Abstract
Objective:
This study attempted to fill the gaps in evidence related to response to clopidogrel treatment in the Turkish population. The study aimed to determine the prevalence, associated risk factors, and clinical outcomes of high on-treatment platelet reactivity (HTPR) of clopidogrel in patients undergoing percutaneous coronary intervention (PCI) in a tertiary cardiovascular hospital in Turkey.
Methods:
In this prospective studied a total of 1.238 patients undergoing PCI were included in the present study. Blood samples were analyzed using a Multiplate analyzer. All patients were examined in the outpatient clinics at the end of the first and sixth months for recording drug therapy compliance and study endpoints.
Results:
Among the study population, 324 (30.2%) patients were found to have HTPR (mean age 58.03±11.88 years, 71.7% men). The incidence of HTPR was higher amongst females than amongst males (38.3% vs. 27%, p=0.010). Hypertension and diabetes mellitus were more frequently observed in the HTPR group (57.7% vs. 48.7%, p=0.004; 35% vs. 29.1%, p=0.040, respectively). When the recorded data were analyzed using multinomial regression analysis, hypertension, hemoglobin level, platelet, lymphocyte, and eosinophil count were independently associated with HTPR.
Conclusion:
On the basis of the results obtained from our study, we conclude that 30.2% of the Turkish population has HTPR. Our results also led us to believe that hypertension is an associated risk factor and decreased hemoglobin level as well as increased platelet counts are laboratory parameters that are strongly associated with the presence of HTPR. However, no differences were observed with regard to cardiovascular mortality and stent thrombosis. (Anatol J Cardiol 2016; 16: 967-73)
Keywords: high on-treatment platelet reactivity, clopidogrel, percutaneous coronary intervention, prevalence, risk factors
Introduction
Aspirin and clopidogrel drug combination was mainstay of antithrombotic treatment in patients with acute coronary syndrome (ACS) and stable angina pectoris who subsequently underwent percutaneous coronary intervention (PCI). However, in 2014 ESC/EACTS myocardial revascularization guideline, ticagrelor and prasugrel were considered as first-line drug therapies before clopidogrel for ST segment elevation myocardial infarction (STEMI) (1). In the same year, ticagrelor and prasugrel were considered as first-line drug therapies along with clopidogrel in patients diagnosed with non-STEMI (NSTEMI) according to ACC/AHA guidelines on the management of NSTEMI (2). Despite the significant progress in defining guidelines, lack of access to new drugs (ticagrelor and prasugrel) is one of the major reasons why clopidogrel is still the most widely used antiplatelet agent in our country. This conclusion is strengthened when crosschecked with data obtained from our hospital. A retrospective investigation of 218 consecutive patients admitted with ACS in 2015 showed that 139 (63.7%) were prescribed clopidogrel, whereas 61 (27.9%) and 18 (8.2%) were prescribed ticagrelor and prasugrel, respectively.
Clopidogrel is a second-generation P2Y12 blocker, which is reportedly safer than the first generation of similar drugs, such as ticlopidine, in terms of side effects on the bone marrow and liver. Clopidogrel is rapidly absorbed from the intestine following ingestion. Hepatic cytochrome P450 enzymes (CYP3A4, CYP3A5, and CYP2C19) convert clopidogrel to a short-lived active metabolite that is capable of binding to the P2Y12 receptor via disulfide bridges, thereby leading to the inhibition of platelet activation (3). Clopidogrel is a noncompetitive selective ADP-receptor antagonist, which functions by blocking ADP and preventing it from binding its receptors. This blockage reduces the activity of glycoprotein (GPIIb/IIIa) in platelets, which is a prerequisite for fibrinogen-platelet adhesion mechanism (4).
In some patients, atherothrombotic complications are known to occur quite frequently despite appropriate aspirin and clopidogrel therapy. This phenomenon gave rise to several questions regarding the efficacy of these drugs in patients. Drug resistance has emerged as the most recognized explanation. High on-treatment platelet reactivity (HTPR) is defined as the failure of the drug molecule to inhibit the target of its action. This is best demonstrated by the evidence of residual post-treatment P2Y12 activity measured by computing ADP-induced platelet aggregation before and after treatment (5). The pharmacodynamic effect of clopidogrel is known to substantially vary between individuals (6). The prevalence of HTPR fluctuates study to study with major published trials reporting that prevalence varies from 41.8% to 49.6% as per two different cut-off values (7). Several clinical trials have demonstrated that HTPR is related to a higher risk of cardiovascular events (7–10).
The prevalence of HTPR has been reported as follows: (i) 21% in patients who presented with STEMI (11), (ii) 22.1% in patients who presented with ACS (12), and (iii) 37.9% (13) to 49% (14) in patients with stable coronary artery disease and undergoing PCI. The aim of this study was to determine the prevalence as well as the associated risk factors and clinical outcomes of HTPR patients diagnosed with ACS and stable coronary artery disease in the Turkish population.
