Abstract
Prosthetic valve thrombosis is one of the major causes of primary valve failure, which can be life-threatening. Multimodality imaging is necessary for determination of leaflet immobilization, cause of underlying pathology (thrombus versus pannus or both), and whether thrombolytic therapy attempt in the patient would be successful or surgery is needed. Current guidelines for the management of prosthetic valve thrombosis lack definitive class I recommendations due to lack of randomized controlled trials, and usually leave the choice of treatment to the clinician’s experience. In this review, we aimed to summarize the pathogenesis, diagnosis, and management of mechanical prosthetic valve thrombosis.
Keywords: prosthetic heart valve, thrombosis, diagnosis, management
Introduction
For six decades, heart valve surgery has been improving the survival and the quality of life of patients with severe valvular disease. However, it has also given rise to development of a new disease—the prosthetic heart valve disease. Although thrombus formation is less frequently observed among new-generation prosthetic valves, the hemodynamic and physical properties of mechanical valves remain thrombogenic (1). Therefore, prosthetic valve thrombosis (PVT) is one of the major causes of primary valve failure. The PVT incidence was reported to be 0.03% in bioprosthetic valves (2), 0.5%–8% in mechanical valves in the mitral and aortic positions, and as high as 20% in mechanical tricuspid valves (3). Recently, it has been reported that approximately 10% of the patients with mechanical heart valves had one episode of PVT per year (4).
PVT may lead to valve dysfunction, and its onset may be acute or gradual, according to the nature of thrombi and involvement of the hinges. The most common cause of PVT is inadequate anticoagulant therapy. Unfortunately, vitamin K antagonists (VKAs) are still the only approved oral anticoagulants in patients with heart valve prostheses. Even with the use of VKA, the risk of thromboembolism is 1%–2% per year, but the risk is consi- derably higher without or inadequate treatment with warfarin (3). There are different therapeutic modalities for PVT, including anticoagulation with heparin, thrombolytic therapy (TT) (4, 5–8), and surgery (9), which are largely influenced by the presence of valvular obstruction, valve location, and clinical features. In this review, we aimed to summarize the pathogenesis, diagnosis, and management of mechanical PVT.
Pathogenesis and clinical findings of PVT
PVT is an obstruction of a prosthesis by noninfective thrombotic material. The etiopathogenesis of PVT is based on several mechanisms. The first mechanism involves the molecular interaction between corpuscular blood components, plasma, and the prosthetic surfaces. The initial adsorption of plasma proteins on the prosthesis is generally followed by platelet adhesion (10). The second mechanism is dependent on the effect of the transprosthetic blood flow on local thrombus formation. The turbulent flow may result in a blood-borne increase in shear stress and may lead to thrombosis. Furthermore, chronic hemolysis may occur as a result of the accelerated destruction of thrombocytes and erythrocytes with shortened intravascular lifespans (11). The third mechanism is ineffective anticoagulation, which is determined by reported valve thrombosis rates for that prosthesis in relation to specific international normalized ratio levels. The other prothrombotic causes are described in Table 1 (12–17). Inherited disorders such as MTHFR A 1298 C and fibrinogen 455G/A polymorphisms may be involved in the pathogenesis of PVT, necessitating further data from large-scale studies (15). Increased levels of specific antibodies, including anticardiolipin and anti–tissue plasminogen activator antibodies have recently been found to be associated with PVT (14, 16). Furthermore, he- parin-induced thrombocytopenia may also lead to PVT (17).
Table 1.
The etiopathogenesis of prosthetic heart valve thrombosis
| I. Molecular interaction between corpuscular blood components and prosthetic surfaces | Initial adsorption of plasma proteins on the prosthesis and adhesion of the platelets (via fibrinogen, fibronectin, von Willebrand factor, vitronectin, thrombospondin, etc.) |
| II. The effect of the transprosthetic blood flow on local thrombus formation | Adenosine diphosphatase, platelet factor 4, beta-thromboglobulin and other proteins are released, which is associated with the increase in blood-borne shear stress |
| III. Ineffective anticoagulation | Subtherapeutic international normalized ratio levels |
| IV. Other prothrombotic factors | Incomplete endothelialization of the sewing ring (the early postoperative period) |
| Atrial fibrillation | |
| Left atrial enlargement | |
| Multiple valve replacement | |
| Ventricular dysfunction | |
| Presence of pannus formation | |
| Sporadic use of drugs (e.g., contraceptives) | |
| Malignancy | |
| Systemic diseases (i.e., systemic lupus erythematosus) | |
| Pregnancy | |
| Potential inherited causes (i.e., methylenetetra- hydrofolate reductase A 1298 C, fibrinogen 455G/A polymorphisms) | |
| Presence of specific antibodies (anticardiolipin, anti-tissue plasminogen activator antibodies, etc.) | |
| Heparin-induced thrombocytopenia |
PVT usually occurs over and under the hinge points of prosthesis and extends unidirectionally or bidirectionally over the annulus or toward the prosthetic orifice. The length and thickness of the thrombus are critical because these features may provide the basis for fresh thrombus attachment with an embolic risk (18).
