table 2.
Table 2.
Index event information and pre-hospital management (medications and/or ECG)
| STEMI (n=514) | UA/NSTEMI (n=520) | |
|---|---|---|
| Pre-hospital care (medications and/or ECG) | ||
| Yes1/No, n (%) | 75 (14.6)/439 (85.4) | 38 (7.3)/482 (92.7) |
| Time from symptom onset to first medical attention (h)2 | ||
| n | 21 | 8 |
| Median (min–max) | 0.92 (0.17–3.00) | 2.00 (0.17–16.00) |
| Place of first medical attention (% of patient with information provided), n (%) | ||
| Ambulance | 9 (40.9) | 0 (0.0) |
| Home | 0 (0.0) | 0 (0.0) |
| Physician office | 3 (13.6) | 2 (20.0) |
| Other | 10 (45.5) | 7 (70.0) |
| Unknown | 0 (0.0) | 1 (10.0) |
| Time from symptom onset to first medical attention or pre-hospital ECG (h)3 | ||
| n | 67 | 33 |
| Median (min–max) | 0.88 (0.03–20.45) | 2.25 (0.02–19.50) |
| Killip classification, n (%) | ||
| Class I | 462 (89.9) | 469 (90.2) |
| Class II | 26 (5.1) | 29 (5.6) |
| Class III | 6 (1.2) | 3 (0.6) |
| Class IV | 1 (0.2) | 0 (0.0) |
| Unknown | 19 (3.7) | 19 (3.7) |
| Pre-hospital medication, n (%) | 23 (4.5) | 7 (1.3) |
| Thrombolytics | 0 (0.0) | 0 (0.0) |
| Antiplatelets | ||
| Acetylsalicylic acid | 23 (4.5) | 6 (1.2) |
| Clopidogrel | 1 (0.2) | 0 (0.0) |
| Anticoagulants | ||
| Unfractioned heparin | 4 (0.8) | 0 (0.0) |
| LMW heparin | 10 (1.9) | 3 (0.6) |
| Fondaparinux | 0 (0.0) | 1 (0.2) |
| First diagnostic ECG | ||
| Time from symptom onset to ECG (h)4 | ||
| n | 477 | 449 |
| Median (min–max) | 2.08 (0.0–117.8) | 3.08 (0.0–720.8) |
| Done in pre-hospital setting, n (%) | 58 (11.3) | 32 (6.2) |
| Abnormal for ischemia, n (%) | 498 (96.9) | 290 (55.8) |
| Any of the ischemic abnormalities was new/presumed new, n (%) | 347 (67.5) | 138 (26.5) |
| Any other abnormalities, n (%) | 33 (6.4) | 8 (1.5) |
| Posterior infarction, n (%) | 20 (3.9) | 1 (0.2) |
| Non-specific ST/T change LBBB, n (%) | 5 (1.0) | 2 (0.4) |
| Atrial fib./flutter, n (%) | 4 (0.8) | 3 (0.6) |
| AV block, n (%) | 10 (1.9) | 0 (0.0) |
| Ventricular tachycardia, n (%) | 3 (0.6) | 2 (0.4) |
| Paced rhythm, n (%) | 1 (0.2) | 0 (0.0) |
AV - atrioventricular; ECG - electrocardiogram; fib - fibrillation; LBBB - left bundle branch block; LMW - low molecular weight; NSTEMI - non-ST-segment elevation myocardial infarction; SD - standard deviation; STEMI - ST-segment elevation myocardial infarction; UA - unstable angina
Patients with an ECG performed pre-hospital and/or any medications administered pre-hospital;
Patients with time of symptom onset and/or time of first medical attention unknown are excluded from this calculation;
Patients with time of symptom onset and/or (time of first medical attention and time of first ECG) unknown are excluded from this calculation;
Patients with time of symptom onset and/or time of first ECG unknown are excluded from this calculation
In-hospital management (diagnostic and therapeutic interventions/procedures)
The rates of diagnostic and therapeutic intervention/procedures) use in the hospital are presented in Table 3. Cardiac catheterization was applied in the majority of patients (94.7%) and in similar percentage of patients with STEMI and UA/NSTEMI. The majority of patients [971 (93.9%)] did not have thrombolysis, which was applied only in 12.1% patients with STEMI but in none of the patients with UA/NSTEMI. The majority of total patients [741 (71.7%)] had PCI with higher percentage of patients with STEMI vs. UA/NSTEMI having the intervention. Coronary artery bypass graft (CABG) was performed only in 24 (2.3%) patients. Time from symptom onset to first PCI in the overall study population was median (min–max) 8.33 (0.0–806.6) h (n=730) which was longer in patients with UA/NSTEMI than in those with STEMI.
Table 3.
In-hospital management (diagnostic and therapeutic interventions/procedures)
| In-hospital management, n (% of patients with information) | STEMI (n=514) | UA/NSTEMI (n=520) |
|---|---|---|
| Thrombolysis | ||
| Yes | 62 (12.1) | 0 (0.0) |
| No | 451 (87.7) | 520 (100.0) |
| Cardiac catheterization | ||
| Yes | 497 (96.7) | 482 (92.7) |
| No | 10 (1.9) | 27 (5.2) |
| Number of procedures | ||
| Any | 497 (96.7) | 482 (92.7) |
| 1 | 460 (92.6) | 468 (97.1) |
| 2 | 37 (7.2) | 14 (2.7) |
| 3 | 3 (0.6) | 1 (0.2) |
| First application | ||
| Type of procedure | ||
| Primary/direct | 372 (74.8) | 44 (9.1) |
| Rescue | 19 (3.8) | 0 (0.0) |
| Facilitated | 5 (1.0) | 9 (1.9) |
| Routine early invasive strategy | 98 (19.7) | 409 (84.9) |
| Other | 3 (0.6) | 20 (4.1) |
| Any PCI | ||
| Yes | 452 (87.9) | 289 (55.6) |
| No | 55 (10.7) | 220 (42.3) |
| Any CABG | ||
| Yes | 8 (1.6) | 16 (3.1) |
| No | 506 (98.4) | 502 (96.5) |
| Reperfusion (PCI and/or thrombolysis) | ||
| Yes | 470 (91.4) | 289 (55.6) |
| No | 41 (8.0) | 220 (42.3) |
| Time from symptom onset to first PCI (h)* | ||
| n | 448 | 282 |
| Median (min–max) | 4.20 (0.0–806.6) | 26.25 (0.0–696.0) |
| Patients with any procedure | 497 (96.7) | 482 (92.7) |
| Coronary stenosis (>50%) | 488 (98.2) | 427 (88.6) |
| Femoral vascular access | 877 (91.5) | 837 (90.6) |
| PCI | 446 (89.7) | 280 (58.1) |
| Number of stents | ||
| 1 | 339 (68.2) | 208 (43.2) |
| 2 | 72 (14.5) | 49 (10.2) |
| Second application | ||
| Patients with any type of procedure | 37 (7.2) | 14 (2.7) |
| Femoral vascular access | 37 (100.0) | 14 (100.0) |
| Percutaneous cardiac intervention | 24 (64.9) | 13 (92.9) |
| Number of stents | ||
| 1 | 17 (45.9) | 5 (35.7) |
| 2 | 2 (5.4) | 2 (14.3) |
| Laboratory testing | ||
| On admission | ||
| White blood count, /uL | ||
| n | 498 | 499 |
| Mean (SD) | 12188.6 (3924.11) | 9114.2 (2761.17) |
| Initial creatinine, mg/dL | ||
| n | 507 | 510 |
| Mean (SD) | 0.943 (0.3719) | 0.971 (0.5373) |
| Blood glucose, mg/dL | ||
| n | 479 | 481 |
| Mean (SD) | 150.0 (69.57) | 139.0 (80.43) |
| Hemoglobin, g/dL | ||
| n | 505 | 515 |
| Mean (SD) | 14.43 (1.661) | 13.92 (1.773) |
| Hematocrit, % | ||
| n | 506 | 514 |
| Mean (SD) | 42.9 (4.86) | 41.4 (5.13) |
| During hospitalization | ||
| Peak creatinine, mg/dL | ||
| n | 493 | 474 |
| Mean (SD) | 1.052 (0.4861) | 1.043 (0.5800) |
| Positive cardiac markers | 498 (96.9) | 266 (51.2%) |
| Other diagnostic and therapeutic procedures | ||
| Echocardiography | 405 (78.8) | 382 (73.5%) |
| Non-Invasive testing | 3 (0.6) | 9 (1.7) |
| Resuscitation | 20 (3.9) | 0 (0.0) |
| Mechanical ventilation | 7 (1.4) | 0 (0.0) |
| Intra-aortic balloon pumping | 3 (0.6) | 3 (0.6) |
| Temporary pacemaker | 8 (1.6) | 1 (0.2) |
| Cardiac resynchronization therapy | 0 (0.0) | 4 (0.8) |
| Cardiac surgery (CABG) | ||
| Total | 8 (1.6) | 16 (3.1) |
| Urgent | 3 (37.5) | 10 (62.5) |
CABG - coronary artery bypass graft; MRI - magnetic resonance imaging; NSTEMI - non-ST-segment elevation myocardial infarction; PCI - percutaneous cardiac intervention; SD - standard deviation; STEMI - ST-segment elevation myocardial infarction; UA - unstable angina.
