Abstract
Objective:
Cardiac uptake of fructose is thought to be mediated by glucose transporter 5 (GLUT5), whereas the uptake of glycerol is facilitated by aquaporin 7 (AQP7). We aimed to investigate the effect of a high-fructose diet (HFD) on GLUT5 and AQP7 levels in the rat heart subjected to exercise.
Methods:
Male Sprague–Dawley rats were allocated to control (C; n=11), exercise (E; n=10), HFD (n=12), and HFD plus exercise (HFD-E; n=12) groups. HFD was started 28 days before euthanasia. From day 24 to 27, rats were subjected to moderate exercise, followed by vigorous exercise on day 28 (groups E and HFD-E). Cardiac GLUT5 and AQP7 mRNA levels were determined using RT-PCR. The protein contents of GLUT5 and AQP7 were immunohistochemically assessed. Paired-t, ANOVA with Bonferroni, Kruskal–Wallis, and Bonferroni-corrected Mann–Whitney U tests were used for statistical analysis.
Results:
GLUT5 mRNA expression and protein content did not differ between the groups. AQP7 mRNA levels significantly increased (4.8-fold) in group E compared with in group C (p<0.001). Compared with group C, no significant change was observed in AQP7 mRNA levels in groups HFD and HFD-E. The AQP7 staining score in group E was significantly higher than that in groups C (p<0.001), E (p<0.001), and HFD-E (p<0.001).
Conclusion:
Our study indicates that exercise enhances cardiac AQP7 mRNA expression and protein content. However, HFD prevents the exercise-induced increase in cardiac AQP7 expression. This inhibitory effect may be related to the competition between fructose and glycerol as energy substrates in the rat heart subjected to 5 days of physical exercise. (Anatol J Cardiol 2016; 16: 916-22)
Keywords: high-fructose diet, exercise, glucose transporter 5, aquaporin 7, heart
Introduction
Nutrients that boost exercise performance in sportsmen and physically active people are nowadays becoming significantly important. While most of the energy in the heart is provided by fatty acid oxidation during rest (1), the contribution of carbohydrates to cardiac energy conversion increases during enhanced workloads of the heart, for instance, during physical exercise. It has been reported that the contributions of glucose, fatty acids, and lactate to total cardiac energy metabolism are 16%, 21%, and 61%, respectively, during exercise (2). Until now, the role of fructose in cardiac energy metabolism is incompletely understood.
Fructose is a common ingredient in sports drinks. A high-fructose diet (HFD) causes a substantial rise in plasma fructose levels (3). Fructose is converted into metabolites such as glucose (~50%), glycogen (>15%), and lactate (~25%) and a small amount of fatty acids in hepatocytes and is used as an energy substrate by a variety of organs (4, 5). Previous studies have shown that HFD increases the aerobic performance of sportsmen and mitigates fatigue compared with food containing solely glucose (6, 7). Although the heart plays an important role in performing strenuous exercise, there are only a limited number of studies regarding the effect of exercise on fructose metabolism in cardiac tissue. It has been shown that cardiac tissue expresses the fructokinase gene, resulting in fructokinase enzymatic activity (8). Since fructose is converted into fructose-1-P by fructokinase, and subsequently into dihydroxyacetone P and glyceraldehyde 3-P, energy can most likely be obtained from fructose via the Krebs cycle and electron transport chain in cardiac muscle cells (5, 9). Additionally, it has been reported that an increase in exogenous fructose modulates cardiomyocyte excitation–contraction coupling, and fructose transport in cardiomyocytes can also be facilitated by glucose transporter 5 (GLUT5) (10). However, it is not clear whether HFD increases GLUT5 expression in cardiomyocytes under exercise conditions. In this study, we first tested the hypothesis that GLUT5 gene expression and protein content of the left ventricle increase during exercise in HFD-supplied rats.
A previous study has shown that the intravenous infusion of fructose reduced the exercise-induced increase in plasma noradrenaline and glycerol concentrations in dogs (11), suggesting an interaction between fructose and glycerol as fuels during physical exercise. On the other hand, it is known that catecholamines stimulate AQP7 to move to the plasma membrane in adipocytes (12). Thus, we considered investigating the effect of exercise as a stimulating factor for catecholamine action on cardiac AQP7 levels. The second hypothesis of this study was that cardiac AQP7 mRNA gene expression and protein content increases by physical exercise. We also postulated that HFD supplied to rats subjected to exercise could block the exercise-induced increase in AQP7 gene expression and protein content. Thus, as the third hypothesis in this study, we tested if HFD could block the exercise-induced increase in AQP7 gene expression and protein content.