Methods
In total, 690 (55.7%) patients diagnosed with ACS and 548 (44.2%) patients diagnosed with stable coronary artery disease were included in the present prospective study. Written informed consent was obtained before enrollment, and the study protocol was approved by the local ethical committee. The exclusion criteria were set as follows: (i) history of previous coronary intervention; (ii) history of coronary artery bypass surgery; (iii) history of using clopidogrel, nonsteroidal anti-inflammatory drugs, and anticoagulant; (iv) age under 18 years; (v) hematocrit value less than 30% and platelet count less than 100.000/mL; (vi) presence of hematological or malignant disorder; (vii) presence of severe renal and hepatic failure; (viii) history of recent bleeding or bleeding diathesis; and (ix) proven allergy to clopidogrel.
In our study, all patients received a daily dose of 300 mg of acetylsalicylic for the first month, followed by 100 mg daily indefinitely. All patients received a daily dose of 75 mg of clopidogrel for at least one year. Patients who presented with stable angina pectoris received a clopidogrel loading dose of 600 mg 4–12 h before stent implantation or a daily dose of 75 mg clopidogrel for at least 7 days. USAP and NSTEMI patients received a clopidogrel loading dose of 600 mg as soon as the diagnosis was made, and PCIs were performed within the next 24 h. STEMI patients received a clopidogrel loading dose of 600 mg just before PCI. In conclusion, blood samples were collected 24 h after the clopidogrel loading dose in STEMI patients and 24–48 h later in USAP and NSTEMI patients.
All PCIs were performed via the femoral route as per current guidelines.
Unfractioned heparin (70 IU/kg) was administered at the beginning of the intervention. An activated clotting time of 250–300 was ensured in the prolonged interventions with an additional dose of heparin. As and when needed, predilation was performed using balloon angioplasty before stent deployment. Bare-metal stents (BMS) were implanted in all patients that presented with STEMI. For other patients, drug-eluting stents or BMS were implanted depending upon the lesion complexity, co-morbidities of patients, patient preference as well as the operator’s prefe-rence. Tirofiban (a glycoprotein IIb/IIIa inhibitor) use was also left to the operator’s discretion.
Routine blood biochemistry, hematological parameters, thyroid function test, and ELISA testing for HIV, HBV, and HCV were performed immediately after admission for all ACS and elective cases. All patient blood samples obtained for analyzing clopidogrel efficiency were drawn post 24 h of stent implantation. Plastics tubes (4 mL) containing lepirudin (25 mg/mL, Refludan, Hirudin blood collection tubes; Dynabyte Medical, Munich, Germany) were used for blood collection. All samples were analyzed using Multiplate analyzer (Dynabyte Medical). Electrical impedance of multiple electrodes was used for measuring platelet aggregation in this test. Under normal circumstances, platelets are nonthrombogenic in the resting phase. Upon activation with 20 mmol/L ADP, they expose the receptors present on their surface, which causes adherence to vessel walls and artificial surfaces. Platelet binding to the sensor wires in the ADP-supplemented Multiplate test tubes causes an increase in the resistance of the sensor, which is interpreted as the computed aggregation unit (AU) in an AU versus time graph. The results obtained using the Multiplate analyzer were expressed in terms of the area under the aggregation curve (15). All the materials used for this purpose were obtained from the above-mentioned manufacturer; HTPR was defined with a cut-off value of 200 and the area under the aggregation curve as described by the manufacturer.
There is no exact data describing the most appropriate time to take blood samples for platelet reactivity after clopidogrel dose in the literature. However, blood samples were obtained within the first 24 h after the loading dose in most studies (11, 16). The GRAVITAS randomized trial attempted to demonstrate the effect of high-dose versus standard-dose clopidogrel on platelet reactivity at 12–24 h and 30 days after clopidogrel dosing (7).
All patients were examined in the outpatient clinic at the end of the first and sixth month for the purpose of revision as well as for monitoring drug therapy compliance and noting study endpoints. A cardiovascular death or stent thrombosis was considered as the endpoint of the study. Cardiovascular death was defined as death from acute myocardial infarction, coronary artery disease, heart failure, or arrhythmia. Diagnosis of stent thrombosis was made according to the criteria defined by the Academic Research Consortium (17).
Statistical analysis
Data obtained in this study were evaluated using the IBM SPSS 22 (IBM SPSS, USA) software. Continuous variables were tested for the normal distribution assumption using Kolmogorov–Smirnov statistics and were reported as mean±SD or median (interquantile range). Student’s t-test was used for normally distributed variables in both groups. The Mann–Whitney U test was used for variables that were not normally distributed. Qualitative variables were evaluated by chi-square, Fisher’s exact test, and continuity (Yates) correction. A p value of 0.05 was accepted as statistically significant. Univariate and multinomial binary logistic regression analysis was performed to investigate independent correlates of HTPR. Variables with p<0.10 in univariate analysis were included in the multinomial regression analysis.