Calculated to the nearest day where time of symptom onset and/or time of PCI are unknown; PCIs with start date prior to symptom onset are excluded from this calculation
In 51 patients with ≥2 procedures, all (37 patients with STEMI and 14 with NSTEMI) had femoral vascular access. Overall 72.5% of these patients underwent PCI, 43.1% received 1 stent, with a higher percentage of patients with STEMI receiving 1 stent than those with UA/NSTEMI.
On admission, the mean values for white blood count, initial creatinine, blood glucose, hemoglobin, and hematocrit were simi- lar between patients with STEMI and
UA/NSTEMI diagnoses during hospitalization, patients with STEMI and UA/NSTEMI had similar levels of peak creatinine, whereas higher percentage of positive cardiac markers was identified in patients with STEMI (96.9%) than in those with UA/NSTEMI (51.2%). Echocardiography was performed in majority of patients (76.1%) and in similar percentages of patients with STEMI and UA/NSTEMI.
In hospital medications
All patients received at least 1 in-hospital medication and all received at least 1 antiplatelet, and majority received dual antiplatelet therapy (DAPT) with ASA (99.5%), followed by clopidogrel (94.4%) and a GP IIb/IIIa inhibitor (13.1%). The majority of patients also received at least 1 anticoagulant (87.3%) with highest percentage of patients receiving LMWH (58.1%) followed by UFH (44.6%). At least one thrombolytic therapy was applied in 13.2% patients with STEMI, mostly with tissue-type plasminogen activator (tPA; 12.1%) followed by streptokinase (1.2%). The majority of total patients (99.7%) had at least 1 other CV therapy, mostly with statins (96.1%) followed by beta blockers (90.3%) and angiotensin-converting-enzyme (ACE) inhibitor/angiotensin receptor blockers (ARBs) (85.6%), and percentages of CV and non-CV therapy were similar between diagnostic groups (Table 4). In-hospital medication use by diagnosis is listed in Table 4.
Table 4.
In-hospital medications
| STEMI (n=514) | UA/NSTEMI (n=520) | |
|---|---|---|
| At least one in-hospital medication | 514 (100.0) | 520 (100.0) |
| Total number of in-hospital | ||
| medications†, median (min–max) | 9.0 (4–15) | 8.0 (3–19) |
| Thrombolytics | ||
| At least one thrombolytic | 68 (13.2) | 0 (0.0) |
| Streptokinase | 6 (1.2) | 0 (0.0) |
| t-PA | 62 (12.1) | 0 (0.0) |
| Total number† | ||
| n* | 68 | |
| Median (min–max) | 1.0 (1.0–1.0) | – |
| Antiplatelets | ||
| At least one antiplatelet | 514 (100.0) | 520 (100.0) |
| Acetylsalicylic acid | 513 (99.8) | 516 (99.2) |
| Ticlopidine | 5 (1.0) | 0 (0.0) |
| Clopidogrel | 505 (98.2) | 471 (90.6) |
| Any GP IIb/IIIa | 103 (20.0) | 32 (6.2) |
| Abciximab | 3 (0.6) | 0 (0.0) |
| Tirofiban | 100 (19.5) | 32 (6.2) |
| Total number† | ||
| n* | 514 | 520 |
| Median (min–max) | 4.0 (1–7) | 3.0 (1–9) |
| Anticoagulants | ||
| At least one anticoagulant | 481 (93.6) | 422 (81.2) |
| Unfractioned heparin | 268 (52.1) | 193 (37.1) |
| Low molecular weight heparin | 317 (61.7) | 284 (54.6) |
| Warfarin/Acenocoumarol | 0 (0.0) | 3 (0.6) |
| Total number† | ||
| n* | 481 | 422 |
| Median (min–max) | 1.0 (1–4) | 1.0 (1–4) |
| Other CV Therapy | ||
| At least one other CV therapy | 513 (99.8) | 518 (99.6) |
| Beta blockers | 459 (89.3) | 475 (91.3) |
| ACE inhibitor/ARB | 436 (84.8) | 449 (86.3) |
| Statins | 496 (96.5) | 498 (95.8) |
| Other anti-lipid agents | 25 (4.9) | 14 (2.7) |
| Aldosterone inhibitors | 29 (5.6) | 25 (4.8) |
| Loop diuretics | 34 (6.6) | 39 (7.5) |
| Other non-loop diuretics | 14 (2.7) | 10 (1.9) |
| Ca-channel blocker | 17 (3.3) | 49 (9.4) |
| Other | 33 (6.4) | 46 (8.8) |
| Total number† | ||
| n* | 513 | 518 |
| Median (min–max) | 3.0 (1–6) | 3.0 (1–7) |
| Other non-CV therapy | ||
| At least one non-CV therapy | 416 (80.9) | 384 (73.8) |
| PPIs | 292 (56.8) | 267 (51.3) |
| Esomeprazole | 87 (16.9) | 115 (22.1) |
| Lansoprazole | 81 (15.8) | 62 (11.9) |
| Omeprazole | 36 (7.0) | 16 (3.1) |
| Pantoprazole | 91 (17.7) | 78 (15.0) |
| Rabeprazole | 2 (0.4) | 0 (0.0) |
| H2-receptor antagonist | ||
| NSAIDs (traditional and coxibs) | ||
| Total number† | ||
| n* | 416 | 384 |
| Median (min–max) | 1.0 (1–2) | 1.0 (1–3) |
ACE - angiotensin-converting-enzyme inhibitor; ARB - angiotensin receptor blockers; Ca - calcium; CV - cardiovascular; NSAIDs - non-steroidal anti-inflammatory drugs; NSTEMI - non-ST-segment elevation myocardial infarction; PPIs - proton pump inhibitors; r - PA-reteplase; SD - standard deviation; STEMI - ST-segment elevation myocardial infarction; TNK-t-PA - tenecteplase; t-PA - tissue-type plasminogen activator; UA-unstable angina
Total number of medication records provided, including multiple dose types or levels for each medication as well as multiple medication types. Number of unique doses recorded. Note- for patients with no in-hospital antiplatelet use recorded, any discharge antiplatelet is assumed to have also been an in-hospital maintenance dose
AMP derivation based on pre and/or in-hospital medication use
Considering overall pre and/or in-hospital medication use, ASA + clopidogrel was the most common type of antiplatelet therapy (81.2%) followed by ASA + clopidogrel + GP IIb/IIIa inhibitor (12.7%). ASA + clopidogrel were applied in slightly higher percentage of patients with UA/NSTEMI, but slightly earlier in patients with STEMI (Table 5). Frequency of the use of antiplatelets alone or in combination and among patients with or without thrombolytic, anticoagulant, and antiplatelet therapy varied by diagnosis (Table 5).
