Abstract
Objective:
Viscum album L. has favorable cardiovascular effects including antihypertensive and vasorelaxant activity, and the nitric oxide (NO) pathway upregulation has been proposed to be the underlying mechanism. NO also plays an important role in the pathophysiology of heart failure. However, its effects on cardiac systolic function are unclear.
Methods:
A total of 30 male Wistar albino rats at 12 weeks of age were randomly divided into three groups: control, isoproterenol-induced heart failure group (ISO), and isoproterenol-induced heart failure + V. album treatment group (VA) groups (n=10 in each group). V. album was orally given at a dose of 250 mg/kg/day by gavage. Parameters of heart failure were compared among the groups. Tamhane’s T2 test, paired sample t-test, and Bonferroni methods were used for statistical analysis.
Results:
V. album resulted in an improvement in all parameters of heart failure including left ventricular diameters (6.34±0.23 mm, 6.98±0.35 mm, and 6.71±0.10 mm for left ventricular end-diastolic diameter in control, ISO, and VA groups, respectively, p<0.05), ejection fraction (73.3±3.1%, 56.7±2.6%, and 65.2±1.5% for control, ISO, and VA groups, respectively, p<0.05), serum NT-proBNP levels, and histopathological changes. V. album treatment resulted in a statistically significant attenuation of increased levels of NO and iNOS (p<0.0001). The levels of hs-CRP were also found to be lower in the VA group compared with the controls and ISO groups (p<0.01).
Conclusion:
V. album exerted favorable effects on left ventricular function in isoproterenol-induced heart failure rats. Upregulation of the NO pathway seems to be the possible pathophysiological mechanism. Favorable vascular outcomes can also be speculated considering the reduction in serum hs-CRP levels.
Keywords: heart failure, Viscum album, isoproterenol
Introduction
Heart failure (HF) remains to be one of the leading causes of morbidity and mortality (1). In fact, it is a progressed end-stage of various cardiac disorders, and the common final outcome is cardiac overload or myocardial injury leading to insufficiency in supplying blood to meet the metabolic needs of the body. The most prominent characteristics of heart failure are impairment of active relaxation and contraction of the left ventricle, ventricular remodeling, ventricular hypertrophy, and reduced ejection fraction (2).
Although current medical treatment options, such as digoxin, diuretics, angiotensin converting enzyme inhibitors, and beta blockers, are widely used, most of them can only provide symptomatic relief. Long-term beta adrenergic blocking is the only pharmacologic intervention that reverses left ventricular remodeling. Nevertheless, whether beta adrenergic blocking prevents or delays left ventricular remodeling is unclear. Moreover, there is still no consensus about the phenotype of patients who will derive a mortality benefit from implantable cardioverter defibrillator therapy (3). On the other hand, sophisticated treatment modalities such as heart transplantation and mechanical circulatory support can only be delivered to a very small fraction of patients with systolic HF. All these warrant more new therapeutic approaches for the treatment of HF.
Aqueous extracts of the European mistletoe Viscum album L. have been widely used as a medicinal plant, especially for the treatment of oncologic disorders, due to their cytotoxicity against tumor cells and immunomodulating activity (4). V. album has also been extensively studied in cardiovascular research, especially in the field of hypertension, and favorable effects have been identified. The reduction in both systolic and diastolic blood pressure was attributed to a mechanism involving upregulation in the nitric oxide (NO) pathway (5). The association between V. album and NO has been supported by other clinical and experimental studies (6, 7). Because it is considered that V. album exerts positive cardiovascular effects via the NO pathway, it may also have favorable effects on HF as NO also plays an important role in the pathophysiology of HF (8).
Methods
This experimental animal study was designed to evaluate the possible effects of V. album on cardiac function through the NO pathway in isoproterenol-induced HF rats.
Animal preparation
This study was conducted in the experimental animal laboratory of Giresun University between March 2015 and May 2015. All protocols and surgical procedures were approved by the Institute of Animal Care and Use Committees. (Approval No: 58380337/370-215). A total of 30 male Wistar albino rats at 12 weeks of age weighing 250±50 g were supplied by Kobay Laboratory Animal Center (Ankara, Turkey). All animals were maintained on standard rat chow and water ad libitum and were individually housed in separate cages in a 12-h dark–light cycle, ambient temperature (21±2°C), and moisture (%40–60)-controlled room. The study conformed to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes or the NIH guidelines.