The clinical presentation of PVT may be variable. Patients with PVT may present with symptoms such as dyspnea, dec- reased exercise capacity, palpitation, chest pain, vertigo, cerebro- vascular accident, or even flank pain (19–22). Occluder clicks are typically muffled or absent during auscultation. Also, stenotic or regurgitant murmurs may be heard. Early detection and diagnosis may often be limited by a progressive or insidious course. The clinical status may depend on the type of the prosthesis. Patients with bileaflet prosthetic valves might be in a good hemodynamic condition due to a well-functioning single leaflet or might be unstable due to bileaflet involvement. Echocardiographic exa- mination should be urgently performed in case of high level of clinical suspicion.
Imaging modalities
Transthoracic echocardiography (TTE) is usually the first modality for detecting PVT. PVT, including large thrombotic masses, may be missed during initial TTE study, and Doppler echocardiography is generally used for evaluation of severity of obstruction (23). The principles of recording of flow velocity through prosthetic valves are similar to those used in assessing native valve stenosis (24). The use of pulsed-wave and continuous-wave Doppler as well as color Doppler are usually necessary during TTE evaluation. Doppler recordings should be performed at a sweep speed of 100 mm/s. Measurements should be taken over one to three cycles in sinus rhythm, and an average of five cycles is recommended in atrial fibrillation (23, 24). Heart rate is an important key point during TTE examination, and Doppler measurements should be performed during periods of physio- logic heart rate (65–85 bpm).
For the aortic position, the Doppler measurements needed are peak velocity, mean gradient, velocity time integral, Dopp- ler velocity index, and effective orifice area by the continuity equation, whereas the measurements needed in the mitral and tricuspid positions are peak velocity, mean pressure gradient, velocity time integral, and pressure half-time (23). Novel Dopp- ler parameters, including ejection systolic parameters such as acceleration time (AT) and ejection time (ET), may also be very helpful during assessment of valve function and identification of prosthetic aortic valve stenosis (25). Increased transprosthetic gradients do not always indicate prosthetic dysfunction, and alternative causes, including pressure recovery in bileaflet mechanical valves, anemia, arteriovenous fistula, and tachycardia, should also be considered. It is important to compare with baseline studies. Figure 1 shows a TTE and Doppler study in a patient with suspected mitral PVT.
Figure 1.
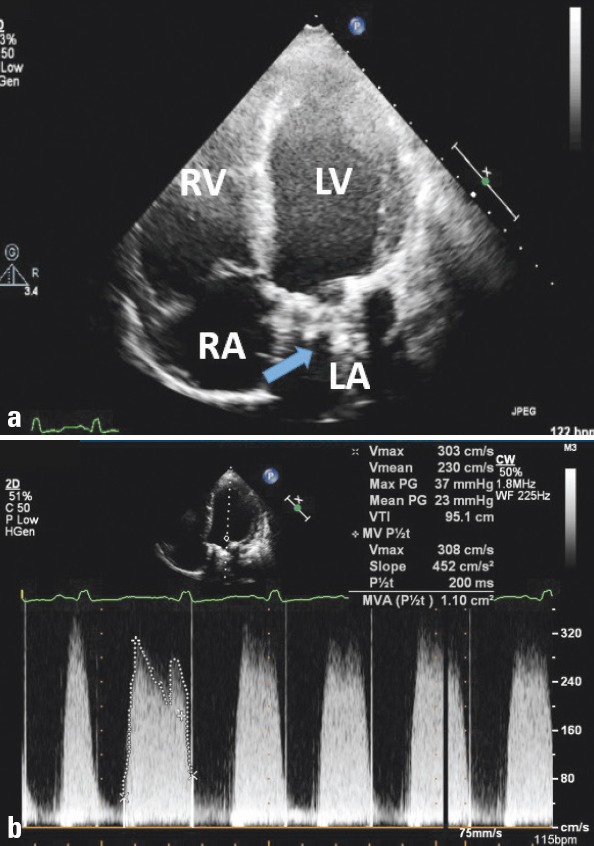
Two-dimensional transthoracic echocardiographic imaging of mitral prosthetic valve thrombosis (arrow) in four-chamber view (a) and increased transvalvular gradients and reduced mitral valve area, as demonstrated by Doppler imaging (b).