Table 5.
AMP derivation based on pre and/or in-hospital medication use: “all patients, by index event final diagnosis”
| STEMI (n=514) | UA/NSTEMI (n=520) | ||
|---|---|---|---|
| Antiplatelets, n (%) | |||
| None | 0 (0.0) | 0 (0.0) | |
| ASA only | 7 (1.4) | 48 (9.2) | |
| Clopidogrel only | 0 (0.0) | 4 (0.8) | |
| ASA + Clopidogrel | 404 (78.6) | 436 (83.8) | |
| ASA + GP IIb/IIIa | 2 (0.4) | 1 (0.2) | |
| Clopidogrel + GP IIb/IIIa | 1 (0.2) | 0 (0.0) | |
| ASA + Clopidogrel + GP IIb/IIIa | 100 (19.5) | 31 (6.0) | |
| By thrombolytic therapy | |||
| YES | 68 (13.2) | 0 (0.0) | |
| None† | 0 (0.0) | 0 (0.0) | |
| ASA + Clopidogrel | 67 (98.5) | 0 (0.0) | |
| ASA + GP IIb/IIIa | 0 (0.0) | 0 (0.0) | |
| Clopidogrel + GP IIb/IIIa | 0 (0.0) | 0 (0.0) | |
| ASA + Clopidogrel + GP IIb/IIIa | 1 (1.5) | 0 (0.0) | |
| NO | 446 (86.8) | 520 (100.0) | |
| None† | 0 (0.0) | 0 (0.0) | |
| ASA only | 7 (1.6) | 48 (9.2) | |
| Clopidogrel only | 0 (0.0) | 4 (0.8) | |
| ASA + Clopidogrel | 337 (75.6) | 436 (83.8) | |
| ASA + GP IIb/IIIa | 2 (0.4) | 1 (0.2) | |
| Clopidogrel + GP IIb/IIIa | 1 (0.2) | 0 (0.0) | |
| ASA + Clopidogrel + GP IIb/IIIa | 99 (22.2) | 31 (6.0) | |
| By anticoagulant therapy | |||
| YES | 482 (93.8) | 422 (81.2) | |
| None† | 0 (0.0) | 0 (0.0) | |
| ASA only | 6 (1.2) | 24 (5.7) | |
| Clopidogrel only | 0 (0.0) | 3 (0.7) | |
| GP IIb/IIIa only | 0 (0.0) | 0 (0.0) | |
| ASA + Clopidogrel | 373 (77.4) | 366 (86.7) | |
| ASA + GP IIb/IIIa | 2 (0.4) | 1 (0.2) | |
| Clopidogrel + GP IIb/IIIa | 1 (0.2) | 0 (0.0) | |
| ASA + Clopidogrel + GP IIb/IIIa | 100 (20.7) | 28 (6.6) | |
| NO | 32 (6.2) | 98 (18.8) | |
| None† | 0 (0.0) | 0 (0.0) | |
| ASA only | 1 (3.1) | 24 (24.5) | |
| Clopidogrel only | 0 (0.0) | 1 (1.0) | |
| ASA + Clopidogrel | 31 (96.9) | 70 (71.4) | |
| ASA + Clopidogrel + GP IIb/IIIa | 0 (0.0) | 3 (3.1) | |
| Number of antiplatelet medications | |||
| Total | 0 | 0 (0.0) | 0 (0.0) |
| 1 | 7 (1.4) | 52 (10.0) | |
| 2 | 402 (78.2) | 437 (84.0) | |
| 3 | 105 (20.4) | 31 (6.0) | |
| 4 | 0 (0.0) | 0 (0.0) | |
| By anticoagulant use | |||
| Unfractioned heparin | YES | 270 (52.5) | 193 (37.1) |
| 0 | 0 (0.0) | 0 (0.0) | |
| 1 | 3 (1.1) | 8 (4.1) | |
| 2 | 195 (72.2) | 171 (88.6) | |
| 3 | 72 (26.7) | 14 (7.3) | |
| 4 | 0 (0.0) | 0 (0.0) | |
| Low molecular weight heparinx | YES | 318 (61.9) | 284 (54.6) |
| 0 | 0 (0.0) | 0 (0.0) | |
| 1 | 4 (1.3) | 20 (7.0) | |
| 2 | 249 (78.3) | 242 (85.2) | |
| 3 | 65 (20.4) | 22 (7.7) | |
ASA - acetylsalicylic acid; GP IIb/IIIa - Glycoprotein IIb/IIIa inhibitors; NSTEMI - non- ST-segment elevation myocardial infarction; STEMI - ST-segment elevation myocardial infarction; UA-unstable angina. Table presents use of any loading or maintenance dose of listed medications pre- or in-hospital
did not take ASA, clopidogrel or a GP IIb/IIIa Inhibitor; may have received prasugrel or ticlopidine; *Time to treatment calculated as; #hours from symptom onset to first in-hospital administration of any of the associated medications, where data available missing dates/times of pre-and in-hospital medications replaced with the imputed date/time of hospital admission. Note-for patients with no in-hospital antiplatelet use recorded, any discharge antiplatelet is assumed to have also been used in-hospital
Discharge medications
Overall, the majority of patients were receiving the antiplatelet agents including ASA (98.4%) and clopidogrel (86.8%) at discharge with similar percentages between patients with STEMI and those with UA/NSTEMI in terms of ASA use, whereas a slightly higher percentage of patients with STEMI (93.0%) than those with UA/NSTEMI (80.6%) was observed under clopidogrel at discharge. The majority of all patients (99.5%) were not recei- ving any anticoagulants at discharge.
The rate of ASA usage at discharge was similar (98.7% vs. 97.5%, respectively) between patients with and without stent placement, whereas clopidogrel use at discharge was higher in patients with than without stent placement (96.5% vs. 65.4%, respectively).
Majority of patients were taking beta blockers (90.1%), lipid lowering drugs (89.3%), and ACE/ARB inhibitors (82.4%) at discharge; percentages were similar between diagnostic groups. Overall, 31.9% patients were taking the non-CV therapy proton pump inhibitor (PPI). Discharge medications by diagnosis are presented in Table 6.
Table 6.