Methods
Animals
Experiments were performed on male Sprague–Dawley rats (20 weeks of age; body weight, 348±43 g at the start of the experiments) from the Animal Laboratory of the Trakya University, Medical Faculty. The animals were allotted to four groups. The control (C; n=11) and exercise (E; n=10) groups received a standard diet for 28 days. HFD was given to animals in the HFD (n=12) and HFD-E (n=12) groups. Exercise was started on day 23 of the nutritional period and was continued until day 28 in the E and HFD-E groups. All rats were allowed to drink tap water ad libidum. The experiments in this study were approved by the Animal Ethics Committee of the Trakya University.
Composition of the diet
A standard diet (Optima Rat Chow, Bolu, Turkey) containing 5.5% fats, 24.0% proteins, and 61.7% carbohydrates (starch) on weight basis was supplied to the rats in the C and E groups. HFD (MBD Rat Chow, Kocaeli, Turkey), containing 5.2% fats, 18.3% proteins, and 60.4% carbohydrates (fructose), was supplied to the rats in the HFD and HFD-E groups. The remaining ingredients in the two diets included minerals, vitamins, and cellulose.
Exercise procedure
Animals in the E and HFD-E groups performed daily exercise during the last 5 days of the nutritional period. Moderate exercise at a speed of 15 m/min for 15 min on a treadmill was performed for 4 consecutive days between 10:00 and 12:00 AM. On the last day of the experimental period (day 28), the rats exercised more vigorously at a speed of 25 m/min for 25 min on a treadmill (13). Thereafter, all animals were intraperitoneally anesthetized with sodium thiopental (100 mg/kg). After making an incision on the midline of the chest, we harvested the hearts for analysis. Heart weight was measured with a microbalance immediately after extirpation. Heart weight was normalized to tibia length (14). The heart weight/tibia length (HW/TL) ratio was used to determine whether exercise induced cardiac hypertrophy during the experimental period.
Assessment of AQP7 and GLUT5 gene expression RNA isolation and cDNA synthesis
Total RNA was extracted from left ventricular tissues using the High Pure FFPET RNA Isolation Kit (Roche, Mannheim, Germany). RNA concentration was measured by a micro-volume spectrophotometer (Denovix DS-11, Wilmington, USA). cDNA was synthesized from RNA samples using the cDNA synthesis kit for real-time PCR (RT-PCR; Bioneer, South-Korea). PCR was performed using a thermal cycler (Bioneer) under the following conditions: primer annealing at 30°C for 5 min, cDNA synthesis at 60°C for 60 min, and heat inactivation at 90°C for 5 min (for 1 cycle).
RT-PCR
Primer sequences used for RT-PCR were the AQP7 gene (F: 5’ ATCCTTGTTTGCGTTCTTGG 3’, R: 5’ GCGTGAATTAAGCCCAGGTA 3’), GLUT5 gene (5’ TCGAGGCCCTGTAATTGGAA 3’, R: 5’ CCCTCCAATGGATCCTCGTT 3’), and GAPDH (reference) gene (F: 5’ AATGTATCCGTTGTGGATCT 3’, R: 5’ CAAGAAGGTGGTGAAGCAGG 3’) (Bioneer). RT-PCR was performed using Accupower® GreenStar qPCR PreMix (Bioneer), a ready-to-use reagent containing all the components required for RT-PCR, including SYBR Green.
The ExiCycler™-96 (Bioneer) RT-PCR device was used in this study. Briefly, the following protocol was applied: PCR-F primer, 2 µL; PCR-R primer, 2 µL (forward and reverse primers for each gene); template (cDNA), 5 µL; and DEPC water, 11 µL were added into the Accupower® GreenStar qPCR PreMix tubes. These tubes were sealed by adhesive optical film. RT-PCR protocol: enzyme activation and denaturation at 95°C for 5 min; 45 cycles of amplification: at 95°C for 20 s and 60°C for 45 s; and signal detection and cooling at 40°C for 30 s. After normalizing the AQP7 and GLUT5 gene expressions to the GAPDH reference gene expression, the statistical results of relative gene expression were calculated by the “Pfaffl” mathematical method using the REST program (Relative Expression Software Tool v2.0.13, 2009, Qiagen, Hilden, Germany) (15).