LA - left atrium; LV - left ventricle; RA - right atrium; RV -right ventricle
Two-dimensional (2-D) transesophageal echocardiography (TEE; 2-D TEE) is limited in evaluating structural abnormalities of prosthetic valves due to attenuation and acoustic shadowing; therefore, further TEE examination is usually required (5, 6). TEE can correctly identify opening and closing angles in most of the patients, regardless of the prosthetic type. Detailed image of the atrial side of the mitral valve prosthesis can be obtained because of the proximity of the esophagus to the heart and absence of interference with lungs and ribs (26). On the other hand, thrombus may not be clearly visualized by TEE in all aortic PVT cases. Acoustic shadowing from prosthetic material may often obscure the anterior part of the aortic prosthesis. This is also similar for the prosthesis on tricuspid position (24). TEE also has an indispensable value to assess thrombus size, mobility, and location, which may help in treatment decisions, such as thrombolysis, anticoagulation, and surgery (5, 6). Moreover, TEE provides direct imaging of the thrombus in the body or the appendage of the left atrium, which usually cannot to be detected with TTE. The presence of a left atrial thrombus is accepted as a contraindication for thrombolysis and should be ruled out by TEE before TT (5, 6).
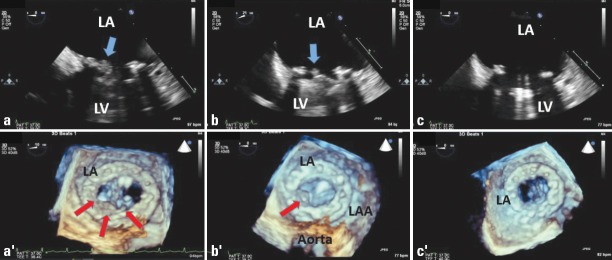
A thrombus was defined as soft and homogeneous, with mobile or fixed echodensity, similar to myocardium, located at the valve occluder, hinges, and/or valve struts (5, 6). The thrombus burden usually contributes to the severity of transvalvular gradients. Larger thrombi are more likely to cause hemodynamic compromise and may result in thromboembolic complications. The thrombus size visualized by TEE is important in deciding on the optimal treatment strategy. PRO-TEE trial has reported that a thrombus area <0.8 cm2 confers a lower risk for embolism or death associated with TT in left-sided obstructive PVT. Therefore, they showed that TEE could predict a low-risk group for TT (18). Figure 2a–c shows serial 2-D TEE images in a patient who received TT due to mitral PVT.
Figure 2.
Two-dimensional TEE demonstrates a soft thrombotic mass (arrow) attached to the hinge of the prosthesis (a). Real-time three-dimensional transesophageal echocardiography from the left atrial side revealed thrombus (arrows) on the prosthetic mitral valve (a’). The thrombus burden was diminished (arrow) after an initial dose of TT (25 mg TPA), shown by 2-D TEE (b) and 3-D TEE (b’). After the second dose of TT, the thrombus size was completely lysed, shown by 2-D TEE (c) and 3-D TEE (c’).
LA - left atrium; LAA - left atrial appendage; LV - left ventricle; TEE - transesophageal echocardiography; tPA - tissue-type plasminogen activator; TT - thrombolytic therapy
Real-time three-dimensional TEE (RT-3-D TEE) has been a milestone in the era of cardiovascular imaging of both native and prosthetic valves (19, 27). It is an excellent tool to obtain spatial information from cardiac structures and visualize cardiac pathologies in real-time. It has also the capacity to section the echogenic mass and visualize it from multiple angles (28).
RT-3-D TEE has especially provided a great insight into the evaluation of PVT. An en face view of the mitral valve from the left atrium can be carefully reconstructed for each patient when the mitral valve is closed because this method provides the best contrast to detect thrombus. Specific echocardiographic acquisition settings, including “gain,” “dynamic range,” “brightness.” and “smoothing” may be adjusted for better depiction of PVT (23). Furthermore, Vision H and Chromo map settings may be used for high-resolution color images. Linear, purple- or violet-colored echodensity on a bright cream-colored base of the endothelialized sewing ring surrounding the prosthetic valve suture line or medial to it suggests thrombus (19, 23). RT-3-D TEE provides a more comprehensive delineation of PVT compared to conventional 2-D TEE, which may underestimate or even miss thrombi, particularly when it is ring-located and nonobstructive “Doppler silent.” It may inform the clinician about the total thrombus burden in detail helping to organize a more strict anticoagulation therapy (27). Therefore, patients may benefit from accurate diagnosis and correct course of therapy with the utility of RT-3-D TEE (Fig. 2a–c). This also avoids unnecessary further diagnostic workup. It must be acknowledged that RT-3-D TEE is a complementary diagnostic tool; its accuracy depends on the quality of the original 2-D images. RT 3-D TEE is time-consuming and requires added training. It has also several limitations such as reduced temporal resolution, poor visualization of anterior structures of the heart such as aortic and tricuspid valves, and poor image quality due to poor electrocardiography gating in patients with arrhythmias (23). Furthermore, it has the problem of acoustic shadowing like 2-D imaging; for instance, it may be difficult to visualize the pathologies (thrombus, pannus, etc.) located on the ventricular side of the mitral prosthesis (29).