Discharge medications
| STEMI (n=514) | UA/NSTEMI (n=520) | |
|---|---|---|
| Antiplatelets, n (%) | ||
| ASA | 508 (98.8) | 509 (97.9) |
| Clopidogrel | 478 (93.0) | 419 (80.6) |
| Combined ASA/clopidogrel use | ||
| ASA only | 36 (7.0) | 97 (18.7) |
| Clopidogrel only | 6 (1.2) | 7 (1.3) |
| ASA + Clopidogrel | 472 (91.8) | 412 (79.2) |
| Neither | 0 (0.0) | 4 (0.8) |
| Ticlopidine | 23 (4.5) | 6 (1.2) |
| Other antiplatelet | 3 (0.6) | 1 (0.2) |
| By stent placement during PCI | ||
| YES | 434 (84.4) | 276 (53.1) |
| ASA | 428 (98.6) | 273 (98.9) |
| Clopidogrel | 415 (95.6) | 270 (97.8) |
| Ticlopidine | 18 (4.1) | 5 (1.8) |
| Other antiplatelet | 3 (0.7) | 0 (0.0) |
| NO | 80 (15.6) | 244 (46.9) |
| ASA | 80 (100) | 236 (96.7) |
| Clopidogrel | 63 (78.8) | 149 (61.1) |
| Ticlopidine | 5 (6.3) | 1 (0.4) |
| Other antiplatelet | 0 (0.0) | 1 (0.4) |
| Anticoagulants (Acenocoumarol/Warfarin) | 2 (0.4) | 3 (0.6) |
| Other CV therapies | ||
| Beta blockers | 466 (90.7) | 466 (89.6) |
| ACE/ARB inhibitors | 439 (85.4) | 413 (79.4) |
| Lipid lowering drugs | ||
| Total | 463 (90.1) | 460 (88.5) |
| Atorvastatin | 337 (65.6) | 307 (59.0) |
| Pravastatin | 1 (0.2) | 2 (0.4) |
| Rosuvastatin | 122 (23.7) | 145 (27.9) |
| Simvastatin | 1 (0.2) | 2 (0.4) |
| Other | 5 (1.0) | 6 (1.2) |
| Diuretics | 48 (9.3) | 42 (8.1) |
| Aldosterone inhibitors | 41 (8.0) | 14 (2.7) |
| Ca-channel blocker | 26 (5.1) | 60 (11.5) |
| Non-CV therapies | ||
| PPIs | ||
| Total | 175 (34.0) | 155 (29.8) |
| Omeprazole | 26 (5.1) | 8 (1.5) |
| Pantoprazole | 99 (19.3) | 106 (20.4) |
| Rabeprazole | 2 (0.4) | 1 (0.2) |
| Esomeprazole | 2 (0.4) | 9 (1.7) |
| Lansoprazole | 47 (9.1) | 31 (6.0) |
| H2-receptor antagonist | 78 (15.2) | 66 (12.7) |
| NSAIDs (traditional and coxibs) | 1 (0.2) | 2 (0.4) |
ACE - angiotensin-converting-enzyme inhibitor; ARB - angiotensin receptor blockers; ASA - acetylsalicylic acid; Ca - calcium; CV - cardiovascular; NSAIDs - non-steroidal anti-inflammatory drugs; NSTEMI - non-ST-segment elevation myocardial infarction; PCI - percutaneous coronary intervention; PPIs - proton pump inhibitors; STEMI – Stsegment elevation myocardial infarction; UA - unstable angina
In-hospital cardiac and hemorrhagic outcomes
Overall, only 8 patients (0.8%) had an in-hospital occurrence of MI, whereas a slightly higher percentage of total patients had an in-hospital occurrence of recurrent ischemia [12 patients (1.2%)]. Both were higher in patients with STEMI than in patients with UA/NSTEMI having no remarkable difference with respect to center type (Table 7). In total, 16 patients (1.5%) experienced non-fatal in-hospital bleeding events with higher incidence in patients with STEMI (Table 7). Bleeding was related to a medical procedure in 12 of total 16 patients (75.0%), in 8 of 12 patients with STEMI (66.7%), and in all of 4 patients with UA/NSTEMI. In the majority of patients, clinical significance (81.3%) and hemodynamic compromise (87.5%) of bleeding was minimal, more commonly in patients with STEMI than in those with UA/NSTEMI (Table 7).
Table 7.
In-hospital cardiac and hemorrhagic outcomes
| STEMI (n=514) | UA/NSTEMI (n=520) | |
|---|---|---|
| Cardiac complications, n (%) | ||
| Cardiac ischemic complications | ||
| Myocardial infarction | 6 (1.2) | 2 (0.4) |
| Recurrent ischemia | 8 (1.6) | 4 (0.8) |
| Other Cardiac complications | ||
| Heart failure | 18 (3.5) | 3 (0.6) |
| Maximum Killip Class | ||
| II | 11 (2.1) | 2 (0.4) |
| III | 7 (1.4) | 0 (0.0) |
| IV | 0 (0.0) | 0 (0.0) |
| Unknown | 0 (0.0) | 1 (0.2) |
| Cardiogenic shock | 1 (0.2) | 0 (0.0) |
| Dyspnea other causes (not HF or CS) | 2 (0.4) | 5 (1.0) |
| Arrhythmias | ||
| Cardiac arrest/VF | 26 (5.1) | 2 (0.4) |
| Atrial fibrillation/flutter | 6 (1.2) | 3 (0.6) |
| Sustained ventricular tachycardia | 10 (1.9) | 2 (0.4) |
| High degree AV block | 3 (0.6) | 0 (0.0) |
| Stroke (ischemic) | 1 (0.2) | 0 (0.0) |
| Other complications | 2 (0.4) | 8 (1.5) |
| All outcomes, by center type | ||
| Regional/Community/Rural | 16 (3.1) | 17 (3.3) |
| Heart failure | 1 (6.3) | 0 (0.0) |
| Cardiac arrest/VF | 1 (6.3) | 0 (0.0) |
| Non-university, General | 130 (25.3) | 95 (18.3) |
| Myocardial infarction | 3 (2.3) | 0 (0.0) |
| Recurrent ischemia | 4 (3.1) | 0 (0.0) |
| Heart failure | 3 (2.3) | 1 (1.1) |
| Cardiogenic shock | 0 (0.0) | 0 (0.0) |
| Dyspnea other causes (not HF or CS) | 0 (0.0) | 1 (1.1) |
| Cardiac arrest/VF | 5 (3.8) | 0 (0.0) |
| Atrial fibrillation/flutter | 1 (0.8) | 1 (1.1) |
| High degree AV block | 2 (1.5) | 0 (0.0) |
| Other complications | 0 (0.0) | 1 (1.1) |
| University, General | 345 (67.1) | 362 (69.6) |
| Myocardial infarction | 2 (0.6) | 1 (0.3) |
| Recurrent ischemia | 4 (1.2) | 2 (0.6) |
| Heart failure | 13 (3.8) | 2 (0.6) |
| Cardiogenic shock | 1 (0.3) | 0 (0.0) |
| Dyspnea other causes (not HF or CS) | 2 (0.6) | 4 (1.1) |
| Cardiac arrest/VF | 20 (5.8) | 2 (0.6) |
| Atrial fibrillation/Flutter | 5 (1.4) | 2 (0.6) |
| Sustained ventricular tachycardia | 10 (2.9) | 2 (0.6) |
| High degree AV Block | 1 (0.3) | 0 (0.0) |
| Stroke | 1 (0.3) | 0 (0.0) |
| Other complications | 2 (0.6) | 7 (1.9) |
| Other center type | 23 (4.5) | 46 (8.8) |
| Myocardial infarction | 1 (4.3) | 1 (2.2) |
| Recurrent ischemia | 0 (0.0) | 2 (4.3) |
| Heart failure | 1 (4.3) | 0 (0.0) |
| Hemorrhagic complications, n (%) | 12 (2.3) | 4 (0.8) |
| Location* | ||
| Vascular access | 6 (50.0) | 2 (50.0) |
| Gastrointestinal | 2 (16.7) | 0 (0.0) |
| Genitourinary | 1 (8.3) | 0 (0.0) |
| Non vascular related hematomas | 1 (8.3) | 1 (25.0) |
| Other | 2 (16.7) | 1 (25.0) |
| Related to a medical procedure* | 8 (66.7) | 4 (100.0) |
| Severity | ||
| Clinical significance* | ||
| Minimal | 11 (91.7) | 2 (50.0) |
| Non-minimal | 1 (8.3) | 2 (50.0) |
| Hemodynamic compromise* | ||
| Minimal | 11 (91.7) | 3 (75.0) |
| Non-minimal | 1 (8.3) | 1 (25.0) |
| Laboratory | ||
| Bleeding management | ||
| Blood transfusion* | 1 (8.3) | 2 (50.0) |
| Red blood* | 1 (8.3) | 2 (50.0) |
| Urgent surgery* | 0 (0.0) | 1 (25.0) |
| Interruption of antithrombotic therapy* | 3 (25.0) | 2 (50.0) |
| Therapy* | ||
| Acetylsalicylic acid | 2 (16.7) | 2 (50.0) |
| Clopidogrel | 1 (8.3) | 0 (0.0) |
| Other | 2 (16.7) | 0 (0.0) |
AV - atrioventricular; CS - coronary syndrome; HF - heart failure; NSTEMI - non-STsegment elevation myocardial infarction; STEMI - ST-segment elevation myocardial infarction; UA - unstable angina; VF - ventricular fibrillation
All figures represent the number of patients with one or more hemorrhagic complications reported in the relevant category; a subject may be reported in multiple categories
Bleeding was at vascular access in 8 patients, gastrointestinal in 2 patients (12.5%, both were patients with STEMI) and characterized to be non-vascular related hematomas in 2 patients (12.5%, 1 patient from each group) (Table 7).