Experimental protocols
The animals were randomly divided into three groups: control, ISO (experimental isoproterenol-induced HF group), and VA (experimental isoproterenol-induced HF + V. album treatment group) groups (n=10 in each group). Isoproterenol (DL-Isoproterenol hydrochloride®, Product Number:I5627, Sigma-Aldrich, USA) was dissolved in normal saline and subcutaneously injected into rats in the ISO and VA groups at a dose of 5 mg, once daily for 14 consecutive days to induce experimental HF as previously described (9). A matched group of control rats simultaneously received saline injections. On the 14th day, all the rats underwent echocardiographic evaluation and 1 mL of blood was obtained via a 26 D branule (BD Neoflon®, Helsingborg, Sweden) for biochemical examination. Starting from the 14th day, rats in Control and ISO groups were given 1 mL/day of saline with a gavage needle for 10 days whereas rats in the VA group were treated orally with aqueous extract of V. album at a dose of 250 mg/kg/day by gavage. The animals in the ISO and VA groups continued to receive subcutaneous injections of 5 mg/kg/day isoproterenol to prevent spontaneous regression of the HF. On day 24, echocardiography and biochemical evaluation of NT-proBNP, hs-CRP, NO products, and iNOS were performed for the last time before rats were sacrificed. Hearts were removed, freed of connective tissue, washed extensively with saline to remove all contaminating blood, and weighed. The received heart samples were placed in 10% formaldehyde solution for histopathological examination. All interventional procedures and echocardiographic evaluation in rats were performed under anesthesia. Anesthesia was induced via 80 mg/kg ketamine hydrochloride (Ketalar® vials, 50 mg/mL, Eczacıbaşı, Co, Ltd, Istanbul, Turkey) intraperitoneally (ip) and 5 mg/kg xylazine hydrochloride (Rompun® vials, 23.32 mg/mL, Bayer, Istanbul, Turkey)ip. Body weights of all rats were recorded in the beginning, on the 14th day, and at the end of the study.
Echocardiographic assessment
On days 14 and 24, transthoracic echocardiography was performed on the rats under ketamine hydrochloride anesthesia (80 mg/kg). Razor blades were used to scrape the chest hair in the left lateral position. Two-dimensional, targeted M-mode tracings were obtained with GE Vivid S5 echocardiographic system (General Electric VingMed Systems, Horten, Norway) equipped with a 12-MHz linear array transducer. The left ventricular end-systolic diameter (LVESD, mm), left ventricular end-diastolic diameter (LVDDD, mm), left ventricular fractional shortening (LVFS, %), and left ventricular ejection fraction (LVEF, %) were measured during five heartbeats, and the values were averaged. LVFS was calculated as (LVEDD % – LVESD)/LVEDD x 100. Ejection fraction was calculated as follows: ejection fraction (%)=[(LVDV – LVSV)/LVDV] x 100, where LVDV and LVSV are left ventricular end-diastolic and end-systolic volumes, respectively. All evaluations were performed by an experienced physician familiar with the study design.
Measurements of NT-proBNP, NO, iNOS, and hs-CRP
Blood samples were collected in tubes containing heparin as an anticoagulant. The plasma samples were separated from the cells by centrifugation for 15 min at 1000 xg at 2–8°C within 30 min of collection. The supernatants were quickly frozen and stored at –80°C until use. Plasma levels of NT-proBNP, inducible nitric oxide synthase (iNOS), and hs-CRP were determined using commercially available sandwich-enzyme linked immunosorbent assay kits (Elabscience Biotechnology Co., Ltd, WuHan, P.R.C.). The minimum detectable doses of hs-CRP, iNOS, and NT-proBNP were 0.1 ng/mL, 0.19 ng/mL, and 18.75 pg/mL, respectively. The reported coefficients of variation for all three parameters were <10%. Plasma levels of NO were spectrophotometrically assayed by measuring the accumulation of nitrite and nitrate that its stable degradation products (10). Nitrite levels were detected after conversion of nitrate to nitrite by metallic cadmium. It was accepted that 1 µmol/L NaNO2 was equivalent to 1 µmol/L NO and the standard curve was represented in NO µmol/L equivalents.
Histopathological analysis and immunofluorescence staining
The hearts were excised and placed in 10% formalin. Heart sections (4–5µm thick) were prepared and stained with hematoxylin–eosin (HE) for histopathology and then visualised using light microscopy. Immunohistochemistry was performed to localize the brain natriuretic peptide (BNP) in cardiomyocytes. Fixed (10% formaldehyde), parafin-embedded sections of control and experimental groups were stained using the BNP primer antibody (Santa Cruz Biotechnology, Inc.) according to the manufacturer’s protocols. Fluorescein isothiocyanate (FITC)-conjugated secondary antibody was used for immunodetection, and fluorescence microscope was used for visualization. Nuclei were counterstained with 4¢,6-diamidino-2-phenylindole (DAPI, Santa Cruz Biotechnology, Inc.). Images were captured at 400x magnification from five fields/case. The positive areas of the histological sections were quantified using an image quantitative digital analysis system (Image-Pro Plus 6.0). In each animal tissue section, cells that radiated FITC were counted and the ratio of the number of these cells to the total cells in the same field was determined. All measurements were performed in a blind manner.