Immunohistochemical staining and microscopic evaluation
Hearts were kept in 10% formalin solution at room temperature for 24 h after the weighing procedure. Tissue sections were cut at a thickness of 3–5 microns and mounted on positively charged slides. Before treatment, all sections were dried in a slide oven at 60°C for 30 min to ensure that the sections adhered to the slides and were completely dry. Sections were deparaffinized in four series of xylene for 5 min each and then rehydrated through a series of graded alcohol, with a final rinse in distilled water. Endogenous peroxides were quenched by soaking sections in two series of 3% H2O2 in methanol for 5 min each.
The following specific antibodies were used: polyclonal rabbit antibody for GLUT5 (sc-271055) and AQP7 (sc-28625) from Santa Cruz Biotechnology (Dallas, Texas, USA). Before the application of AQP7 and GLUT4 antibodies, sections were treated with antigen retrieval to facilitate antibody binding to the antigen [“EDTA buffer 10X pH 8.0,” ab64216 Lot.GR171628-2; Avidin Biotin Complex (ABC)].
The sections were examined under an Olympus BX-51 model light microscope. Images of the cross-sections were taken and recorded using MC80 DX programs. For assessing the immunohistochemical staining, the pathologist made an objective evaluation without knowing the group characteristics. All cardiomyocytes to be evaluated were randomly chosen. The immune reaction, if any, was studied using a microscope in a randomly chosen 10 high-power field (HPF) area (400x). The extent of staining was determined as the percentage immunopositive cardiomyocytes of the total number of cardiomyocytes seen in each section (16). Stained-cell prevalence was graded as 0 (0%–5%), 1 (6%–24%), 2 (25%–49%), 3 (50%–74%), and 4 (≥75% of the total number of cells). Staining intensity was evaluated as 0 (absent), 1 (mild), 2 (medium), and 3 (strong). The grades of stained-cell prevalence and staining intensity were multiplied, yielding an immunoreactivity score ranging from 0 to 12 (16, 17).
Statistical analysis
The one-sample Kolmogorov–Smirnov test was applied to determine whether a sample came from a population that was normally distributed. One-way analysis of variance was used to compare the groups (with the Bonferroni test for posthoc comparison), and for parametric data, the paired t-test was applied for comparisons in a group. Non-normally distributed continuous data were evaluated using the Kruskal–Wallis variance analysis test. The posthoc test used was the Bonferroni-corrected Mann–Whitney U test. The significance was taken as a=0.008 (P<0.05/comparison number). P values <0.05 were considered statistically significant.
Data are reported as mean±standard error or as median and range. All statistical analyses were conducted using the SPSS [Statistical Package for Social Sciences) for Windows v.21.0 (New York, USA)] package program. The GraphPad Prism for Windows v6.03 (California, USA) program was used for drawing the graphics.
Results
Before feeding, no significant differences in body weight were present between the four groups of rats: C (346.5±12.8 g), E (356.5±14.5 g), HFD (337.0±11.0 g), and HFD-E (355.7±11.7 g) groups (Fig. 1). A 7.2% increase in body weight was observed in the HFD group during the feeding period (361.5±16.3 g after feeding versus 337.0±11.0 g before feeding; p<0.001). No significant change in body weight was observed during the feeding period in the other three groups.
Figure 1.
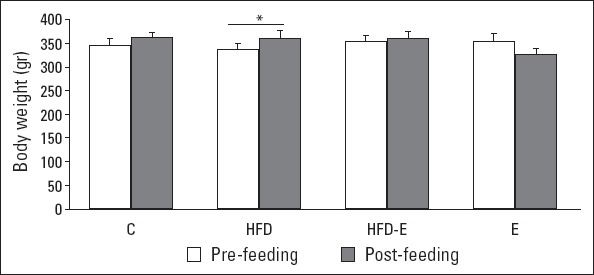
Body weight before and after the feeding period. Values are expressed as mean±SE.
*Paired t-test, P<0.001. C - control group; E - exercise group; HFD - high fructose diet group; HFD-E - high fructose diet plus exercise group
No statistical difference was observed in the HW/TL ratio between the four groups, indicating the absence of a hypertrophic response (Table 1).
Table 1.