For the management of bleeding event, blood transfusion was applied in 3 patients (18.0%) in the form of RBC transfusion and urgent surgery was performed in 1 patient (6.3%), while antithrombotic therapy was discontinued in 5 patients (31.3%) (Table 7).
Functional outcomes
For the majority of patients, ACS did not result in permanent disability [1010 of 1034 patients (97.7%)] or a changed degree of dependence since hospital admission [1019 of 1034 patients (98.6%)] with similar percentages between patients diagnosed with STEMI or UA/NSTEMI. For the 15 patients (1.5%) with a changed degree of dependence since hospital admission the majority (73.3%) had non-severe dependence. All patients (n=11) who became newly dependent become depended on a relative, regardless of the STEMI or UA/NSTEMI diagnosis (Table 8).
Table 8.
Functional outcomes
| STEMI (n=514) | UA/NSTEMI (n=520) | |
|---|---|---|
| Functional outcomes, n (%) | ||
| ACS resulted in permanent disability | ||
| Yes | 10 (1.9) | 14 (2.7) |
| No | 497 (96.7) | 495 (95.2) |
| Degree of dependence changed since admission* | ||
| Yes | 10 (1.9) | 5 (1.0) |
| No | 490 (95.3) | 495 (95.2) |
| If yes: New dependence degree | ||
| No dependence | 3 (30.0) | 0 (0.0) |
| Non severe dependence | 6 (60.0) | 5 (100) |
| Severe dependence | 0 (0.0) | 0 (0.0) |
| Unknown | 1 (10.0) | 0 (0.0) |
| Shift in dependence degree | ||
| Increased dependence | ||
| None - Non severe | 6 (60.0) | 5 (100) |
| None - Severe | 0 (0.0) | 0 (0.0) |
| Non severe - Severe | 0 (0.0) | 0 (0.0) |
| Reduced dependence | ||
| Severe - Non severe | 0 (0.0) | 0 (0.0) |
| Non severe - None | 0 (0.0) | 0 (0.0) |
| Unknown | ||
| None - Unknown | 1 (10.0) | 0 (0.0) |
| Non severe - Unknown | 0 (0.0) | 0 (0.0) |
| Unknown - Non severe | 0 (0.0) | 0 (0.0) |
| Unknown - None | 3 (30.0) | 0 (0.0) |
| If newly dependent: depends on | ||
| Relative | 6 (100.0) | 5 (100.0) |
| Hired person | 0 (0.0) | 0 (0.0) |
ACS - acute coronary syndrome; NSTEMI - non-ST-segment elevation myocardial infarction; STEMI - ST-segment elevation myocardial infarction; UA - unstable angina
Includes patients where the investigator has indicated a change and/or patients with differing enrollment and discharge dependencies
Discussion
Findings from the Turkish cohort of patients are in agreement with the overall population recruited in the EPICOR study that showed slightly (on average 4–5 years) younger age of ACS presentation than that seen in registries including the contemporary FAST-MI 2010 registry (13) and GRACE registry (14). Although this is most likely explained by the exclusion of patients who died in hospital, usually older and sicker patients, from EPICOR (12), it should be noted that analysis of the differences between Turkey and other European countries included in EUROASPIRE III survey also revealed higher percentage of young patients with myocardial infarction (<50 years, 20% vs. 12.7%) in the Turkish cohort (15). The rate for current smoking was higher (45.7% vs. 38.7%), and obesity (17.0% vs. 29.1%) was lower in Turkish cohort as compared with overall Eastern European cohort. In both cohorts higher rate for current smoking and lesser rate for CV disease were noted in patients with STEMI than in those with UA/NSTEMI along with similar rates of obesity in the two diagnostic groups (unpublished observations).
The rate of smoking among Turkish patients with ACS was also higher than that reported in GRACE (28%) and Gulf RACE (37%) registries (14), while it was similar to the rate (40%) reported in CREATE registry (16) and Maghreb countries from ACCESS registry (17). These high smoking rates may explain the younger age of ACS.
The percentage of patients who did not receive pre-hospital care after symptoms onset was higher in Turkish patients than in overall Eastern European cohort including Poland, Romania, Slovenia, and Turkey (89.1% vs. 57.3%), with higher likelihood of patients with STEMI than those with UA/NSTEMI to receive pre-hospital care (unpublished observations). Earlier induction of first medical attention or pre-hospital ECG after symptom onset (after 2.9 h vs. 4.8 h) was noted in Turkish than in Eastern European cohort, while in patients with STEMI than with UA/NSTEMI in both cohorts.
Based on data available in 67 patients, an average of 125 min passed from symptom onset to first medical attention or pre-hospital ECG in our patients with STEMI, as compared with 140–170 min before presenting to the emergency department reported in GRACE registry (18) and Euro Heart surveys (19, 20).
Pre-hospital management included drug treatment only in 2.9% (ASA in 2.8%) of Turkish patients as compared with 13.4% patients (ASA in 12.9%) in the overall Eastern European cohort, with higher rates for patients with STEMI than for those with UA/NSTEMI initiating drug treatment before hospitalization in both cohorts (unpublished observations). Pre-hospital fibrinolysis was given in none of the patients with STEMI in Turkish cohort and only in 0.2% patients with STEMI in the Eastern European cohort, while none of the patients in the Turkish cohort received ticlopidine, prasugrel or any GP IIb/IIIa other than trifoban and also bivalirudin, warfarin/acenocoumarol or dabigatran. Similarly, none of the patients in the Eastern European cohort received ticlopidine, prasugrel or GP IIb/IIIa other than eptifibatide, bivalirudin or dabigatran in the pre-hospital period (unpublished observations).
Only 8.7% patients in the Turkish cohort, whereas 40.5% patients in the Eastern European cohort received their first ECG in the pre-hospital setting and in both cohorts ECG findings were abnormal in more than 75.0% patients, more commonly in patients with STEMI than in those with UA/NSTEMI. Average time from symptom onset to ECG was 6.69 h in the overall study population and found to be longer in UA/NSTEMI (9.20 h) than in STEMI (4.31 h) patients which seems consistent with data from overall Eastern European cohort (unpublished observations).
Implementation of pre-hospital ECG in less than 10% patients in the Turkish cohort seems notable given that early recording and interpretation of an ECG in any suspected ACS is critical to trigger life-saving treatment via primary PCI, particularly in STEMI with direct impact of delays on the outcome (21).
Reducing the time from symptoms onset to activation of the emergency medical service (patient delay) as well as the time from activation of calling the emergency service to reperfusion (system delay) are the key targets for improving outcomes in STEMI (22). In this regard, given that from symptom onset, median 2.08 h passed to pre-hospital ECG, and 4.20 h to PCI in our STEMI patients, our findings emphasize that not only the usage rate but also the timing of immediate pre-hospital ECG from symptom onset should be improved in Turkish STEMI patients.
Considering in-hospital management, cardiac catheterization was applied in the majority (94.7%) of patients, mainly with PCI (71.7%) and more commonly (87.9 vs. 55.6%) and much earlier (4.2 vs. 26.25 h) from the symptom onset in patients with STEMI vs. UA/NSTEMI. These findings are consistent with data from Eastern European cohort (unpublished observations).
High rates for PCI implementation in our cohort of patients with ACS seems notable given the significant decrease shown in mortality after STEMI in countries switching from fibrinolysis to primary PCI (23).