Results
This single-center, cross-sectional, prospective study was conducted between February 2012 and June 2014 in our tertiary cardiovascular surgery hospital. Our study included 1,238 patients (28.2% women) diagnosed with ACS (n=690, 55.7%) and stable coronary artery disease (n=548, 44.2%). Among the patients, 374 (30.2%) had HTPR (mean age 58.03±11.88 years), whereas 864 (69.8%) were found to be sensitive to clopidogrel (mean age 58.05±11.72 years).
A comparison of baseline demographics and study endpoint properties is summarized in Table 1. HTPR was observed to have a higher incidence amongst females compared with males (38.3% vs. 27%, p=0.01). Hypertension and diabetes mellitus (DM) occurred with a higher frequency in the HTPR group than in the control group (57.7% vs. 48.7%, p=0.004; 35% vs. 29.1%, p=0.04, respectively). A larger number of patients in the HTPR group presented with ACS compared with the control group (62% vs. 53%, p=0.01); however, the occurrence of stable angina pectoris was found to be lower in the HTPR group than in the control group (37.9% vs. 46.9%, p=0.01). Tirofiban (a glycoprotein IIb/IIIa inhibitor) usage was higher in the control group than in the HTPR group (30% vs. 18.9%, p=0.01). There was no statistically significant difference between the study and control groups in terms of stent thrombosis (2.9% vs. 2.6%, p=0.820) and cardiovascular mortality (2.9% vs. 4%, p=0.34).
Table 1.
Demographic characteristics and risk factors identification of patients
| HTPR (n=374) (30.2%) | Controls (n=864) (69.8%) | P | |
|---|---|---|---|
| Age, years | 58.03±11.88 | 58.05±11.72 | 0.971 |
| Female gender, (%) | 134/350 (38.3) | 216/350 (61.7) | 0.001 |
| Male gender, (%) | 240/888 (27) | 648/888 (73) | |
| Hypertension, (%) | 216 (57.7) | 421 (48.7) | 0.004 |
| Diabetes mellitus, (%) | 131 (35) | 252 (29.1) | 0.040 |
| Acute coronary syndrome, (%) | 232 (62) | 458 (53) | 0.010 |
| Stable angina pectoris, (%) | 142 (37.9) | 406 (46.9) | 0.010 |
| Tirofiban, (%) | 71 (18.9) | 260 (30) | 0.010 |
| Stent thrombosis, (%) | 11 (2.9) | 23 (2.6) | 0.820 |
| Cardiovascular mortality, (%) | 11 (2.9) | 35 (4) | 0.340 |
HTPR - high on-treatment platelet reactivity of clopidogrel
There was no statistically significant difference between the groups in terms of estimated event-free days from stent thrombosis (173.273 vs. 172.751 days, p=0.851). Further, stent thrombosis time graph showed parallel distribution of stent thrombosis between two groups (Fig. 1).
Figure 1.
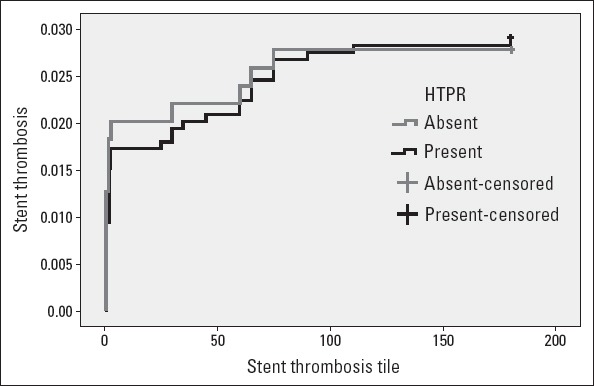
Stent thrombosis time graph showing parallel distribution of stent thrombosis between two groups
The incidence of HTPR was higher amongst patients who presented with ACS than amongst those who presented with stable angina pectoris (62% vs. 37.9%, p=0.01).
Hematological and biochemical parameters of the study groups are shown in Table 2. In the HTPR group, CRP (6.14±21.44 vs. 3.93±14.65, p<0.01), TSH (1.94±2.11 vs. 1.58±1.54, p<0.01), fas-ting plasma glucose (130.93±56.03 vs. 121.87±51.38, p<0.01), total cholesterol level (187.39±50.12 vs. 177.44±45.19, p<0.01), platelet counts (266.68±81.71 vs. 240.6±84.69, p<0.01), and eosinophil counts (0.23±0.21 vs. 0.19±0.17, p<0.01) were significantly higher than in the control group. In the HTPR group, hemoglobin level (12.78±1.96 vs. 13.29±1.89, p<0.01) and lymphocyte counts (2.13±0.77 vs. 2.54±1.33, p<0.01) were significantly lower than those in the control group.
Table 2.