Heart weight/tibia length ratio
| C (n=11) | E (n=10) | HFD (n=12) | HFD-E (n=12) | P* | |
|---|---|---|---|---|---|
| HW/TL, mg/mm | 25.0±0.7 | 27.1±0.5 | 25.1±1.0 | 26.3±0.7 | 0.192 |
Values are expressed as mean±SEM.
ANOVA, posthoc Bonferroni. C - control group; E - exercise group; HFD- high-fructose diet group; HFD-E - high-fructose diet plus exercise group; HW - heart weight; TL - tibia length
GLUT5 and AQP7 gene expression
The relative expressions of GLUT5 mRNA levels in the left ventricle in the E, HFD, or HFD-E groups were not different compared with those in the C group (Fig. 2). A significant 4.8-fold increase in AQP7 mRNA expression was found in the E group compared with in the C group (p<0.001). In contrast, no significant differences were observed in AQP7 mRNA expression in the HFD and HFD-E groups mutually and between these two groups and the C group (Fig. 3).
Figure 2.
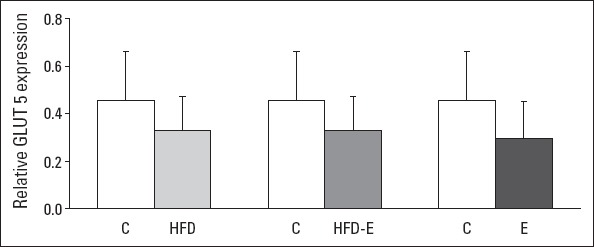
Relative expression of GLUT5 mRNA levels of the left ventricle. Values are expressed as mean±SEM. Kruskal–Wallis and Bonferroni-corrected Mann–Whitney U test.
C - control group; E - exercise group; HFD - high fructose diet group; HFD-E - high fructose diet plus exercise group; GLUT5 - glucose transporter 5
Figure 3.
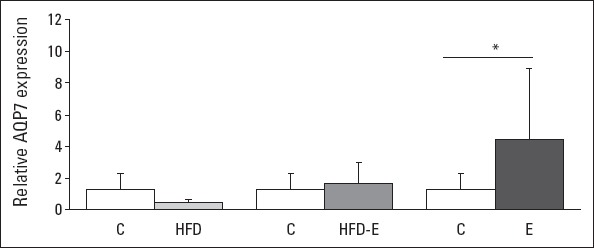
Relative expression of AQP7 mRNA levels of the left ventricle. Values are expressed as mean±SEM.
*Kruskal-Wallis and Bonferroni corrected Mann-Whitney U test, P<0.001; AQP7: aquaporin 7. C - control group; E - exercise group; HFD - high fructose diet group; HFD-E - high fructose diet plus exercise group
Immunohistochemical staining of GLUT5 and AQP7
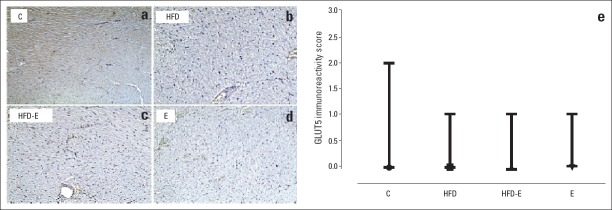
Figure 4a–d shows examples of GLUT5 staining in the four groups. Staining was absent in the sections of 7 of 11 rats in the C group, 7 of 10 rats in the E group, 8 of 12 rats in the HFD group, and 8 of 12 rats in the HFD-E group. Weak-intensity staining of the left ventricle cardiomyocytes was only observed in four rats of the C group, three of the E group, four of the HFD group, and four of the HFD-E group. No significant difference was present in the immunoreactivity scores between the four groups (Fig. 4e).
Figure 4.
GLUT5 staining and immunoreactivity scores in left ventricle sections. (a) Weak-intensity staining and 25%–49% stained-cell prevalence of left ventricle cardiomyocytes in the C group; (b–d) No staining in the HFD, HFD-E, and E groups (100x); (e) Immunoreactivity scores in the four groups under investigation. Median values and ranges are indicated.