However, if the time from symptom onset is greater than 6 h, it has been considered that there is less time dependence to reperfusion and on the basis of growing body of evidence on the beneficial effects of reperfusion therapy even in patients presenting more than 12 h from symptom onset, recent recommendations emphasize primary PCI a strong indication in this group of patients (24).
Accordingly, PCI was applied in 87.9% patients with STEMI after a median of 4.2 h from symptom onset, consistent with data from the recent studies indicating that a reperfusion strategy can be applied in more than 90% patients with STEMI (25–27).
In this regard, our findings indicate adherence to current practice guidelines on use of intervention-based strategy for the management of patients with STEMI.
Considering overall pre and/or in-hospital medication use, our findings are consistent with data from Eastern European cohort (unpublished observations).
Similar to Eastern European cohort, antiplatelet therapy was based on dual therapy in majority (81.1%) of total patients and also in patients under UFH (79.0%) or LMWH (81.6%) therapy, while more commonly in patients with UA/NSTEMI than in those with STEMI in the Turkish cohort. Consistent with threefold increase in major bleeding and thus lower prevalence of triple than dual antithrombotic therapy reported in patients following ACS (28), triple antiplatelet therapy was applied in lesser percentage (13.2%) of total patients and patients under anticoagulant therapy, while more commonly in patients with STEMI than in patients with UA/NSTEMI.
Accordingly, our findings support the statement that ASA and clopidogrel are currently the antiplatelet agents most frequently used in the early management of ACS (29). Real-life clinical practice regarding in-hospital medications in Turkey seems in accordance with both the guidelines that recommend the admi- nistration of DAPT with both ASA and clopidogrel to medium- or high-risk patients with NSTE-ACS at the time of presentation, to all patients with STEMI regardless of the reperfusion strategy, and to all patients directed to primary PCI (at the time of first medical contact) along with intravenous bolus injection of UFH (25, 29).
Similar to recent trends reported by other ACS registries (21, 30–32) our study showed high use of guidelines-recommen- ded medications including ASA and invasive procedures, while lower rates of anticoagulants and GP IIb/IIIa inhibitor use than reported in developed countries (16, 18, 30–34) and lower rates for thrombolysis (30% to 93%) reported in STEMI populations in other registries (17, 20, 35).
Notably, in a recent study with 39 291 patients from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) registry with non-ST-segment elevation ACS, the association between hospital guideline adherence, dosing safety, and outcomes was evaluated among patients with ACS. Authors indicated that performance metrics should be based on not only the application of guideline-indicated medications but also the antithrombotic dosing safety, since guideline adherence and dosing safety appeared to provide independent and complementary information on hospital bleeding and mortality in patients with ACS (36).
Lesser use of UFH in patients with UA/STEMI than in those with STEMI in our study supports the preference to use the LMWH over UFH reported in patients with NSTEMI (17). Notably, while GP IIb/IIIa inhibitors were shown to reduce the relative risk of death or myocardial infarction among patients with NSTEMI (37) in-hospital use of GP IIb/IIIa inhibitors (tirofiban) was noted only in 6.2% patients with NSTEMI in the present study.
Although the first decade of the 21st century refers to a decade of PCIs from the ACS perspective, the availability of new, more potent and faster-acting oral antiplatelets such as pra- sugrel or ticagrelor has been currently changing the treatment of ACS and this has been reflected on the new guidelines (38).
Reassuringly high prescription of DAPT as well as therapy for relief of ischemic symptoms at discharge among our patients seems consistent with guidelines in the management of patients with ACS in terms of pharmacological secondary prevention treatments This is noteworthy given the under-use of recommended pharmacological secondary prevention treatments in patients with ACS indicated in French [OPERA (39), CONNECT (40)], European regional [Euro Heart (19), EUROASPIRE II (41)], US (42) and multinational [GRACE (43)] registry studies. Notably consistent with our findings, data from more recent follow-up registry studies have reported better use of pharmacological treatments in the management of ACS (20, 44).
Cardiac ischemic complications including in-hospital occurrence of MI (0.8%) and recurrent ischemia (1.2%) were quite rare in Turkish patients as were other cardiac complications, while all were higher in patients with STEMI than in those with UA/NSTEMI. In the Eastern European cohort, slightly higher rates for MI (1.4%) and recurrent ischemia (4.1%) as well as other cardiac complications were noted; all were also higher in patients with STEMI than in those with UA/NSTEMI (unpublished observations).
Non-fatal in-hospital bleeding events were also rare in Tur- kish patients (1.5%) with higher rates in patients with STEMI than in those with UA/NSTEMI. Being mostly at vascular access with minimal clinical significance and hemodynamic compromise, in-hospital bleeding led discontinuation of antithrombotic therapy in one third of cases. In the Eastern European cohort, slightly higher rates for non-fatal in-hospital bleeding events (2.3%) were noted with characteristic similar to identified in the Turkish cohort.
Data from the GRACE registry showed that the overall incidence of major bleeding was 3.9% in patients with STEMI, 4.7% in patients with NSTEMI, and 2.3% in patients with UA (45). In this regard, low incidence of bleeding in our cohort seems notable since bleeding itself is associated with an increased risk for mortality (5, 46).
Besides, given that the risk of bleeding is likely to be higher among patients encountered in clinical practice who likely have more comorbidities than those enrolled in controlled clinical trials (46, 47), identification of bleeding complication only in 1.5% of our patients is notable, while support that PCIs are associated with improved outcomes with fewer bleeding complications (48).
Study limitations
Certain limitations to this study should be considered. First, because of observational nature, non-randomized allocation to exposure of interest and thus strong likelihood of bias and confounding are possible along with the possibility of data to be incomplete and of poorer quality and outcomes to be not validated. Second, absence of accurate data on place of first medical attention as well as the time from symptom onset to first medical attention or ECG in majority of our patients is another limitation which otherwise would extend the knowledge achieved in the current study. Nevertheless, despite these certain limitations, given the paucity of the solid information available on this area, our findings represent a valuable contribution to the literature and provide insights into the clinical characteristics, risk factors, management and outcomes for patients hospitalized for an ACS.