Preparation of the extract
Leaves of V. album ssp. album were harvested in March from the host plant, Pyrus communis L. ssp. communis (common pear) in the center of Giresun, a city in the east of the Black Sea region of Turkey. The dried and powdered leaves of the plant V. album ssp. album (500 g) were extracted with 80% methanol (25 L) at room temperature several times, and the process was checked using thin layer chromatography. The extract was concentrated under reduced pressure in a rotary evaporator to yield a gum-like brown extract and then refrigerated at 4°C until use. A portion of the extract was reconstituted in 0.9% NaCl before the experiment. The duration of pretreatment and test doses of V. album ssp. album were determined on the basis of preliminary studies. The dose was determined as 250 mg/kg/day orally as previously described (11).
Statistical analysis
Number Cruncher Statistical System 2004 (NCSS systems, Kaysville, UT) and MedCalc (MedCalc software, Broekstraat, Mariakerke, Belgium) were used for statistical analysis. The Kolmogorov–Smirnov and Shapiro–Wilk tests were used for testing the normality of the distributions. Results were expressed as mean±standard deviation (SD). Statistical comparisons among independent groups were performed using one-way analysis of variance (ANOVA). Repeated measure ANOVA or paired sample t-test was used for statistical comparisons among paired groups. Posthoc analysis was performed using Tamhane’s T2 test for independent groups or Bonferroni method for paired groups. A probability value (p) of <0.05 was considered statistically significant.
Results
Animal mortality
The mortality in both the experiment groups was 20% throughout the protocol. Deaths occurred at the 10th and 13th days and at the 11th and the 13th days in the ISO and VA groups, respectively. All rats in the control group were alive before sacrifice. Initial body weight measurements were compared among three groups, each containing 10 rats. The 14th-day and final evaluations were carried out considering 10, 8, and 8 animals in the control, ISO, and VA groups, respectively.
General characteristics
The initial body weights of the animals in the three groups were similar (Table 1, n=10 for each group). On day 14, body weights of the controls significantly increased compared with initial values, and this increase was more prominent at the end of the study (n=10). No significant change was seen in the experiment groups at the 14th and 24th days (n=8 for each group). Moreover, the final body weights did not differ compared with each other and controls. Secondary to hypertrophy of the ventricles, the heart weight was much more in the ISO group than in controls. Although the animals in the VA group also had heavier hearts, the increase in the VA group was lower than in the ISO group. Similarly, both experiment groups had higher heart weight/body weight ratio compared with controls. The heart weight/body weight ratio was lower in the VA group compared with the ISO group, but the difference was not statistically significant.
Table 1.
Effects of V. album on body weight and heart weight in rats with heart failure (mean±S.D.)
| Groups | Body weight, g | Heart weight, g | Heart weight/body weight | |||
|---|---|---|---|---|---|---|
| Initial day¶ | 14th day | Final day | P# | |||
| Control, n=10 | 279.1±29.0 | 290.6±25.5d | 296.5±23.8e | =0.0020 | 1.29±0.12 | 4.36±0.41 |
| ISO, n=8 | 292.8±35.0 | 286.3±40.1 | 286.9±37.4 | =0.5530 | 1.65±0.13c | 5.82±0.77b |
| VA, n=8 | 286.9±30.2 | 282.6±29.7 | 275.9±28.4 | =0.2590 | 1.50±0.16a | 5.46±0.58b |
| P* | =0.6650 | =0.8690 | =0.3630 | <0.0001 | <0.0001 | |
SO - isoproterenol group; VA - V. album treatment group.
The initial day body weight comparison was performed between three groups each containing 10 rats;
P was calculated using one-way ANOVA;
P was calculated using repeated measure ANOVA;
Posthoc: P<0.05 vs. control group;
Posthoc: P<0.01 vs. control group;
Posthoc: P<0.000 vs. control group;
Posthoc: P<0.01 vs. initial day;
EPosthoc: P<0.001 vs. initial day
Effects of V. album on echocardiographic parameters
Tables 2 and 3 show the echocardiographic parameters. On the 14th day, interventricular septum and posterior wall thickness were significantly higher in both the experiment groups compared with controls (n=10, n=8, and n=8 for the control, ISO, and VA groups, respectively). This profile was consistent till the end of the experiment. Final values in the ISO group were higher compared with those on the 14th day, whereas there was an evident reduction in values in the VA group at the end compared with those on the 14th day. Similarly, LVEDD and LVESD were higher in ISO and VA groups on day 14 compared with controls. LVESD was higher at the end in both experiment groups compared with controls. LVEDD was higher in the ISO group on the 24th day compared with that on the 14th day, whereas a slight reduction in LVEDD was observed in the VA group compared with the 14th day. Mean LVEF was similar in the control group on days 14 and 24. There was a significant LVEF reduction in the ISO group on day 24 compared with that day 14. The animals in the ISO group also had lower LVEF compared with controls. Although there was a slight reduction in LVEF in the VA group on the 24th day, the mean value was significantly higher compared with the ISO group. The control group had similar heart rates on the 14th and 24th day. The heart rate in the ISO group was also similar on the 14th and 24th day but was significantly higher compared with controls. The heart rate in the VA group on the 14th day was also higher compared with controls. However, there was a significant reduction in the heart rate on the 24th day.