Kruskal-Wallis and Bonferroni corrected Mann-Whitney U test. C - control group; E - exercise group; HFD - high fructose diet group; HFD-E - high fructose diet plus exercise group; GLUT5 - glucose transporter 5. ●, ■, ▲, and ▼: median values of staining scores in the C, E-HFD, and HFD-E groups, respectively
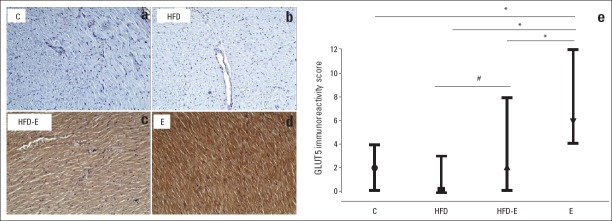
The staining scores for AQP7 varied from 0 to 4 in the C group, 4 to 12 in the E group, 0 to 3 in the HFD group, and 0 to 8 in the HFD-E group. Examples of AQP7 staining are shown in Figs. 5a–d. The corresponding median values of the staining scores were as follows: two for the C group, six for the E group, zero for the HFD group, and two for the HFD-E group (Fig. 5e). The immunoreactivity score for AQP7 was significantly higher in the E group than in the C, HFD, and HFD-E groups (Fig. 5e). It is noteworthy that the immunoreactivity score of AQP7 was significantly higher in the HFD-E group than in the HFD group (p=0.003).
Figure 5.
AQP7 staining and immunoreactivity scores in left ventricle sections. (a) No staining in the left ventricle cardiomyocytes in the C group; (b) No staining in the left ventricle cardiomyocytes in the HFD group; (c) Moderate staining with the AQP7 antibody in more than 75% of the left ventricle cardiomyocytes in the HFD-E group; (d) Strong staining with the AQP7 antibody in more than 75% of the left ventricle cardiomyocytes in the E group; (100x); (e) Immunoreactivity scores of the four groups. Median values and ranges are indicated.
*,#: Kruskal-Wallis and Bonferroni corrected Mann-Whitney U test *P<0.001, #P=0.003. AQP7-aquaporin 7; C - control group; E - exercise group; HFD - high fructose diet group; HFD-E - high fructose diet plus exercise group. ●,■,▲, and ▼ median values of the staining scores in the C, E, HFD, and HFD-E groups, respectively
Discussion
Since GLUT5 promotes the cellular entry of carbohydrates such as fructose, we postulated that a fructose-rich diet would enhance the cardiac expression of GLUT5 when the rats were subjected to exercise. Secondly, since catecholamines stimulate the transfer of AQP7 to the plasma membrane (12), we hypothesized that physical exercise would increase the expression of AQP7 in cardiac tissue. Our study indicated that we must dismiss the first hypothesis that HFD increases cardiac GLUT5 expression; however, we can accept the second hypothesis that exercise enhances cardiac AQP7 expression and protein content. Finally, the results of the present study showed an inhibition of exercise-induced increase in cardiac AQP7, confirming the third hypothesis.
Previous studies have indicated that nutritional fructose results in an improved aerobic performance and decreased fatigue (6, 7). Fructose is metabolized in the liver but can also be used as an energy substrate in the heart. Although it is known that cardiomyocytes express the fructose-specific GLUT5 transporter and that fructose transport in enterocytes and hepatocytes is stimulated by GLUT5, it is uncertain whether GLUT5 is facilitating fructose transport in the rat heart subjected to exercise (6, 10). In hepatocytes, fructose can be converted into glycerol 3-P and subsequently used for energy metabolism (5, 9). In the present study on rats, we did not find any effect of high-fructose feeding on GLUT5 gene expression and protein content in cardiac tissue of rats fed with HFD for 28 days, corroborating the earlier findings of Mellor et al. (3) in mice. These findings, however, do not exclude that the glucose/hexose transporters (18, 19) are involved in fructose transport through the membrane of cardiac muscle cells but strongly suggest that fructose is not able to modulate GLUT5 and hence the facilitated uptake of fructose by this particular transport protein in cardiac tissue.