Conclusion
In conclusion, although the preference of centers that perform PCI would be potential bias, our findings in a real-life population of patients with ACS enrolled into the Turkey arm of the EPICOR study, revealed in-hospital and at-discharge management to be mainly based on DAPT with ASA + clopidogrel overall, along with use of interventional strategies in majority of patients with STEMI. In this regard, our findings emphasize adherence to guidelines in terms of in-hospital care including antiplatelet therapy and interventional strategies as well as pharmacological secondary prevention treatments, while pre-hospital management should be improved in terms of usage and earlier induction of ECG and medications from symptom onset in addition to the time to PCI from symptom onset. Turkish cohort of patients with EPICOR seems to be advantageous in terms of reassuringly high rates of primary PCI as well as lower rate for CV risk factors and ischemic/bleeding complications, which in fact should encourage the utilization of newer antiplatelet and anticoagulant agents. Further follow-up of these patients will help to determine whether these practice patterns affect outcome
Collaborators (34)*: Murat Çaylı1, Ahmet Camsan2, Enbiya Aksakal3, Emin Alioğlu4, Hasan Pekdemir5, Murat Yeşil6, Sema Güneri7, Abdurrahman Oğuzhan8, Refik Emre Altekin9, Timuçin Altın10, Merih Kutlu11, Ertuğrul Ercan12, Rojhat Altindağ13, Mahmut Şahin14, Fatih Sinan Ertaş15, Ceyhun Ceyhan16, Murat Ersanlı17, Mesut Demir18, Necmi Ata19, Alpay Turan Sezgin20, Ilgın Karaca21, Ekrem Yeter22, Zeki Öngen23, Mustafa Cemri24, Osman Bolca25, Mehmet Yazıcı26, Mehmet Aksoy27, İzzet Tandoğan28, Neşe Cam29, Serdar Payzın30, Mehmet Sıddık Ülgen31, Mehmet Zihni Bilik32, Gönül Zeren33, Kamil Adalet34
*EPICOR Study Group (by the number of patients enrolled): 1Adana Numune Training and Research Hospital, Adana, 2Mersin University Faculty of Medicine, Mersin, 3Atatürk University Aziziye Research Hospital, Erzurum, 4İzmir Central Hospital, İzmir, 5İnonu University Faculty of Medicine, Malatya, 6İzmir Atatürk Training and Research Hospital, İzmir, 79 Eylül University Faculty of Medicine, İzmir, 8Erciyes University Faculty of Medicine, Kayseri, 9Akdeniz University Faculty of Medicine, Antalya, 10Ankara University Faculty of Medicine, Ankara, 11Karadeniz Technical University Faculty of Medicine, Trabzon, 1218 Mart University Faculty of Medicine, Çanakkale, 13Diyarbakır Training and Research Hospital, Diyarbakır, 1419 Mayıs University Faculty of Medicine, Samsun, 15Ankara University Faculty of Medicine, İbni Sina Hospital, Ankara, 16Adnan Menderes University Faculty of Medicine, Aydın, 17İstanbul University, Institute of Cardiology, İstanbul, 18Adana Çukurova Faculty of Medicine, Adana, 19Osmangazi University Faculty of Medicine, Eskişehir, 20Adana Başkent University Practice and Research Center, Adana, 21Fırat University Faculty of Medicine, Elazığ, 22Ankara Dışkapı Yıldırım Beyazıt Training and Research Hospital, Ankara, 23İstanbul University Cerrahpaşa Faculty of Medicine, İstanbul, 24Gazi University Faculty of Medicine, Ankara, 25Prof Dr. Siyami Ersek Chest Diseases and Thoracic Surgery Training and Research Hospital, İstanbul, 26Selçuk University Faculty of Medicine, Konya, 27Gaziantep University Faculty of Medicine, Gaziantep, 28Cumhuriyet University Faculty of Medicine, Sivas, 29Prof Dr. Siyami Ersek Chest Diseases and Thoracic Surgery Training and Research Hospital, İstanbul, 30Ege University Faculty of Medicine, İzmir, 31Dicle University Faculty of Medicine, Diyarbakır, 32Diyarbakır Training and Research Hospital, Diyarbakır, 33Aksaray State Hospital, Aksaray, 34İstanbul University İstanbul Faculty of Medicine, İstanbul
Acknowledgements
The study is funded by Astra-Zeneca Turkey. Authors would like to thank to Prof. Şule Oktay, MD, PhD and Çağla Ayhan, MD from KAPPA Consultancy Training Research Ltd (Istanbul, Turkey) who provided medical writing support funded by funded by Astra-Zeneca Turkey.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.

From Prof. Dr. Arif Aksit’s collection
References
- 1.Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–81. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, et al. 2009 focused updates:ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–41. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Davies MJ. The pathophysiology of acute coronary syndromes. Heart. 2000;83:361–6. doi: 10.1136/heart.83.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. American College of Cardiology;American Heart Association. Committee on the Management of Patients With Unstable Angina. ACC/AHA 2002 guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction-summary article: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (Committee on the Management of Patients With Unstable Angina) J Am Coll Cardiol. 2002;40:1366–74. doi: 10.1016/s0735-1097(02)02336-7. [DOI] [PubMed] [Google Scholar]
- 5.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–45. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 6.Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol. 2005;46:937–54. doi: 10.1016/j.jacc.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 7.Fox KA. Coronary disease. Acute coronary syndromes:presentation-clinical spectrum and management. Heart. 2000;84:93–100. doi: 10.1136/heart.84.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 9.Fox KA, Carruthers KF, Dunbar DR, Graham C, Manning JR, De Raedt H, et al. Underestimated and under-recognized: the late consequences of acute coronary syndrome (GRACE UK–Belgian Study) Eur Heart J. 2010;31:2755–64. doi: 10.1093/eurheartj/ehq326. [DOI] [PubMed] [Google Scholar]
- 10.Fox KA, Clayton TC, Damman P, Pocock SJ, de Winter RJ, Tijssen JG, et al. Long-term outcome of a routine versus selective invasive strategy in patients with non–ST-segment elevation acute coronary syndrome a meta-analysis of individual patient data. J Am Coll Cardiol. 2010;55:2435–45. doi: 10.1016/j.jacc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Harnek J, Nilsson J, Friberg O, James S, Lagerqvist B, Hambraeus K, et al. The 2011 outcome from the Swedish Health Care Registry on Heart Disease (SWEDEHEART) Scand Cardiovasc J. 2013;47:1–10. doi: 10.3109/14017431.2013.780389. [DOI] [PubMed] [Google Scholar]
- 12.Bueno H, Danchin N, Tafalla M, Bernaud C, Annemans L, Van de Werf F. EPICOR (long-tErm follow-up of antithrombotic management Patterns In acute CORonary syndrome patients) study:rationale, design, and baseline characteristics. Am Heart J. 2013;165:8–14. doi: 10.1016/j.ahj.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Hanssen M, Cottin Y, Khalife K, Hammer L, Goldstein P, Puymirat E, et al. French registry of acute ST-elevation and non ST-elevation myocardial infarction 2010 FAST-MI 2010. Heart. 2012;98:699–705. doi: 10.1136/heartjnl-2012-301700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Awad HH, Zubaid M, Alsheikh-Ali AA, Al Suwaidi J, Anderson FA, Jr, Gore JM, et al. Comparison of characteristics, management practices, and outcomes of patients between the global registry and the Gulf registry of acute coronary events. Am J Cardiol. 2011;108:1252–8. doi: 10.1016/j.amjcard.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 15.Tokgözoğlu L, Kaya EB, Erol C, Ergene O EUROASPIRE III Turkey Study Group. [EUROASPIRE III: a comparison between Turkey and Europe] Turk Kardiyol Dern Ars. 2010;38:164–72. [PubMed] [Google Scholar]
- 16.Xavier D, Pais P, Devereaux PJ, Xie C, Prabhakaran D, Reddy KS, et al. CREATE registry investigators. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–42. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 17.Moustaghfir A, Haddak M, Mechmeche R. Management of acute coronary syndromes in Maghreb countries: The ACCESS (ACute Coronary Events - a multinational Survey of current management Strategies) registry. Arch Cardiovasc Dis. 2012;105:566–77. doi: 10.1016/j.acvd.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Steg PG, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Flather MD, et al. GRACE Investigators. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002;90:358–63. doi: 10.1016/s0002-9149(02)02489-x. [DOI] [PubMed] [Google Scholar]