Table 2.
Effects of V. album on left ventricular diameters in heart failure rats (mean±S.D.)
| Parameters | Measurement time | Control, n=10 | ISO, n=8 | VA, n=8 | P* |
|---|---|---|---|---|---|
| IVS, mm | 14th day | 1.26±0.11 | 1.91±0.15c | 1.88±0.09c | <0.0001 |
| Final day | 1.24±0.08 | 1.98±0.10c | 1.79±0.06c,d | <0.0001 | |
| P# | =0.3430 | =0.0110 | =0.0060 | ||
| PW, mm | 14th day | 1.32±0.11 | 1.91±0.14c | 1.98±0.12c | <0.0001 |
| Final day | 1.28±0.10 | 1.96±0.13c | 1.75±0.05c,d | <0.0001 | |
| P# | =0.1040 | =0.0330 | =0.0020 | ||
| LVEDD, mm | 14th day | 6.34±0.26 | 6.71±0.15a | 6.73±0.18a | <0.0010 |
| Final day | 6.34±0.23 | 6.98±0.35a | 6.71±0.10b | <0.0001 | |
| P# | =1.000 | =0.0320 | =0.7320 | ||
| LVESD, mm | 14th day | 3.63±0.19 | 3.99±0.06c | 3.98±0.14b | <0.0001 |
| Final day | 3.59±0.17 | 4.84±0.21c | 4.25±0.09c,e | <0.0001 | |
| P# | =0.1040 | <0.0001 | <0.0001 |
ISO - isoproterenol group; IVS - interventricular septum; LVEDD - left ventricular end-diastolic diameter; LVESD - left ventricular end-systolic diameter; PW - posterior wall; VA - V. album treatment group.
P was calculated using one-way ANOVA;
P was calculated using paired sample t-test;
Posthoc: P<0.01 vs. control group;
Posthoc: P<0.001 vs. control group;
Posthoc: P<0.0001 vs. control group
Table 3.
Effects of V. album on heart rate, left ventricular volume, and function in heart failure rats (mean±S.D.)
| Parameters | Measurement time | Control, n=10 | ISO, n=8 | VA, n=8 | P* |
|---|---|---|---|---|---|
| Heart rate | 14th day | 318±16 | 358±15c | 353±10c | <0.0001 |
| Final day | 315±9 | 358±15c | 337±9c,d | <0.0001 | |
| P# | =0.2640 | =0.9650 | =0.0020 | ||
| LVEDV | 14th day | 203.8±19.5 | 232.0±11.7a | 228.5±22.1 | =0.0060 |
| Final day | 203.7±17.0 | 253.8±30.0a | 232.0±7.9b | <0.0001 | |
| P# | =0.9730 | =0.0380 | =0.6960 | ||
| LVESV | 14th day | 53.8±9.7 | 69.0±1.9a | 68.5±5.4a | <0.0001 |
| Final day | 54.2±6.3 | 109.3±11.6c | 80.8±4.1c,e | <0.0001 | |
| P# | =0.8430 | <0.0001 | <0.0001 | ||
| LVFS | 14th day | 42.4±3.3 | 40.5±1.2 | 40.9±2.1 | =0.2280 |
| Final day | 43.3±2.9 | 30.6±1.8c | 36.6±1.2c,e | <0.0001 | |
| P# | =0.0650 | <0.0001 | <0.0001 | ||
| LVEF | 14th day | 72.6±3.7 | 70.2±1.1 | 69.8±3.9 | =0.1590 |
| Final day | 73.3±3.1 | 56.7±2.6c | 65.2±1.5c,e | <0.0001 | |
| P# | =0.1120 | <0.0001 | =0.0140 |
ISO - isoproterenol group; LVEDV - left ventricular end-diastolic volume; LVEF - left ventricular ejection fraction; LVESV - left ventricular end-systolic volume; LVFS - left ventricular fractional shortening; VA - V. album treatment group.
P was calculated using one-way ANOVA;
P was calculated using paired sample t-test;
Posthoc: P<0.01 vs. control group;
Posthoc: P<0.001 vs. control group;
Posthoc: P<0.000 vs. control group;
Posthoc: P<0.05 vs. isoproterenol group;
Posthoc: P<0.000 vs. isoproterenol group
Biochemical evaluation
Serum levels of NT-proBNP were similar on the 14th and 24th day in the control group (n=10). The rats in the ISO group (n=8) had significantly higher values on the 14th and 24th day and this was indicative of HF development. The VA group (n=8) also had significantly higher values on the 14th and 24th day compared with controls. However, compared with the ISO group, serum levels of NT-proBNP were significantly lower in the VA group on the 24th day. NO products also did not differ in the control group between the 14th and 24th day. The mean values were significantly higher in the ISO group on both the 14th and 24th day compared with controls. The VA group also had significantly higher values compared with controls, but the levels were significantly lower on the 24th day compared with the ISO group. The same was true for the serum levels of iNOS. Although the serum levels of iNOS were lower on the 24th day in the VA group compared with the ISO group, this could not reach a statistical significance. The animals in the control and ISO groups had similar serum levels of hs-CRP on the 14th and 24th day. There was no significant difference when the three groups were compared with each other; however, a significant reduction was prominent in the VA group on the 24th day compared with the 14th day (Table 4).