Comparison of hematological and blood chemistry parameters of the two groups
| HTPR (n=374, 30%) | Controls (n=864, 70%) | P | |
|---|---|---|---|
| ATR, AU/min | 533.32±(381-615) | 154.14±(64-222) | 0.001 |
| CRP, mg/L | 6.14±(0.4-3.4) | 3.93±(0.20-2.22) | 0.001 |
| TSH, U/mL | 1.94±(0.9-2.3) | 1.58±(0.82-1.95) | 0.006 |
| Fasting plasma glucose, mg/dL | 130.93±(94-151) | 121.87±(92-134) | 0.008 |
| HbA1C,% | 6.86±(5.7-7.7) | 6.61±(5.7-7.1) | 0.066 |
| Sodium, mEq/L | 139.08±(138-141) | 139.1±(137-141) | 0.932 |
| Potassium, mEq/L | 4.43±(4.1-4.6) | 4.38±(4.0-4.7) | 0.057 |
| Calcium, mg/dL | 9.32±(9.0-9.7) | 9.3±(9.0-9.7) | 0.462 |
| HDL, mg/dL | 39.97±(31-45) | 38.94±(32-44) | 0.215 |
| LDL, mg/dL | 109.69±(81-129) | 106.71±(77-128) | 0.254 |
| VLDL, mg/dL | 34.89±(21-41) | 35.18±(23-43) | 0.832 |
| Triglyceride, mg/dL | 176.7±(106-200) | 171.76±(112-205) | 0.480 |
| Total cholesterol, mg/dL | 187.39±(146-218) | 177.44±(144-205) | 0.002 |
| Hemoglobin, g/dL | 12.78±(11.0-14.2) | 13.29±(12.0-14.7) | 0.001 |
| Platelet, 103 /μL | 266.68±(214-299) | 240.6±(187-280) | 0.001 |
| Neutrophil, 103 /μL | 6.17±(4.2-7.3) | 6.2±(4.2-7.8) | 0.861 |
| Lymphocyte, 103 /μL | 2.13±(1.6-2.5) | 2.54±(1.7-3.0) | 0.001 |
| Monocyte, 103 /μL | 0.73±0.34 | 0.77±0.35 | 0.049 |
| Eosinophil, 103 /μL | 0.23±(0.1-0.3) | 0.19±(0.10-0.25) | 0.001 |
| MCV, fL | 87.26±(83.4-91.4) | 87.42±(84.7-91.0) | 0.680 |
| RDW, % | 14.33±(12.5-15.0) | 14.15±(13-15) | 0.211 |
| MPV, fL | 8.81±(8.1-9.4) | 8.88±(8.2-9.4) | 0.369 |
ATR - on treatment reactivity; AU - aggregation units; CRP - c-reactive protein; HDL - high density lipoprotein; HTPR - high on-treatment platelet reactivity of clopidogrel; LDL - low density lipoprotein; MCV - mean corpuscular volume; MPV - mean platelet volume; RDW - red cell distribution width; TSH - thyroid stimulating hormone; VLDL - very low density lipoprotein
Univariate and multivariate binary logistic regression analysis was performed to investigate the independent correlates of HTPR in the study population. In univariable model, hypertension, DM, TSH, fasting plasma glucose, HbA1c, and total cholesterol levels and platelet, lymphocyte, monocyte, and eosinophil counts were found to correlate with the presence of HTPR (Table 3). In the multivariable model, hypertension, hemoglobin level, platelet eosinophil, and lymphocyte counts were found to be independently associated with HTPR (Table 4).
Table 3.
Univariate regression analysis for the predictors of HTPR of Clopidogrel
| β | Odds ratio (95% CI) | P | |
|---|---|---|---|
| Age | 0.000 | –0.002 - 0.002 | 0.971 |
| Hypertension | 0.076 | 0.025 - 0.127 | 0.003 |
| Diabetes mellitus | 0.058 | 0.002 - 0.113 | 0.041 |
| CRP | 0.002 | 0.000 - 0.004 | 0.096 |
| TSH | 0.026 | 0.009 - 0.043 | 0.003 |
| Fasting plasma glucose | 0.001 | 0.000 - 0.001 | 0.006 |
| HbA1c | 0.024 | –0.001 - 0.049 | 0.056 |
| Sodium | 0.000 | –0.006 - 0.006 | 0.932 |
| Potassium | 0.052 | –0.004 - 0.108 | 0.068 |
| Calcium | 0.019 | –0.031 - 0.068 | 0.462 |
| HDL | 0.002 | –0.001- 0.004 | 0.176 |
| LDL | 0.000 | 0.000 - 0.001 | 0.254 |
| Triglyceride | 0.000 | 0.000 - 0.000 | 0.480 |
| Total cholesterol | 0.001 | 0.000 - 0.002 | 0.001 |
| Hemoglobin | –0.029 | –0.042 - (–0.015) | <.0.010 |
| Platelet | 0.001 | 0.000-0.001 | <0.010 |
| Neutrophil | –0.001 | –0.008 - 0.007 | 0.861 |
| Lymphocyte | –0.059 | –0.080 - (–0.038) | <0.010 |
| Monocyte | –0.075 | –0.149 - 0.00 | 0.049 |
| Eosinophil | 0.246 | 0.106 - 0.386 | 0.001 |
| MCV | –0.001 | –0.005 - 0.003 | 0.680 |
| RDW | 0.010 | –0.033 - 0.022 | 0.148 |
| MPV | –0.012 | –0.035 - 0.011 | 0.316 |
CRP- C -reactive protein; HDL - high density lipoprotein; HTPR - high on-treatment platelet reactivity of clopidogrel; LDL - low density lipoprotein; MCV - mean corpuscular volume; MPV - mean platelet volume; RDW - red cell distribution width; TSH - thyroid stimulating hormone
Table 4.