- 19.Hasdai D, Behar S, Wallentin L, Danchin N, Gitt AK, Boersma E, et al. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin;the Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS) Eur Heart J. 2002;23:1190–201. doi: 10.1053/euhj.2002.3193. [DOI] [PubMed] [Google Scholar]
- 20.Mandelzweig L, Battler A, Boyko V, Bueno H, Danchin N, Filippatos G, et al. Euro Heart Survey Investigators. The second Euro Heart Survey on acute coronary syndromes:Characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basın in 2004. Eur Heart J. 2006;27:2285–93. doi: 10.1093/eurheartj/ehl196. [DOI] [PubMed] [Google Scholar]
- 21.Gersh BJ, Stone GW, White HD, Holmes DR., Jr Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA. 2005;293:979–86. doi: 10.1001/jama.293.8.979. [DOI] [PubMed] [Google Scholar]
- 22.Lars Wallentin L, Kristensen SD, Anderson JL, Tubaro M, Sendon JL, Granger CB, et al. How can we optimize the processes of care for acute coronary syndromes to improve outcomes? Am Heart J. 2014;168:622–31. doi: 10.1016/j.ahj.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Zahn R, Schiele R, Schneider S, Gitt AK, Wienbergen H, Seidl K, et al. Decreasing hospital mortality between 1994 and 1998 in patients with acute myocardial infarction treated with primary angioplasty but not in patients treated with intravenous thrombolysis Results from the pooled data of the Maximal Individual Therapy in Acute Myocardial Infarction (MITRA) Registry and the Myocardial Infarction Registry (MIR) J Am Coll Cardiol. 2000;36:2064–71. doi: 10.1016/s0735-1097(00)00981-5. [DOI] [PubMed] [Google Scholar]
- 24.Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 25.Fitchett DH, Theroux P, Brophy JM, Cantor WJ, Cox JL, Gupta M, et al. Assessment and management of acute coronary syndromes (ACS): a Canadian perspective on current guideline-recommended treatment-part 2:ST-segment elevation myocardial infarction. Can J Cardiol. 2011;27:S402–12. doi: 10.1016/j.cjca.2011.08.107. [DOI] [PubMed] [Google Scholar]
- 26.Juliard JM, Golmard JL, Ducrocq G, Himbert D, Aubry P, Garbarz E, et al. Universal reperfusion therapy can be implemented: lessons from 20 years of management of patients admitted within 6 hours of symptom onset with ST-segment elevation acute myocardial infarction. Arch Cardiovasc Dis. 2009;102:259–67. doi: 10.1016/j.acvd.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Charpentier S, Celse D, Cambou JP, Lauque D, Carrie D, Galinier M, et al. Evaluation of therapeutic strategies for myocardial infarction: the ESTIM Midi-Pyrenees survey. Arch Mal Coeur Vaiss. 2005;98:1143–8. [PubMed] [Google Scholar]
- 28.Smith JG, Wieloch M, Koul S, Braun OÖ, Lumsden J, Rydell E, et al. Triple antithrombotic therapy following an acute coronary syndrome:prevalence, outcomes and prognostic utility of the HAS-BLED score. EuroIntervention. 2012;8:672–8. doi: 10.4244/EIJV8I6A105. [DOI] [PubMed] [Google Scholar]
- 29.Fitchett DH, Theroux P, Brophy JM, Cantor WJ, Cox JL, Gupta M, et al. Assessment and management of acute coronary syndromes (ACS): a Canadian perspective on current guideline-recommended treatment-part 1:non-ST-segment elevation ACS. Can J Cardiol. 2011;27:S387–401. doi: 10.1016/j.cjca.2011.08.110. [DOI] [PubMed] [Google Scholar]
- 30.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA, Jr, Granger CB, et al. Decline in rates of death and heart failure in acute coronary syndromes 1999–2006. JAMA. 2007;297:1892–900. doi: 10.1001/jama.297.17.1892. [DOI] [PubMed] [Google Scholar]
- 31.Yan AT, Yan RT, Tan M, Fung A, Cohen EA, Fitchett DH, et al. Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch Intern Med. 2007;167:1009–16. doi: 10.1001/archinte.167.10.1009. [DOI] [PubMed] [Google Scholar]
- 32.Rosengren A, Wallentin L, Simoons M, Gitt AK, Behar S, Battler A, et al. Age, clinical presentation, and outcome of acute coronary syndromes in the Euro Heart Acute Coronary Syndrome Survey. Eur Heart J. 2005;27:789–95. doi: 10.1093/eurheartj/ehi774. [DOI] [PubMed] [Google Scholar]
- 33.Goodman SG, Huang W, Yan AT, Budaj A, Kennelly BM, Gore JM, et al. The expanded global registry of acute coronary events: baseline characteristics, management practices, and hospital outcomes of patients with acute coronary syndromes. Am Heart J. 2009;158:193–201. doi: 10.1016/j.ahj.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Amsterdam EA, Peterson ED, Ou FS, Newby LK, Pollack CV, Jr, Gibler WB, et al. Comparative trends in guidelines adherence among patients with non-ST segment elevation acute coronary syndromes treated with invasive versus conservative management strategies: results from the CRUSADE quality improvement initiative. Am Heart J. 2009;158:748–54. doi: 10.1016/j.ahj.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 35.The ACCESS Investigators. Management of acute coronary syndromes in developing countries: acute coronary events-a multinational survey of current management strategies. Am Heart J. 2011;162:852–9. doi: 10.1016/j.ahj.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 36.Mehta RH, Chen AY, Alexander KP, Ohman EM, Roe MT, Peterson ED. Doing the right things and doing them the right way: association between hospital guideline adherence, dosing safety, and outcomes among patients with acute coronary syndrome. Circulation. 2015;131:980–7. doi: 10.1161/CIRCULATIONAHA.114.013451. [DOI] [PubMed] [Google Scholar]
- 37.King S, Short M, Harmon C. Glycoprotein IIb/IIIa inhibitors: The resurgence of tirofiban. Vascul Pharmacol. 2016;78:10–6. doi: 10.1016/j.vph.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Grove EL, Würtz M, Thomas MR, Kristensen SD. Antiplatelet therapy in acute coronary syndromes. Expert Opin Pharmacother. 2015;16:2133–47. doi: 10.1517/14656566.2015.1079619. [DOI] [PubMed] [Google Scholar]
- 39.Montalescot G, Dallongeville J, Van Belle E, Rouanet S, Baulac C, Degrandsart A, et al. STEMI and NSTEMI: are they so different? 1-year outcomes in acute myocardial infarction as defined by the ESC/ACC definition (the OPERA registry) Eur Heart J. 2007;28:1409–17. doi: 10.1093/eurheartj/ehm031. [DOI] [PubMed] [Google Scholar]
- 40.Sabouret P, Asseman P, Dallongeville J, Dujardin JJ, Philippe F, Herrmann MA, et al. CONNECT Study Investigators. Observational study of adherence to European clinical practice guidelines for the management of acute coronary syndrome in revascularized versus non-revascularized patients - the CONNECT Study. Arch Cardiovasc Dis. 2010;103:437–46. doi: 10.1016/j.acvd.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 41.EUROASPIRE II Study Group. Lifestyle and risk factor management and use of drug therapies in coronary patients from 15 countries;principal results from EUROASPIRE II Euro Heart Survey Programme. Eur Heart J. 2001;22:554–72. doi: 10.1053/euhj.2001.2610. [DOI] [PubMed] [Google Scholar]
- 42.Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–20. doi: 10.1001/jama.295.16.1912. [DOI] [PubMed] [Google Scholar]
- 43.Budaj A, Brieger D, Steg PG, Goodman SG, Dabbous OH, Fox KA, et al. Global patterns of use of antithrombotic and antiplatelet therapies in patients with acute coronary syndromes: insights from the Global Registry of Acute Coronary Events (GRACE) Am Heart J. 2003;146:999–1006. doi: 10.1016/S0002-8703(03)00509-X. [DOI] [PubMed] [Google Scholar]
- 44.Kotseva K, Wood D, De Backer G, De Bacquer D, Pyorala K, Keil U. Cardiovascular prevention guidelines in daily practice: a comparison of EUROASPIRE I, II, and III surveys in eight European countries. Lancet. 2009;373:929–40. doi: 10.1016/S0140-6736(09)60330-5. [DOI] [PubMed] [Google Scholar]
- 45.Moscucci M, Fox KA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2003;24:1815–23. doi: 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 46.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 47.Macie C, Forbes L, Foster GA, Douketis JD. Dosing practices and risk factors for bleeding in patients receiving enoxaparin for the treatment of an acute coronary syndrome. Chest. 2004;125:1616–21. doi: 10.1378/chest.125.5.1616. [DOI] [PubMed] [Google Scholar]
- 48.Waters RE, 2nd, Mahaffey KW, Granger CB, Roe MT. Current perspectives on reperfusion therapy for acute ST-segment elevation myocardial infarction: integrating pharmacologic and mechanical reperfusion strategies. Am Heart J. 2003;146:958–68. doi: 10.1016/S0002-8703(03)00439-3. [DOI] [PubMed] [Google Scholar]