Table 4.
Evaluation of biochemical parameters
| Parameters | Measurement time | Control, n=10 | ISO, n=8 | VA, n=8 | P* |
|---|---|---|---|---|---|
| NT-proBNP, pg/mL | 14th day | 163.4±5.6 | 246.7±21.4c | 242.5±21.8c | <0.0001 |
| Final day | 163.1±6.7 | 351.1±30.5c | 286.2±15.9c,d | <0.0001 | |
| P# | =0.8940 | <0.0001 | <0.0001 | ||
| NO equivalents, μmol/L | 14th day | 17.8±2.8 | 33.2±4.4c | 29.5±4.3c | <0.0001 |
| Final day | 19.0±3.3g | 48.5±2.3c | 37.8±3.1c,e | <0.0001 | |
| P# | <0.0010 | <0.0001 | <0.0010 | ||
| iNOS, ng/mL | 14th day | 2.83±0.53 | 3.89±0.31c | 3.81±0.25b | <0.0001 |
| Final day | 2.57±0.47 | 4.67±0.95b | 3.90±0.35c | <0.0001 | |
| P# | =0.1930 | =0.0450 | =0.2660 | ||
| hs-CRP, ng/mL | 14th day | 0.164±0.022 | 0.159±0.018 | 0.172±0.052 | =0.7320 |
| Final day | 0.183±0.034 | 0.155±0.025 | 0.133±0.023a | =0.0040 | |
| P# | =0.2340 | =0.5340 | =0.1360 |
hs-CRP - high sensitive C reactive protein; iNOS - inducible nitric oxide synthase; ISO - isoproterenol group; NO - nitric oxide equivalents; NT - proBNP-N-terminal pro-brain natriuretic peptide; VA - V. album treatment group.
P was calculated using one-way ANOVA;
P was calculated using paired sample t-test;
Posthoc: P<0.01 vs. control group;
Posthoc: P<0.001 vs. control group;
Posthoc: P<0.0001 vs. control group;
Posthoc: P<0.001 vs. isoproterenol group;
Posthoc: P<0.0001 vs. isoproterenol group
Histomorphological and immunohistochemical findings
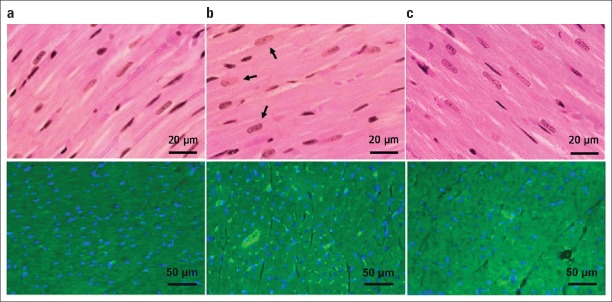
The rats in the ISO group had obviously large and rectangular-shaped nuclei compared with the other groups in the hematoxylin–eosin staining (n=10, n=8, and n=8 for the control, ISO, and VA groups, respectively). In the VA group, the size of the nuclei in cardiomyocytes was observed to be similar to that of those in the control group. The presence of BNP in cardiomyocytes was shown by immunofluorescence staining (Fig. 1). Cells that radiated green with FITC in the cell cytoplasm were counted in a section in each group. All cells were also counted in the same field. The ratio of positively stained cells to total number of cardiomyocytes was calculated to be 71%, 99%, and 80% in the control, ISO, and VA groups, respectively (Fig. 2).
Figure 1.
Histological analysis of the left ventricle via hematoxylin–eosin staining (above). A represents myocardium of a normal rat (n=10). B represents myocardium of a rat in the ISO group (n=8). The arrows show large and rectangular-shaped nuclei. C represents myocardium of a rat in the VA group (n=8). Images were acquired at 400x magnification. Fluorescence micrographs of the left ventricular myocytes of the control, ISO, and VA groups (Below). Yellow represents cells that radiate FITC. Blue shows the nuclei of cardiomyocytes which were counterstained with DAPI. A. Rat cardiomyocyte in the control group (n=10). B. Increased FITC radiation representing increased BNP secretion in the isoproterenol-induced HF (n=8). C. Isoproterenol-induced HF and hence increased BNP secretion was attenuated by V. album treatment (n=8)
Figure 2.