Multinomial regression analysis for the predictors of HTPR of clopidogrel
| OR | 95% CI | P | |
|---|---|---|---|
| Hypertension | 1.584 | 1.027 - 2.443 | 0.037 |
| Hemoglobin | 0.865 | 0.773 - 0.968 | 0.012 |
| Platelet | 1.003 | 1.001 - 1.006 | 0.014 |
| Lymphocyte | 0.621 | 0.487 - 0.792 | <0.010 |
| Eosinophil | 4.599 | 1.366 - 15.486 | 0.014 |
Discussion
The main findings of our study can be summarized as follows: (i) In the Turkish population, 30.2% of patients diagnosed with ACS or stable coronary artery disease and undergoing PCI therapy were found to have HTPR, (ii) hypertension was found to be an associated risk factor, and decreased hemoglobin level as well as increased platelet counts were found to be laboratory parameters associated with HTPR, (iii) stent thrombosis and cardiovascular mortality rates were found to be similar between the two groups.
The CAPRIE study showed that the combined risk of ischemic stroke, myocardial infarction, and vascular death was lower with long-term consumption of 75 mg clopidogrel per day (18). Clopidogrel has a crucial role for platelet inhibition in patients with coronary heart disease. However, variability in individual platelet response to clopidogrel is a well-documented problem, which can cause deleterious clinical events. The prevalence of this phenomenon is observed to vary study to study and population to population, and various reports in the literature have estimated that it occurs in 5%–56% of coronary stent-implanted patients (19, 20). In our study, 30.2% (n=1.238) of patients who underwent PCI were found to have HTPR. The prevalence of HTPR can vary depending upon the cut-off value of the analyzer device used for the study; however, the prevalence we have reported for our country was similar with values reported in previous studies. However, the effect of dietary habitude, cigarette smoking, and genetic differences cannot be denied, and they might interfere with values demonstrated in our study.
The exact mechanism behind the development of HTPR remains unclear. Multifactorial interactions between genetics and the clinical situation of the patient are hypothesized to have a dominant role in determining the response. For example, intestinal epithelial cells expressing P-glycoprotein can influence the absorption of clopidogrel from the digestive tract into the blood stream, thus affecting the efficacy of the drug (1, 21).
Because clopidogrel is a pro-drug and requires activation by liver enzymes, any factor affecting the activity of liver enzymes directly affects the response. The polycyclic aromatic hydrocarbons present in cigarette smoke can activate cytochrome P450 isoenzymes 3A4 and 1A2, which alter the clopidogrel response (22).
It is controversial whether some or all proton pump inhibitors reduce the effectiveness of clopidogrel via drug interaction through cytochrome P450 2C19 enzyme. Clopidogrel is a pro-drug that is metabolized to an active form, primarily by cytochrome P450 2C19, which is inhibited by proton pump inhibitors (23). This interaction leads to a reduction in the activity of clopidogrel when used together with proton pump inhibitors including omeprazole and lansaprazole. The same interaction of the cytochrome P450 2C19 enzyme inhibition is observed with the use of paroxetine or fluoxetine, which in turn could result in the reduction of the activity of clopidogrel (23). Calcium channel blockers inhibit P450 CYP3A4, which also converts clopidogrel to active metabolites (24). This also means a reduced clopidogrel activity with the concomitant use of calcium channel blockers. Statins (atorvastatin) also interact with cytochrome P450 CYP3A4 and decrease clopidogrel activity (25). There is no proven direct drug interaction among clopidogrel with beta-blockers, angiotensin-converting enzyme inhibitors, or aldosterone receptor blockers yet.