Cinefluoroscopy (CF) is a low-cost, noninvasive imaging technique, which is readily available in most centers and can be performed rapidly, particularly in unstable patients, for detecting stuck valves (30, 31). In the case of bileaflet valves, the disks can be directly visualized, and opening and closing angles measured using a tangential view (31). Although the role of CF has declined since the introduction of TEE, it still serves as a complementary method to echocardiography in evaluation of prosthetic valve obstruction (30). It may be particularly utilized as an easily repeatable modality to follow stable patients for evaluation of valve motions during TT. CF has also limitations; it is not useful in distinguishing pannus from thrombus since neither pannus nor thrombus can be identified fluoroscopically. Therefore, TEE should be performed to confirm the findings obtained by CF.
Multidetector cardiac computed tomography (MDCT) is a promising technique for functional evaluation of bileaflet mechanical valves. Opening and closing leaflet angles can be accurately assessed. Currently, TEE is still the most reliable method in the diagnosis of PVT, but MDCT can be used as a complementary diagnostic method for a definitive diagnosis in case of clinical suspicion. It may be helpful especially in patients with double left-sided mechanical valves because acoustic shadowing can occur even during TEE study and make the interpretation difficult. It has also been proven to be a useful method for the differential diagnosis of masses amenable to TT in patients with prosthetic valve dysfunction (32) (see next section) (Fig. 3).
Figure 3.
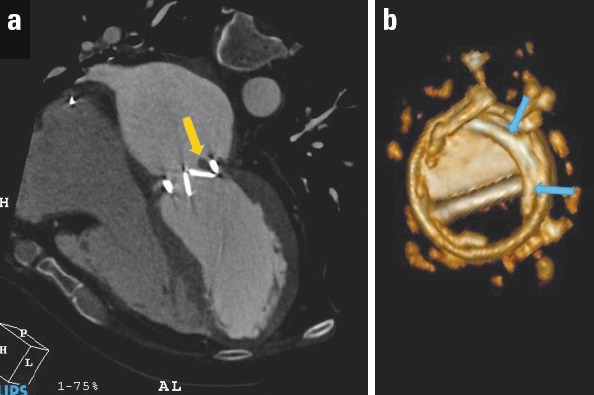
Multidetector computed tomography revealed a periprosthetic mass with HU: 65 (favors thrombus), which restricted the mobility of one of the leaflets (a). Volume rendering demonstration of pannus over the removed prosthetic valve (b). HU-Hounsfield unit
Differential diagnosis of PVT
The distinction between PVT and other prosthesis-related pathologies, such as pannus, vegetation, and prosthesis–patient mismatch (PPM), is important to choose the optimal treatment (23, 25, 33). Differential diagnosis based on clinical presentation may be challenging, and multimodality imaging, including echocardiography, CF, and MDCT is usually required. The masses related to prosthetic heart valves (thrombus, pannus, and vegetation) are compared in Table 2.
Table 2.
Comparison of prosthetic heart valve related-masses
| Thrombus | Pannus | Vegetation | |
|---|---|---|---|
| Echo predictors | Soft echo density with irregular shape, homogeneous, similar to myocardium | (Semi) circular, bright, and hard echo dense structures, sometimes containing focal calcific deposits | Pedunculated mass initially similar to thrombus, echodensity increases with increasing vegetation age |
| Localization | Mainly hinges, valve occluder, and/or valve struts |
Mitral: Atrial and/or ventricular sides Aortic: Aortic and/or left ventricular outflow tract side |
Typically attached on the low pressure side of the prosthesis |
| Mobility | Fixed or mobile | Immobile | Oscillating or nonoscillating (Mobility usually increases with increasing vegetation size) |
| 3-D echo predictors | Linear, purple- or violet-colored echo density on a bright cream color base of the endothelialized sewing ring surrounding the prosthetic valve suture line or medial to it | (Semi)circular mass narrowing circumferentially the inflow and outflow aspect of the prosthesis by extending into both sides of the prosthesis | Provides the entire morphology of the vegetation and determine its maximum size more accurately, leading to a better prediction of risk of embolism in IE patients compared to 2-D echo |
| Videointensity ratio | ≤0.4 | ≥0.7 | Unavailable data |
| MDCT | Low attenuation levels (Hounsfield units <90) Favors thrombus | High attenuation levels (Hounsfield units ≥145) Favors pannus formation | Combined with FDG-PET results in high-resolution anatomical and metabolic imaging of the prosthesis and its surrounding anatomy |
| Associated abnormalities | Pannus, LA and LAA thrombi, strands | Thrombus | Abscess, pseudoaneurysm, perforation, fistula, paravalvular leak, dehiscence |
2-D/3-D-dimensional; FDG-PET - 18-fluorine-fluorodesoxyglucose positron emission tomography; IE - infective endocarditis; LA - left atrium; LAA - left atrial appendage; MDCT - multide-tector-row computed tomography
Pannus is an overgrowth of fibrous tissue. It is relatively more common in the aortic position. Since surgery can be avoided for some patients with mechanical valve obstruction secondary to thrombosis, but not pannus, the distinction between these two etiologies needs specific concern (23, 32). Certain findings, such as the echocardiographic homogeneity of the mass, reduced transmitral gradients, and evolution of thrombus morphology with therapeutic anticoagulation, integrated with clinical (presen- ce of inadequate anticoagulation or evidence of thromboembolism) observations, help distinguish thrombosis from pannus overgrowth (19, 23). Previously introduced RT-3-D TEE provides visualization of the atrial and ventricular sides of the prosthesis, improves understanding of the relation between cardiac structures, and helps discriminating pannus from thrombus (34). Furthermore, the role of 64-slice MDCT in the differential diagnosis of thrombus versus pannus has been recently investigated by Gündüz et al. (32). They showed that high-attenuation (HU ≥145) periprosthetic masses are resistant to TT and predict pannus whereas the low-attenuation (HU <90) periprosthetic masses are susceptible to TT and predict thrombus (Table 2). Briefly, differentiation of these two etiologies is now easier with advanced multimodality imaging. Figure 4 shows echocardiographic and postoperative view of pannus overgrowth on mitral prosthesis.