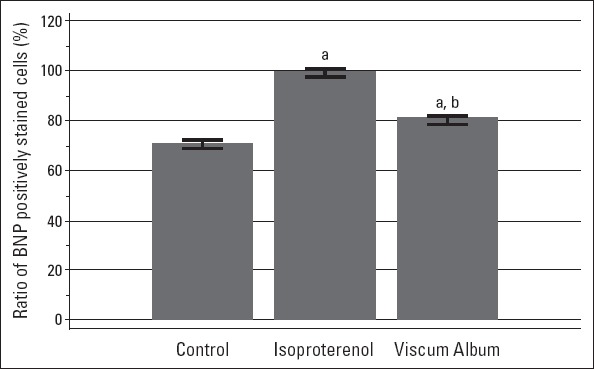
Bars represent 95% CI of mean. The means of ratio of BNP positively stained cells were 71.0%, 99.2%, and 80.7% for the control (n=10), ISO (n= 8), and VA (n=8) groups, respectively.
aTamhane’s T2 test, P<0.0001 vs. control group
bTamhane’s T2 test, P<0.0001 vs. isoproterenol group
Discussion
The present study demonstrated that V. album administration was effective in improving left ventricular dysfunction; reducing the serum levels of NT-pro-BNP, NO, iNOS; and ameliorating cardiac hypertrophy and histopathological changes in isoproterenol-induced HF rats. Upregulation of the NO pathway seems to be the underlying pathophysiological mechanism. We first showed that emergence of HF via administration of isoproterenol led to an increase in NO expression. We subsequently found that V. album treatment improved histopathological changes, hypertrophy of the left ventricles, and biochemical parameters including NO.
Although various noninvasive and invasive experimental models are available, the isoproterenol-induced HF is one of the most commonly used experimental models to investigate the effects of several drugs on HF. The cardiac pathophysiological and morphologic alterations of this noninvasive myocardial necrotic rat model are comparable with those taking place in human HF (12). The basic pathophysiological mechanism in this model is that acute isoproterenol administration produces tachycardia associated with relative ischemia due to an imbalance between increased myocardial oxygen demand and reduced coronary blood supply (13).
The prominent impact of V. album on cardiovascular system is the vasorelaxant activity (14-16). In addition, antihypercholesterolaemic effects are demonstrated (17). Moreover, recent clinical trials have demonstrated the favorable effects of V. album on hypertension (18). Reduction in the cyclophosphamide-induced cardiotoxicity via administration of V. album was reported by Şekeroğlu et al. (11). This finding was significant in terms of speculating positive effects of V. album on cardiac functions. Consistently, Tenorio-Lopez et al. (6) reported that V. album aqueous extract induces NOS-2 and NOS-3 overexpression in Guinea pig hearts. These findings were promising as impaired NOS activity and reduced NO bioavailability are common initiators of cardiovascular dysfunction (19). Three different NO synthase (NOS) isoforms (neuronal, inducible, and endothelial NOS) synthesize NO in the human cardiovascular system (20). Dysfunction of NOS (i.e., altered expression, location, coupling, and activity) exists in various cardiac disease conditions, such as HF, contributing to contractile dysfunction, adverse remodeling, and hypertrophy. In fact, NO is necessary for normal cardiac physiology and has a protective role in the ischemic heart; however, it is overproduced in the failing myocardium as a result of the increased expression and activity of the inducible isoform of NOS (20, 21). This results in an un-coupling between NO and reactive oxygen species leading to loss of antioxidant properties. On the other hand, neuronal and endothelial NOS predominantly represent a physiological role such that favorable effects of many drugs are thought to depend on upregulation of endothelial NOS and neuronal NOS (19). Lectins and viscotoxins, which are well-described and most active phytochemicals identified in V. album, play a substantial role in cancer treatment because of their apoptotic and cytotoxic effects. Phenolic acids, phenylpropanoids, and flavonoids with antioxidant and anti-inflammatory activities, which are another group of compounds found in mistletoe, have been shown to decrease blood pressure and may be responsible for favorable cardiovascular effects observed in our study (22). The extraction method of the plant was also selected considering these compounds. Direct effects of V. Album on left ventricular function have not been studied yet and remain to be identified. In the present study, the left ventricle was hypertrophied, the left ventricular systolic function was deteriorated, and serum level of NT-pro-BNP was increased in all rats in the ISO and VA groups on the 14th day, suggesting that the isoproterenol-induced HF model was successfully achieved. Low-dose ISO-induced hypertrophy ceases rapidly after termination of isoproterenol administration (23). Thus, application of low-dose (5 mg/kg/day) isoproterenol was continued for 10 more days simultaneously with V. album in the VA group and singly in the ISO group after induction of the HF model in 14 days in both groups. It was aimed to prevent the spontaneous regression of left ventricular hypertrophy. Therefore, the regression in the left ventricular hypertrophy can be attributed to use of V. album rather than diminishment of isoproterenol effect. Prolonged treatment using low dose of isoproterenol (5 mg/kg for 21 days) is also an option for this model. Furthermore, it was demonstrated that an electrically and structurally compensated cardiac hypertrophy model can be produced by a short-term treatment of the animals with isoproterenol, while a long-term treatment causes a decompensated HF (24). In our study, generating a decompensated HF model and then starting the therapy was also significant in terms of monitoring the therapeutic effects of V. album in established HF. Reduction in the heart weight/body weight ratio, left ventricular wall thickness, serum NT-pro-BNP, NO and iNOS levels, cell count in immune-staining, and increase in the left ventricular ejection fraction were all compatible with each other supporting the favorable effects of V. album. Concomitance of biochemical, immune-histochemical, and radiological improvement was noteworthy. Although having prognostic significance in HF, hs-CRP is not a marker for developing cardiac systolic dysfunction (25). It rather reflects low-grade inflammation and provides prognostic information about cardiovascular events. Similarity in the hs-CRP levels in three groups on the 14th day can be thus explained. The reduction in the VA group on the 24th day is of importance since it enables to speculate favorable effects of V. album on cardiovascular outcomes in addition to systolic function.