Various studies have indicated that glycerol is used as a fuel for cardiac energy conversion (12, 20). Catecholaminergic stimuli increase the plasma concentration of glycerol, resulting in increased glycerol uptake by the heart (21). It is known that circulating catecholamines are elevated during physical exercise (22); thus, exercise may be considered as an effective factor for glycerol uptake by the heart. Apart from the increased plasma concentrations of glycerol as a driving force for enhanced glycerol uptake, the elevated expression of AQP7, a membrane-associated protein known to facilitate the transport of glycerol through cellular membranes (21), could be instrumental in increased cardiac glycerol utilization during exercise. Here we showed that physical exercise indeed increases both the mRNA expression and the protein content of AQP7 in cardiac tissue, indicating that exercise not only stimulates the transfer of this glycerol translocator to the plasma membrane (12) but also increases the total content of this transport protein in the heart by enhanced genetic expression. The corollary of this finding is that the increased energy demand due to the increased workload during exercise is most likely met by the enhanced uptake of glycerol from the blood compartment. It is interesting to note that Basco and coworkers (23) reported a relationship between contractile activity and AQP4 in skeletal muscles during prolonged exercise.
Glycerol is used as an energy source in the heart with increased workload during physical exercise (20). It can be speculated that the fructose taken up by cardiac tissue may compete with glycerol as the energy-rich fuel during exercise and hence mitigate glycerol uptake by the heart, facilitated by AQP7. Therefore, we have explored whether HFD supplied to rats subjected to exercise blocks the exercise-induced increase in AQP7 expression. The present study showed that physical exercise for 5 days resulted in an increased gene expression of cardiac AQP7 (Fig. 3). Interestingly, this increase was largely blocked when the animals were fed with HFD. Similarly, the cardiac protein content of AQP7 increased during exercise, an alteration also mitigated by fructose-rich feeding (Fig. 5e). However, no effect of fructose was seen in the sedentary HFD group compared with in the C group. Therefore, the findings of this study may point to the competition between fructose and glycerol as an energy substrate in cardiac tissue at the level of transmembrane-facilitated transport, especially under conditions of increased cardiac load.
Nutrition and physical activity are important in preserving the energy balance of the body. Exercise may be less effective in weight management when it is not supported by diet (24). An interaction between HFD and physical activity has been shown with postprandial lipidemia and inflammation in humans (25). Other studies revealed that HFD results in obesity (26, 27) and increases body weight (28–31). Accordingly, here we showed that the body weight increased during 4 weeks of a fructose-rich diet. The findings also suggest that 5 days of physical exercise effectively inhibited the fructose-induced weight gain (Fig. 1). It is noteworthy that the time duration of exercise to prevent weight gain in the present investigation is considerably shorter than that published in previous studies, i.e., 5 (27) or 10 weeks (29) of exercise after a period of fructose feeding.
Both dynamic and static exercise causes hypertrophy of the heart. The degree of hypertrophy appears to depend on the type of sport and its duration and intensity (32). In our study, physical exercise did not result in cardiac hypertrophy. The reason may be the relatively short duration of exercise. Cardiac hypertrophy was determined in a great number of studies where the subjects were exposed to a long duration of exercise and/or intense exercise (32, 33).
Study limitations
A limitation of the present study is that the fructose and glycerol levels in the blood as well as the uptake of these substrates by the myocardium were not measured. Therefore, no firm conclusions can be drawn on the effect of exercise and high-fructose feeding on the cardiac uptake rate of these two energy substrates in vivo.
Conclusion
In conclusion, the present study has shown that a fructose-rich diet for 28 days did not increase the amount of GLUT5 channels in cardiac tissue, which may exclude the regulatory effect of fructose on this fructose transport protein in rat hearts exposed to increased amounts of fructose. In addition, our findings showed that physical exercise increased cardiac AQP7 gene expression and protein content in rats. However, HFD decreased cardiac AQP7 gene expression and protein content in rats subjected to exercise. Fructose-induced mitigation of cardiac AQP7 expression after physical exercise strongly suggests a competition between fructose and glycerol in cardiac energy metabolism at the level of glycerol transport into cardiomyocytes. AQP7, as an aquaglyceroporin, should be taken into account when planning further studies that investigate the effect of exercise and HFD on cardiac energy metabolism.
Acknowledgments
The study was financially supported by the Trakya University Scientific Research Projects Unit (2013/76).
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.K., S.A.V.; Design – A.K., O.P., S.A.V.; Supervision – S.A.V., A.K., F.N.T.; Fundings – Trakya University Research Fone, A.K.; Materials – O.P., E.T.; Data collection &/or processing – O.P., E.T., A.K.; Analysis &/or interpretation – F.N.T., E.T., O.P., A.K.; Literature search – A.K.; Writing – S.A.V., A.K.; Critical review – S.A.V., A.K.; Other – O.P., E.T., F.N.T.