In our study, HTPR was found to be more prevalent among females. Several previously published studies have also reported similar findings, (26, 27) but no precise explanation for this phenomenon has been put forward till date. Another clinical risk factor found to be closely associated with HTPR was DM; several previously reported studies have demonstrated this association (28, 29). Increased exposure to ADP, cytosolic levels of calcium, and platelet turnover are suggested to facilitate the development of HTPR in diabetic patients (30). In diabetic patients, nonenzymatic glycation of platelet GPs results in altered platelet structure, conformation, and membrane lipid dynamics (31), which could lead to increased platelet reactivity. In addition, decreased platelet sensitivity to insulin is associated with reduced P2Y12 receptor inhibition and increased platelet reactivity (32). In multivariate regression analysis, HTPR was found to be significantly associated with hypertension. Similarly, Aktürk et al. (13) and Kim et al. (33) have also identified high blood pressure as a risk factor for HTPR development. Vascular shear stress, high adhesivity, and agreeability are possible mechanisms suggested to explain the increased incidence of HTPR in this population.
In contrast to previous studies, our results indicated that the rate of occurrence of stent thrombosis and mortality were similar between the HTPR and control groups. Reports published by Matetzky et al. (34) showed that in patients with STEMI, HTPR was associated with a 40% probability of recurrence of cardiovascular events within a time period of 6 months (34). In another study conducted on 192 patients undergoing PCI, recurrence of an ischemic event within 6 months of stent implantation was significantly higher in patients with a high reactivity to ADP (35). However, in our findings, there was no difference observed in terms of occurrence of stent thrombosis and mortality between the groups. Our patient group included both ACS and stable coronary artery disease patients. In this aspect, we believe that our study population is more representative of the whole cohort compared with previous studies. Price et al. observed no apparent benefit of a higher dose of clopidogrel (150 vs. 75 mg) in patients with HTPR (7). Moreover, a study conducted to demonstrate the potential additive predictive value of several platelet tests in the assessment of risk and/or recurrence of ischemic events concluded that platelet tests contributed a comparatively minor additive value to ischemic risk assessment in contrast to clinical and angiographic risk models (36).
On the basis of the results of our study, we do not recommend routine checking of clopidogrel response because there was no significant difference in terms of mortality and stent thrombosis between the test and control groups; in addition, clopidogrel HTPR was found in approximately one out of three patients (30.2%). Moreover, the new ESC/ACC guidelines recommend shifting the drug usage from clopidogrel to ticagrelor or prasugrel in patients presenting with STEMI and NSTEMI (1, 2). Shift from clopidogrel to ticagrelor or prasugrel could also be a reasonable option in patients with HTPR as evidenced by GRAVITAS trial, which failed to show any apparent benefit of a doubled daily dose of clopidogrel (150 mg) versus a single daily dose (75 mg) (7).
Increased levels of CRP, TSH, fasting plasma glucose, and total cholesterol were observed in the HTPR group. An association of increased CRP levels with HTPR in patients with STEMI and stable coronary artery disease undergoing PCI (37) and in those with atherosclerotic cerebrovascular disease undergoing stent-assisted angioplasty (38) has been shown earlier. Our findings are in agreement with those of these studies. Lundström et al. (39) showed an association between increased plasma glucose in HTPR in patients with minor ischemic stroke or transient ischemic attack. In our study, we observed similar association in patients with coronary artery disease. High total cholesterol levels were also associated with HTPR in patients with cerebral infarct undergoing clopidogrel treatment (40). Moreover, we observed the same association in patients with coronary artery disease. Increased TSH, which could be taken as a predictor of subclinical hypothyroidism, was found to be associated with HTPR in our study. Platelet hyper-reactivity in patients with overt or sub-clinical hypothyroidism has been previously reported and is thought to be a result of elevated vWF levels. One of our findings in multivariate regression analysis was an association between low levels of hemoglobin and HTPR. It is thought that anemia is an in vitro artifact of VerifyNow P2Y12 assay results; however, there is no such information available for experiments conducted using the Multiplate analyzer.
The incidence of HTPR was higher amongst patients who presented with ACS than amongst those who presented with stable angina pectoris. This difference was attributed to increased platelet turnover and massive platelet activation previously (41).
Study limitations
Our study had several limitations: (i) HTPR status was assessed only 24 h after PCI. Multiple assessments may be needed for more accurate results because HTPR status is liable to change during follow-up. (ii) The study was not blinded to the investigators. This could have resulted in a certain degree of bias during clinical follow-up. (iii) It was a single-center study. A single-center experience may not be an accurate reflection of the whole cohort. (iv) ASA resistance was not studied. There is a possibility for it to be associated with HTPR. (v) Unfortunately in our dataset, there is no clear drug list to compare drug interaction over HTPR. (vi) Stent deployment without OCT and IVUS is a limitation because one of the major causes of stent thrombosis is malapposition. (vi) Our study did not have access to the data regarding the size and type of implanted stents. These data have the potential to affect the clinical results.
Conclusion
In the Turkish population, 30.2% of patients undergoing PCI therapy were found to have HTPR. Hypertension was demonstrated to be an associated risk factor and decreased hemoglobin level and increased platelet counts were found to be laboratory parameters that are associated strongly with HTPR. There were no differences in terms of cardiovascular mortality and stent thrombosis.