Study limitations
Whether these positive effects are secondary to blood pressure lowering activity of V. album is controversial since blood pressure was not monitored in our study. This can be considered as a limitation. On the other hand, there is evidence demonstrating the independency between cardiac hypertrophy and systolic blood pressure. Therefore, it is also possible that the efficacy of V. album in preventing cardiac hypertrophy through the NO pathway is independent of blood pressure. Lack of the chemical analysis of extract content was also another limitation of our study.
Conclusion
Consequently, V. album provided favorable effects on left ventricular function in isoproterenol-induced HF rats. Although the study is limited to a rat model, applying these results to humans is rational because isoproterenol-induced HF in rats and known data from human HF are similar in many aspects.
Acknowledgements
The authors thank Ümit Şengül, Asst. Prof. and Selçuk Takır, Asst. Prof. for their valuable contribution in the preparation of the Viscum album L. extract. We also thank Bekir Erol, MD for sharing his experience with the medicinal plant.
Footnotes
Funding: This work was supported by the Coordinatorship of Scientific Research Projects of Giresun University (SAĞ-BAP-A-250414-71).
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.K., A.V., E.T., S.K.; Design – A.K., A.V., S.K., M.U., B.T.; Supervision – A.K., A.V., E.T., T.S., M.U.; Fundings- M.U., T.S., B.T., E.T.; Materials – B.T., E.T., T.S.; Data collection &/or processing – A.K., A.V., S.K., E.T.; Analysis &/or interpretation – A.K., A.V., S.K., E.T.; Literature search – A.K., S.K., E.T.; Writing – A.K., S.K., B.T., M.U.; Critical review – A.K., S.K., A.V., M.U., B.T., T.S.; Other – E.T.
References
- 1.Thompson KA, Bharadwaj P, Philip KJ, Schwarz ER. Heart failure therapy: beyond the guidelines. J Cardiovasc Med. 2010;11:919–27. doi: 10.2459/JCM.0b013e32833d3566. [DOI] [PubMed] [Google Scholar]
- 2.Johnson FL. Pathophysiology and etiology of heart failure. Cardiol Clin. 2014;32:9–19. doi: 10.1016/j.ccl.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Samson R, Ramachandran R, Le Jemtel TH. Systolic heart failure: knowledge gaps, misconceptions, and future directions. Ochsner J. 2014;14:569–75. [PMC free article] [PubMed] [Google Scholar]
- 4.Maldacker J. Preclinical investigations with mistletoe (Viscum album L.) extract Iscador. Arzneimittelforschung. 2006;56:497–507. doi: 10.1055/s-0031-1296817. [DOI] [PubMed] [Google Scholar]
- 5.Poruthukaren KJ, Palatty PL, Baliga MS, Suresh S. Clinical evaluation of Viscum album mother tincture as an antihypertensive: a pilot study. J Evid Based Complementary Altern Med. 2014;19:31–5. doi: 10.1177/2156587213507726. [DOI] [PubMed] [Google Scholar]
- 6.Tenorio-Lopez FA, Valle Mondragon LD, Olvera GZ, Torres Narvaez JC, Pastelin G. Viscum album aqueous extract induces NOS-2 and NOS-3 overexpression in Guinea pig hearts. Nat Prod Res. 2006;20:1176–82. doi: 10.1080/14786410600898979. [DOI] [PubMed] [Google Scholar]
- 7.Mossalayi MD, Alkharrat A, Malvy D. Nitric oxide involvement in the anti-tumor effect of mistletoe (Viscum album L.) extracts Iscador on human macrophages. Arzneimittelforschung. 2006;56:457–60. doi: 10.1055/s-0031-1296812. [DOI] [PubMed] [Google Scholar]
- 8.Tang L, Wang H, Ziolo MT. Targeting NOS as a therapeutic approach for heart failure. Pharmacol Ther. 2014;142:306–15. doi: 10.1016/j.pharmthera.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal A, Kumar S, Seth S, Dinda AK, Maulik SK. Effect of U50, 488H, a k-opioid receptor agonist on myocardial a-and b-myosin heavy chain expression and oxidative stress associated with isoproterenol-induced cardiac hypertrophy in rat. Mol Cell Biochem. 2010;345:231–40. doi: 10.1007/s11010-010-0577-4. [DOI] [PubMed] [Google Scholar]