From Prof. Dr. Arif Aksit’s collection
References
- 1.Van der Vusse GJ, Glatz JF, Stam HC, Reneman RS. Fatty acid homeostasis in the normoxic and ischemic heart. Physiol Rev. 1992;72:881–40. doi: 10.1152/physrev.1992.72.4.881. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH. The Heart;Fuels: aerobic and anaerobic metabolism. In: Lionel H Opie., editor. Heart Physiology: from cell to circulation. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 306–9. [Google Scholar]
- 3.Mellor KM, Wendt IR, Ritchie RH, Delbridge LM. Fructose diet treatment in mice induces fundamental disturbance of cardiomyocyte Ca2+ handling and myofilament responsiveness. Am J Physiol Heart Circ Physiol. 2012;15:H964–72. doi: 10.1152/ajpheart.00797.2011. [DOI] [PubMed] [Google Scholar]
- 4.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 5.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012;23:203–8. doi: 10.1016/j.jnutbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Byars A, Keith S, Simpson W, Mooneyhan A, Greenwood M. The influence of a pre-exercise sports drink (PRX) on factors related to maximal aerobic performance. J Int Soc Sports Nutr. 2010;7:12. doi: 10.1186/1550-2783-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeukendrup AE. Carbohydrate and exercise performance: the role of multiple transportable carbohydrates. Curr Opin Clin Nutr Metab Care. 2010;13:452–7. doi: 10.1097/MCO.0b013e328339de9f. [DOI] [PubMed] [Google Scholar]
- 8.Noh HL, Hu Y, Park TS, DiCioccio T, Nichols AJ, Okajima K, et al. Regulation of plasma fructose and mortality in mice by the aldose reductase inhibitor lidorestat. J Pharmacol Exp Ther. 2009;328:496–503. doi: 10.1124/jpet.108.136283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinman RD, Fine EJ. Fructose in perspective. Nutr Metab (Lond) 2013;10:45. doi: 10.1186/1743-7075-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mellor KM, Bell JR, Wendt IR, Davidoff AJ, Ritchie RH, Delbridge LM. Fructose modulates cardiomyocyte excitation-contraction coupling and Ca2+ handling in vitro. PLoS One. 2011;6:e25204. doi: 10.1371/journal.pone.0025204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozlowski S, Nazar K, Brzezinska Z, Stephens D, Kaciuba-Uscilko H, Kobryn A. Mechanism of sympathetic activation during prolonged physical exercise in dogs. The role of hepatic glucoreceptors. Pflugers Arch. 1983;399:63–7. doi: 10.1007/BF00652523. [DOI] [PubMed] [Google Scholar]
- 12.Hibuse T, Maeda N, Nakatsuji H, Tochino Y, Fujita K, Kihara S, et al. The heart requires glycerol as an energy substrate through aquaporin 7, a glycerol facilitator. Cardiovasc Res. 2009;83:34–41. doi: 10.1093/cvr/cvp095. [DOI] [PubMed] [Google Scholar]
- 13.Dickson EW, Hogrefe CP, Ludwig PS, Ackermann LW, Stoll LL, Denning GM. Exercise enhances myocardial ischemic tolerance via an opioid receptor-dependent mechanism. Am J Physiol Heart Circ Physiol. 2008;294:H402–H8. doi: 10.1152/ajpheart.00280.2007. [DOI] [PubMed] [Google Scholar]
- 14.Weeks KL, Gao X, Du XJ, Boey EJ, Matsumoto A, Bernardo BC, et al. Phosphoinositide 3-kinase p110 alpha is a master regulator of exercise-induced cardioprotection and PI3K gene therapy rescues cardiac dysfunction. Circ Heart Fail. 2012;5:523–34. doi: 10.1161/CIRCHEARTFAILURE.112.966622. [DOI] [PubMed] [Google Scholar]
- 15.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Jiang S, Hu Y, Zheng X, Zou S, Wang Y, et al. The expression of aquaporin 8 and aquaporin 9 in fetal membranes and placenta in term pregnancies complicated by idiopathic polyhydramnios. Early Hum Dev. 2010;86:657–63. doi: 10.1016/j.earlhumdev.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Medina Villaamil V, Aparicio Gallego G, Valbuena Rubira L, Garcia Campelo R, Valladares-Ayerbes M, Grande Pulido E, et al. Fructose transporter GLUT5 expression in clear renal cell carcinoma. Oncol Rep. 2011;25:315–23. doi: 10.3892/or.2010.1096. [DOI] [PubMed] [Google Scholar]