Figure 4.
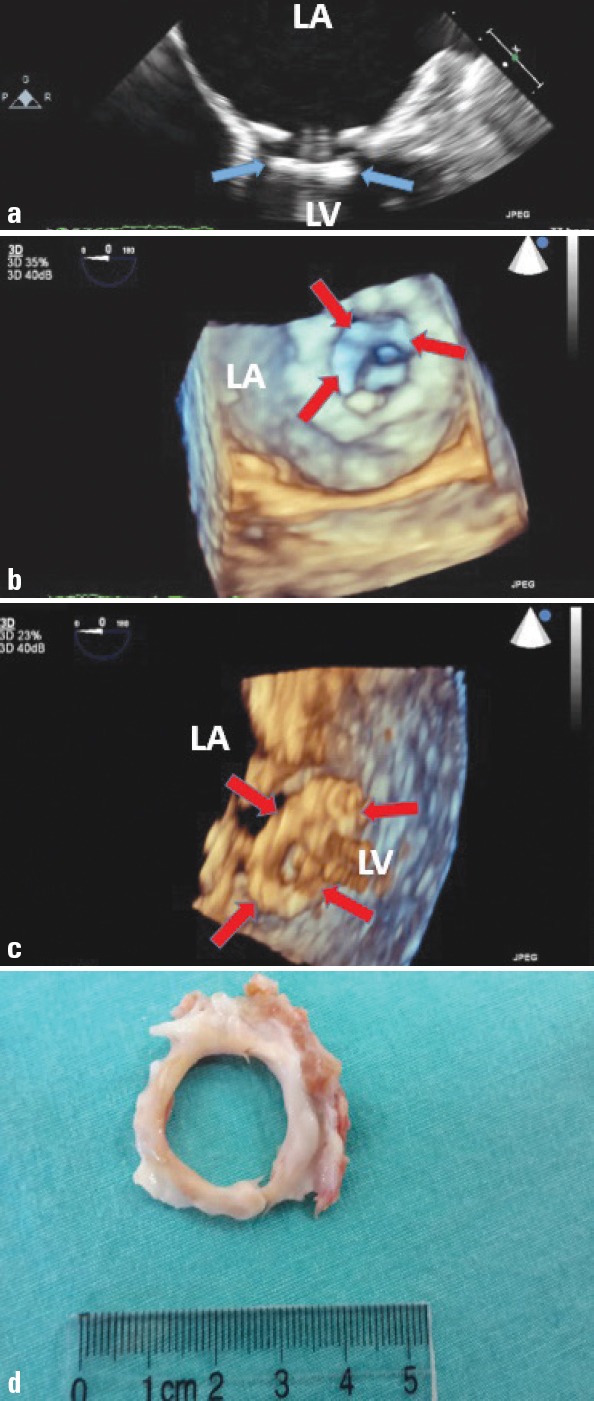
Two-dimensional transesophageal echocardiography (TEE) revealed a hyperechogenic circular mass on the left ventricular side of the prosthesis (a). Real-time three-dimensional TEE left atrial (b) and left ventricular (c) views showed circular left ventricular side pannus formation. Postoperative specimen of pannus formation (left ventricular-sided) is demonstrated (d)
Vegetation is another entity that should be considered in differential diagnosis of PVT (Table 2). They cannot be distinguished by echocardiography alone; depiction of these sessile or pedunculated masses with the presence of full clinical picture may lead to right diagnosis (23). Vegetation is more likely in febrile patients and in the presence of clinical signs of infective endocarditis, perivalvular destruction, leak, or abscess formation. Recently, positron emission tomography–computed tomography has proven its additive role in the diagnosis of infective endocarditis in patients with negative/inconclusive echocardiography (35).