Acknowledgements
There is no financial support associated with this study to acknowledge.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.İ.T., C.T., E.B.B., E.B., M.A.T.; Design – A.K., E.B.B., A.G.; Supervision – Y.Ç., E.B.B., A.G., E.B., M.A.T.; Fundings – A.K., C.T., A.G., M.A.T., A.T.A.; Materials – A.İ.T., Ö.Y.; Data collection &/or processing – Y.Ç., K.K., Ö.Y., A.T.A.; Analysis &/or interpretation – Y.Ç., C.T., K.K., Ö.Y.; Literature search – M.İ.H.; Writing – A.K., M.İ.H., K.K.; Critical review – A.İ.T., M.İ.H., Ö.Y., E.B., A.T.A.
References
- 1.Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Gurbel PA, Tantry US. Clopidogrel resistance? Thromb Res. 2007;120:311–21. doi: 10.1016/j.thromres.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy GK. Clopidogrel bisulfate. AHFS Drug Information. 2008:1520–4. [Google Scholar]
- 5.Ray S. Clopidogrel resistance: the way forward. Indian Heart J. 2014;66:530–4. doi: 10.1016/j.ihj.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Price MJ, Angiolillo DJ, Teirstein PS, Lillie E, Manoukian SV, Berger PB, et al. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) Trial. Circulation. 2011;124:1132–7. doi: 10.1161/CIRCULATIONAHA.111.029165. [DOI] [PubMed] [Google Scholar]
- 8.Gurbel PA, Bliden KP, Samara W, Yoho JA, Hayes K, Fissha MZ, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol. 2005;46:1827–32. doi: 10.1016/j.jacc.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 9.Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicentre randomized prospective study. J Am Coll Cardiol. 2008;51:1404–11. doi: 10.1016/j.jacc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 10.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Eng J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 11.Bozbeyoğlu E, Satılmış S, Aksu H, Yıldırımtürk O, Nurkalem Z. Impact of clopidogrel resistance on ST-segment resolution and no-reflow in acute myocardial infarction with ST-elevation patients treated with a primary percutaneous coronary intervention. Coron Artery Dis. 2012;23:523–7. doi: 10.1097/MCA.0b013e3283596c29. [DOI] [PubMed] [Google Scholar]
- 12.Uzel H, Özpelit E, Badak O, Akdeniz B, Barış N, Aytemiz F, et al. Diagnostic accuracy of mean platelet volume in prediction of clopidogrel resistance in patients with acute coronary syndrome. Anadolu Kardiyol Derg. 2014;14:134–9. doi: 10.5152/akd.2014.4433. [DOI] [PubMed] [Google Scholar]
- 13.Aktürk IF, Çağlar FN, Ertürk M, Tuncer N, Yalçın AA, Sürgit O, et al. Hypertension as a risk factor for aspirin and clopidogrel resistance in patients with stable coronary artery disease. Clin Appl Thromb Hemost. 2014;20:749–54. doi: 10.1177/1076029613481102. [DOI] [PubMed] [Google Scholar]
- 14.Arı H, Özkan H, Karaçınar A, Arı S, Koca V, Bozat T. The EFFect of hIgh-dose ClopIdogrel treatmENT in patients with clopidogrel resistance (the EFFICIENT trial) Int J Cardiol. 2012;157:374–80. doi: 10.1016/j.ijcard.2010.12.083. [DOI] [PubMed] [Google Scholar]
- 15.Cardinal DC, Flower RJ. The electronic aggregometer: a novel device for assessing platelet behavior in blood. J Pharmacol Methods. 1980;3:135–58. doi: 10.1016/0160-5402(80)90024-8. [DOI] [PubMed] [Google Scholar]
- 16.Mangiacapra F, Barbato E, Patti G, Gatto L, Vizzi V, Ricottini E, et al. Point-of-care assessment of platelet reactivity after clopidogrel to predict myonecrosis in patients undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2010;3:318–23. doi: 10.1016/j.jcin.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Academic Research Consortium. Clinical end points in coronary stent trials a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 18.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 19.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting. Response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–13. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 20.Muller I, Besta F, Schulz C, Massberg S, Schonig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003;89:783–7. [PubMed] [Google Scholar]
- 21.Zevin S, Benowitz NL. Drug interactions with tobacco smoking. An uptade. Clin Pharmacokinet. 1999;36:425–38. doi: 10.2165/00003088-199936060-00004. [DOI] [PubMed] [Google Scholar]