- 18.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–5. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–38. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gambert S, Helies-Toussaint C, Grynberg A. Extracellular glycerol regulates the cardiac energy balance in a working rat heart model. Am J Physiol Heart Circ Physiol. 2007;292:H1600–6. doi: 10.1152/ajpheart.00563.2006. [DOI] [PubMed] [Google Scholar]
- 21.Hibuse T, Maeda N, Nagasawa A, Funahashi T. Aquaporins and glycerol metabolism. Biochim Biophys Acta. 2006;1758:1004–11. doi: 10.1016/j.bbamem.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Rogers PJ, Tyce GM, Weinshilboum RM, O’Connor DT, Bailey KR, Bove AA. Catecholamine metabolic pathways and exercise training. Plasma and urine catecholamines, metabolic enzymes, and chromogranin-A. Circulation. 1991;84:2346–56. doi: 10.1161/01.cir.84.6.2346. [DOI] [PubMed] [Google Scholar]
- 23.Basco D, Blaauw B, Pisani F, Sparaneo A, Nicchia GP, Mola MG, et al. AQP4-dependent water transport plays a functional role in exercise-induced skeletal muscle adaptations. PLoS One. 2013;8:e58712. doi: 10.1371/journal.pone.0058712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns DJ, Hartmann-Boyce J, Jebb SA, Aveyard P. Behavioural Weight Management Review Group Diet or exercise interventions vs. combined behavioral weight management programs: a systematic review and meta-analysis of direct comparisons. J Acad Nutr Diet. 2014;114:1557–68. doi: 10.1016/j.jand.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bidwell AJ, Fairchild TJ, Redmond J, Wang L, Keslacy S, Kanaley JA. Physical activity offsets the negative effects of a high-fructose diet. Med Sci Sports Exerc. 2014;46:2091–8. doi: 10.1249/MSS.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bursac BN, Djordjevic AD, Vasiljevic AD, Milutinovic DD, Velickovic NA, Nestorovic NM, et al. Fructose consumption enhances glucocorticoid action in rat visceral adipose tissue. J Nutr Biochem. 2013;24:1166–72. doi: 10.1016/j.jnutbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Rippe JM, Angelopoulos TJ. Sucrose high-fructose corn syrup, and fructose, their metabolism and potential health effects: what do we really know? Adv Nutr. 2013;4:236–45. doi: 10.3945/an.112.002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moraes-Silva IC, Mostarda C, Moreira ED, Silva KA, dos Santos F, de Angelis K, et al. Preventive role of exercise training in autonomic, hemodynamic, and metabolic parameters in rats under high risk of metabolic syndrome development. J Appl Physiol. 2013;15(114):786–91. doi: 10.1152/japplphysiol.00586.2012. [DOI] [PubMed] [Google Scholar]
- 29.Mostarda C1, Moraes-Silva IC, Salemi VM, Machi JF, Rodrigues B, De Angelis K, et al. Exercise training prevents diastolic dysfunction induced by metabolic syndrome in rats. Clinics. 2012;67:815–20. doi: 10.6061/clinics/2012(07)18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morvan E, Lima NE, Machi JF, Mostarda C, De Angelis K, Irigoyen MC, et al. Metabolic, hemodynamic and structural adjustments to low intensity exercise training in a metabolic syndrome model. Cardiovasc Diabetol. 2013;12:89. doi: 10.1186/1475-2840-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beigy M, Vakili S, Berijani S, Aminizade M, Ahmadi-Dastgerdi M, Meshkani R. Alternate-day fasting diet improves fructose-induced insulin resistance in mice. J Anim Physiol Anim Nutr. 2013;97:1125–31. doi: 10.1111/jpn.12022. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes T, Soci UP, Oliveira EM. Eccentric and concentric cardiac hypertrophy induced by exercise training: microRNAs and molecular determinants. Braz J Med Biol Res. 2011;44:836–47. doi: 10.1590/s0100-879x2011007500112. [DOI] [PubMed] [Google Scholar]
- 33.Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008;40:2023–39. doi: 10.1016/j.biocel.2008.02.020. [DOI] [PubMed] [Google Scholar]