PPM is an important cause of elevated velocity and gradients across normally functioning prosthetic valves. It is present when the effective orifice area (EOA) of the inserted prosthetic valve is too small in relation to body size. It is common (20%–70% of aortic valve replacements) and has been shown to be associated with worse hemodynamic function (33). The indexed EOA ≤0.65 and ≤0.90 favors PPM in aortic and mitral prosthesis, respectively (for those with body mass index <30 kg/m2). Although EOA determination is crucial in evaluating PPM, systolic time interval parameters, AT and AT/ET could be also very helpful, especially in differentiation of PPM from prosthetic aortic valve stenosis (25).
Management of PVT
Treatment modalities for PVT include anticoagulation with heparin, TT, surgery, or even in some cases only watchful wai- ting (23).
Anticoagulation
Conclusive data is lacking regarding the effectiveness of anticoagulation in resolution of PVT. It has been previously shown that the prognosis is favorable with medical therapy by optimization of anticoagulant treatment (short-term intravenous unfractionated heparin followed by warfarin adjustment and aspirin addition) for small asymptomatic thrombi (length <10 mm) (36). Lengyel et al. (37) demonstrated a low rate of success with heparin treatment in cases with nonobstructive PVT, but a more recent study authored by Laurent et al. (38) has reported the effectiveness of prolonged heparin with oral anticoagulation in preventing embolic events in patients with early nonobstructive PVT of size <5 mm after mechanical prosthetic mitral valve replacement. Our preliminary experience shows that unfractionated heparin could be successful in 73% of the nonobstructive PVT patients who have contraindications to TT (39). In current literature, the use of low-molecular-weight heparin in left-sided NOPVT is not clear yet.
TT versus surgery
Until the 1990s, the treatment of choice for mechanical valve obstruction was surgery, but over the last decade, TT has been used increasingly (40, 41). Unfortunately, randomized controlled trials to address the initial treatment strategy are lacking. The most recent European (42) and American guidelines (43) recommend surgery for patients with NYHA functional classes III and IV unless surgery is high risk (Class IIa). Thrombolysis is given a IIa indication in patients with right-sided valve thrombosis and a Class IIb indication in patients with a left-sided but small thrombus. The European Society of Cardiology guidelines (42) also recommend surgery for critically ill patients and restrict thrombolysis to patients with high surgical risk and/or right-si- ded valve thrombosis. On the other hand, TT is recommended as the first-line treatment for all patients with left-sided PVT by the Society for Heart Valve Disease guidelines and for patients with low thrombus burden (<0.8 cm2) (40). The diagnostic and mana- gement strategies of PVT are summarized in Figure 5.
Figure 5.
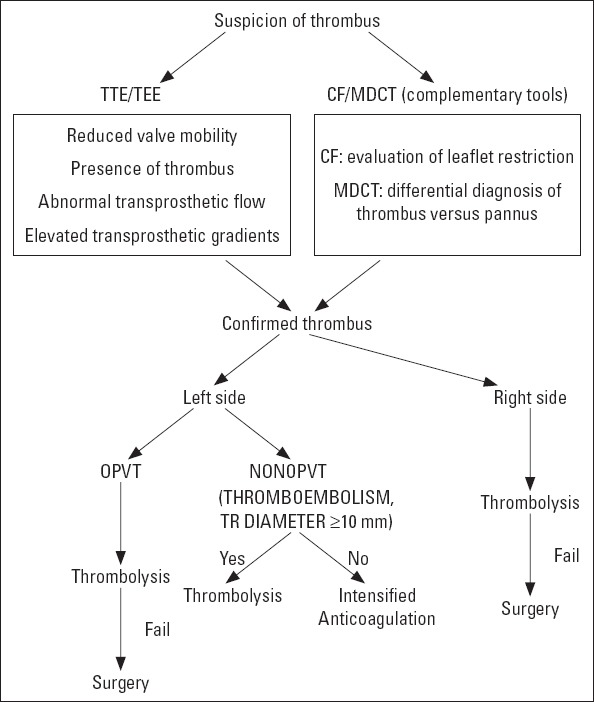
Diagnostic and therapeutic algorithm for prosthetic valve thrombosis.
CF - cinefluoroscopy; MDCT - multidetector computed tomography; NOPVT - nonobstructive prosthetic valve thrombosis; OPVT - obstructive prosthetic valve thrombosis; TEE - transesophageal echocardiography; TTE - transthoracic echocardiography
Recently, several meta-analyses and systematic reviews have been published. Karthikeyan et al. (44) evaluated seven studies with 690 episodes of PVT (446 treated with surgery and 244 with TT) and found no significant differences in the main outcome (restoration of valve functions) or death between patients treated surgically and with TT. They stated that urgent surgery should probably be preferred over TT in experienced centers.
On the basis of previous data, surgical mortality may reach 69%, depending on NYHA class and need for emergency surgery (6, 9, 44-46), whereas the reported death rate is 8%–13.5% in patients undergoing TT for PVT (44–46).