- 10.Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–3. [PubMed] [Google Scholar]
- 11.Şekeroğlu V, Aydın B, Şekeroğlu ZA. Viscum album L. extract and quercetin reduce cyclophosphamide-induced cardiotoxicity, urotoxicity and genotoxicity in mice. Asian Pac J Cancer Prev. 2011;12:2925–31. [PubMed] [Google Scholar]
- 12.Grimm D, Elsner D, Schunkert H, Pfeifer M, Griese D, Bruckschlegel G, et al. Development of heart failure following isoproterenol administration in the rat: role of renin-angiotensin system. Cardiovas Res. 1998;37:91–100. doi: 10.1016/s0008-6363(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 13.Nichtova Z, Novotova M, Kralova E, Stankovicova T. Morphological and functional characteristics of models of experimental myocardial injury induced by isoproterenol. Gen Physiol Biophys. 2012;31:141–51. doi: 10.4149/gpb_2012_015. [DOI] [PubMed] [Google Scholar]
- 14.Tenorio FA, del Valle L, González A, Pastelín G. Vasodilator activity of the aqueous extract of Viscum album. Fitoterapia. 2005;76:204–9. doi: 10.1016/j.fitote.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Tenorio López FA, del Valle Mondragón L, Zarco Olvera G, Torres Narváez JC, Pastelín Hernández G. Viscum album aqueous extract induces inducible and endothelial nitric oxide synthases expression in isolated and perfused guinea pig heart. Evidence of the coronary vasodilation mechanism. Arch Cardiol Mex. 2006;76:130–9. [PubMed] [Google Scholar]
- 16.Mojiminiyi FB, Owolabi ME, Igbokwe UV, Ajagbonna OP. The vasorelaxant effect of Viscum album leaf extract is mediated by calcium-dependent mechanism. Niger J Physiol Sci. 2008;23:115–20. doi: 10.4314/njps.v23i1-2.54947. [DOI] [PubMed] [Google Scholar]
- 17.Avcı G, Küpeli E, Eryavuz A, Yeşilada E, Küçükkurt I. Antihypercholesterolaemic and antioxidant activity assessment of some plants used as remedy in Turkish folk medicine. J Ethnopharmacol. 2006;107:418–23. doi: 10.1016/j.jep.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 18.Poruthukaren KJ, Palatty PL, Baliga MS, Suresh S. Clinical evaluation of Viscum album mother tincture as an antihypertensive: a pilot study. J Evid Based Complementary Altern Med. 2014;19:31–5. doi: 10.1177/2156587213507726. [DOI] [PubMed] [Google Scholar]
- 19.Vanhoutte PM, Gao Y. Beta blockers, nitric oxide and cardiovascular disease. Curr Opin Pharmacol. 2013;13:265–73. doi: 10.1016/j.coph.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Turko IV, Murad F. Protein nitration in cardiovascular diseases. Pharmacol Rev. 2002;54:619–34. doi: 10.1124/pr.54.4.619. [DOI] [PubMed] [Google Scholar]
- 21.Mihm MJ, Coyle CM, Schanbacher BL, Weinstein DM, Bauer JA. Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res. 2001;49:798–807. doi: 10.1016/s0008-6363(00)00307-2. [DOI] [PubMed] [Google Scholar]
- 22.Nazaruk J, Orlikowski P. Phytochemical profile and therapeutic potential of Viscum album L. Nat Prod Res. 2016;30:373–85. doi: 10.1080/14786419.2015.1022776. [DOI] [PubMed] [Google Scholar]
- 23.Golomb E, Abassi ZA, Cuda G, Stylianou M, Panchal VR, Trachewsky D, et al. Angiotensin II maintains, but does not mediate, isoproterenol-induced cardiac hypertrophy in rats. Am J Physiol. 1994;267:1496–506. doi: 10.1152/ajpheart.1994.267.4.H1496. [DOI] [PubMed] [Google Scholar]
- 24.Mészáros J, Lévai G. Ultrastructural and electrophysiological alterations during the development of catecholamine-induced cardiac hypertrophy and failure. Acta Biol Hung. 1990;41:289–307. [PubMed] [Google Scholar]
- 25.Araújo JP, Lourenço P, Azevedo A, Friões F, Rocha-Gonçalves F, Ferreira A, et al. Prognostic value of high-sensitivity C-reactive protein in heart failure: a systematic review. J Card Fail. 2009;15:256–66. doi: 10.1016/j.cardfail.2008.10.030. [DOI] [PubMed] [Google Scholar]