- 22.Gros P, Ben Neriah YB, Croop JM, Housman DE. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986;323:728–31. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- 23.Douglas IJ, Evans SJ, Hingorani AD, Grosso AM, Timmis A, Hemingway H, et al. Clopidogrel and interaction with proton pump inhibitors: comparison between cohort and within person study designs. BMJ. 2012;345:e4388. doi: 10.1136/bmj.e4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siller-Matula JM, Lang I, Christ G, Jilma B. Calcium-channel blockers reduce the antiplatelet effect of clopidogrel. J Am Coll Cardiol. 2008;52:1557–63. doi: 10.1016/j.jacc.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 25.Lau WC, Waskell LA, Watkins PB, Neer CJ, Horowitz K, Hopp AS, et al. Atorvastatin reduces the ability of clopidogrel to inhibit platelet aggregation a new drug–drug interaction. Circulation. 2003;107:32–7. doi: 10.1161/01.cir.0000047060.60595.cc. [DOI] [PubMed] [Google Scholar]
- 26.Hobson AR, Qureshi Z, Banks P, Curzen N. Gender and responses to aspirin and clopidogrel: insights using short thrombelastography. Cardiovasc Ther. 2009;27:246–52. doi: 10.1111/j.1755-5922.2009.00106.x. [DOI] [PubMed] [Google Scholar]
- 27.Lev EI, Patel RT, Maresh KJ, Guthikonda S, Granada J, DeLao T, et al. Aspirin and clopidogrel drug response in patients undergoing percutaneous coronary intervention: the role of dual drug resistance. J Am Coll Cardiol. 2006;47:27–33. doi: 10.1016/j.jacc.2005.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Sabaté M, Jimenez-Quevedo P, et al. Platet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–5. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Wang X, Chen F. Clopidogrel resistance is associated with long-term thrombotic events in patients implanted with drug-eluting stents. Drugs in R D. 2010;10:219–24. doi: 10.2165/11539580-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferroni P, Basili S, Falco A, Davì G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost. 2004;2:1282–91. doi: 10.1111/j.1538-7836.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 31.Winocour PD, Watala C, Perry DW, Kinlough-Rathbone RL. Decreased platelet membrane fluidity due to glycation or acetylation of membrane proteins. Thromb Haemost. 1992;68:577–82. [PubMed] [Google Scholar]
- 32.Ferreira IA, Mocking AI, Feijge MA, Gorter G, van Haeften TW, Heemskerk JW, et al. Platelet inhibition by insulin is absent in type 2 diabetes mellitus. Arterioscler Thromb Vasc Boil. 2006;26:417–22. doi: 10.1161/01.ATV.0000199519.37089.a0. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Lee HK, Han K, Jeon HK. Prevalence and risk factors for aspirin and clopidogrel resistance in patients with coronary artery disease or ischemic cerebrovascular disese. Ann Clin Lab Sci. 2009;39:289–94. [PubMed] [Google Scholar]
- 34.Matetzky S, Shenkman B, Guetta V, Shechter M, Beinart R, Goldenberg I, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–5. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 35.Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, et al. Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING Study al. J Am Coll Cardiol. 2005;46:1820–6. doi: 10.1016/j.jacc.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 36.Malek LA, Grabowski M, Spiewak M, Filipiak KJ, Szpotanska M, Imiela T, et al. Relation between impaired antiplatelet response to clopidogrel and possible pleiotropic effects. J Thromb Thrombolysis. 2007;24:301–5. doi: 10.1007/s11239-007-0026-8. [DOI] [PubMed] [Google Scholar]
- 37.Grdinic A, Vojvodic D, Djukanovic N, Colic M, Grdinic AG, Ignjatovic V, et al. PCI and clopidogrel: antiplatelet responsiveness and patient characteristics. Acta Cardiol. 2011;66:333–40. doi: 10.2143/AC.66.3.2114133. [DOI] [PubMed] [Google Scholar]
- 38.Rho GJ, Shin WR, Kong TS, Kim MS, Lee CJ, Lee BH. Significance of clopidogrel resistance related to the stent-assisted angioplasty in patients with atherosclerotic cerebrovascular disease. J Korean Neurosurg Soc. 2011;50:40–4. doi: 10.3340/jkns.2011.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundström A, Laska AC, Von Arbin M, Jörneskog G, Wallén H. Glucose intolerance and insulin resistance as predictors of low platelet response to clopidogrel in patients with minor ischemic stroke or TIA. Platelets. 2014;25:102–10. doi: 10.3109/09537104.2013.777951. [DOI] [PubMed] [Google Scholar]
- 40.Zhou BR, Shi HT, Wang R, Zhang M, Guan HT, Liu ZF, et al. Dynamic changes and associated factors of clopidogrel resistance in patients after cerebral infarction. J Neurol. 2013;260:2928–37. doi: 10.1007/s00415-013-7140-7. [DOI] [PubMed] [Google Scholar]
- 41.Cavallari I, Nusca A, Ricottini E, Di Sciascio G. Prognostic role of platelet reactivity in patients with acute coronary syndromes. Cardiol Rev. 2014;22:313–8. doi: 10.1097/CRD.0000000000000034. [DOI] [PubMed] [Google Scholar]