Recently, Castilho et al. (9) evaluated 26 studies reporting 1107 episodes of PVT treated by TT and 27 studies reporting 1132 surgeries for PVT. They reported much higher mortality rates with surgery compared with TT in the management of PVT (18.1% versus 6.6%). Nevertheless, it could be misleading to compare TT and surgery with respect to mortality rates without a head-to-head randomized trial.
Recent TT studies for PVT have shown much promise, with the results suggesting that such treatment modality might be the initial choice in these patients (6–8, 40, 47). There is no consensus regarding the optimal treatment strategy, neither the type, nor the dose or route of administration of thrombolytic agents. Due to its high fibrin specificity, recombinant tissue-type plasminogen activator (rt-PA) is widely used in the management of PVT (6–8). On the other hand, because of the relatively higher cost of tPA treatment, streptokinase is still used for TT in the developing countries. Although accelerated protocols seem attractive as they may induce more rapid lysis of the thrombus, they increase the risk of serious thromboembolism and bleeding events (4, 45).
The TROIA (Comparison of Different TRansesophageal Echocardiography Guided thrOmbolytic Regimens for prosthetIc vAlve Thrombosis) study (6), which includes the largest cohort published to date (182 consecutive patients with PVT in 220 different episodes), evaluated a strategy of TEE-guided fibrinolysis with rapid infusion of streptokinase (Group 1) versus slow infusion of streptokinase (Group II) versus full-dose t-PA (100 mg) (Group 3) versus half dose (50 mg) slow infusion of t-PA (Group IV) versus low-dose (25 mg) slow infusion of t-PA (Group 5). This was a monocentric, prospective, nonrandomized study. The authors reported successful thrombolysis in 83.2% of cases without a significant difference between thrombolytic protocols (68.8%, 85.4%, 75.0%, 81.5%, and 85.5%, respectively; p = 0.46). Assessment of complication rates by groups showed a statistically lower combined complication rate in low-dose slow infusion group. Therefore, the authors suggested that lower dose, TEE-guided, repeated, slow administration of a fibrinolytic agent could be equally efficacious with fewer complications. Although no mortality was reported in this regimen, nonfatal major complications were similar between the regimens. Therefore, in order to reduce nonfatal major complications, safety of ultra-slow TT regimen was investigated in 114 PVT patients—the PROMETEE trial (8). This study showed that, ultra-slow (25 hours) infusion of low-dose (25 mg) t-PA without bolus was associated with quite low nonfatal complications and mortality for PVT patients except for those with NYHA class-IV, without compromising effectiveness.
The main limitation associated with TT of left-sided PVT includes cerebral thromboembolism (48) and bleeding. In TROIA study (6), the rate of intracranial bleeding was 0.8% in PVT patients undergoing low-dose slow infusion t-PA strategy. In the PROMETEE study (8), none of the patients suffered intracranial hemorrhage, and the noncerebral major bleeding rate was also quite low (1.7%). These two recent studies have shown that low-dose slow infusion of tPA can be an effective and safe therapy in the management of PVT.
While tPA is widely used as a thrombolytic agent, infusion of rt-PA may trigger the production of anti-tissue plasminogen activator (tPA) antibodies (ATA), which may interfere with the success of TT, necessitating a higher dose of rt-PA for complete success. It has also been shown that some patients with PVT have increased baseline ATA levels, which is also associated with higher risk for rethrombosis (16).
Specific population
Pregnant women
PVT in pregnancy is a double jeopardizing event for both mother and fetus. The main treatment strategies include anticoagulation with heparin, TT, and redo surgery, each having its own pros and cons. Although no evidence-based guidelines for pregnant patients complicated with PVT are currently available, recommendations of guidelines for this complication are similar to the management of PVT in nonpregnant patients (42) and missing definitive class I recommendations because of lack of randomized controlled trials. Cardiac surgery in pregnancy is associated with very high maternal and fetal mortality (6% and 30%, respectively) and morbidity (24% and 9%, respectively) (49). Due to a recent study by Özkan et al. (7), which consisted of the largest series of pregnant patients with PVT reported to date, low-dose, slow infusion of tPA with repeated doses as needed that was guided by TEE was associated with a successful thrombolysis in all episodes, with no maternal deaths and a fetal mortality rate of 20%. The authors indicated that the incidence of maternal and fetal adverse events (mortality, abortion, etc.) with this protocol was lower than with surgery or medical therapy on the basis of the available published data and suggested that it should be used as first-line therapy for PVTs in pregnant women. The high success rate of TT was attributed to the fresh and rapid development of clot in this specific patient population compared with nonpregnant patients with gradually developed and organized thrombus.
Elderly
Elderly individuals are the rapidly growing segment of the population and choosing the optimal management for PVT could be challenging due to safety concerns. Surgical treatment may carry high risk especially in elderly patients with multiple comorbidities. Furthermore, older age also poses an increased risk of hemorrhage in patients undergoing TT. In a recent single-center study, Gündüz et al. (50) has demonstrated that slow or ultra-slow infusions of low-dose thrombolytic agents (mostly t-PA) provide considerably high lysis rates with low major adverse event rates in elderly patients with PVT. Consequently, TT is also a promising therapy in elderly population.
Patients with ischemic stroke
The risk of cerebral embolism can be up to 5%–6% for left-sided PVT (40). CT should be performed in patients who admit with initial suspicion of acute ischemic stroke to exclude any intracranial bleeding and these patients may be considered for TT only if they are stable by neuroradiologic assessment after 3–4 weeks of anticoagulation with UFH (8). Ischemic stroke may also occur during TT and may be managed with continuing low-dose and prolonged infusion of TT in eligible patients (48).
Patients with coronary embolism
It is a potential complication of prosthetic valves and should be suspected in patients with prosthetic valves who admit with acute coronary syndrome (20). Intracoronary thrombolysis, stent implantation, and embolectomy can be performed as reperfusion strategies. Furthermore, intravenous TT can be successfully performed for the management of PVT and concurrent coronary embolism in eligible patients as the fresh nature of the embolic material may easily respond to TT (20).
Conclusion
Despite technological advancements, the hemodynamic and physical properties of mechanical valves remain thrombogenic, and patients with prosthetic heart valves, therefore, are prone to developing PVT. Unfortunately, VKAs are still the only approved oral anticoagulants in patients with heart valve prostheses and today clinicians, worldwide, are expecting for an antithrombotic agent that is at least as effective but safer and more convenient in daily clinical practice. The diagnosis of PVT and other prosthetic valve dysfunctions is now easier with the use of multimodality imaging, including RT-3-D TEE and MDCT. There is still a debate about the optimal treatment strategy for PVT. Guidelines lack definitive NYHA class I recommendations, have significant disparities, and—in most cases—leave the decision to the clinician’s experience. The favorable clinical outcomes of TT comparing with the surgical approach have made TT the first-line treatment in many of the developing countries. Surgical treatment could be left for patients in which TT is contraindicated, or in those where it has already failed. Currently, the superiority of one over other remains speculative due to absence of a head-to-head randomized controlled trial between TT and surgery. However, the recently initiated randomized and multicenter study (NCT02243839), which compares TT (with tPA) versus surgery for the management of patients with PVT, will provide essential data.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.O.G., M.K., M.Ö., S.K.; Design – M.O.G., M.Y., E.B., M.Ö.; Supervision – M.O.G., M.K., M.Y., S.G., M.Ö.; Materials – M.K., M.Y., E.B., S.G., M.Ö.; Data collection &/or processing – M.O.G., M.K., M.Y., S.K., E.B., S.G., M.Ö.; Analysis &/or interpretation – M.O.G., M.K., M.Y., S.K., E.B., S.G., M.Ö.; Literature search – M.O.G., M.K., M.Ö.; Writing – M.O.G., M.Ö.; Critical review – M.O.G., M.K., M.Ö
References
- 1.Hermans H, Vanassche T, Herijgers P, Meuris B, Herregods MC, Van de Werf F, et al. Antithrombotic therapy in patients with heart valve prostheses. Cardiol Rev. 2013;21:27–36. doi: 10.1097/CRD.0b013e3182638578. [DOI] [PubMed] [Google Scholar]
- 2.Grunkemeier GL, Rahimtoola SH. Artificial heart valves. Annu Rev Med. 1990;41:251–63. doi: 10.1146/annurev.me.41.020190.001343. [DOI] [PubMed] [Google Scholar]
- 3.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–41. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 4.Karthikeyan G, Math RS, Mathew N, Shankar B, Kalaivani M, Singh S, et al. Accelerated infusion of streptokinase for the treatment of left-sided prosthetic valve thrombosis: a randomized controlled trial. Circulation. 2009;120:1108–14. doi: 10.1161/CIRCULATIONAHA.109.876706. [DOI] [PubMed] [Google Scholar]
- 5.Özkan M, Kaymaz C, Kırma C, Sönmez K, Özdemir N, Balkanay M, et al. Intravenous thrombolytic treatment of mechanical prosthetic valve thrombosis: a study using serial transesophageal echocardiography. J Am Coll Cardiol. 2000;35:1881–9. doi: 10.1016/s0735-1097(00)00654-9. [DOI] [PubMed] [Google Scholar]
- 6.Özkan M, Gündüz S, Biteker M, Astarcıoğlu MA, Çevik C, Kaynak E, et al. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: The TROIA Trial. JACC Cardiovasc Imaging. 2013;6:206–16. doi: 10.1016/j.jcmg.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Özkan M, Çakal B, Karakoyun S, Gürsoy OM, Çevik C, Kalçık M, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation. 2013;128:532–40. doi: 10.1161/CIRCULATIONAHA.113.001145. [DOI] [PubMed] [Google Scholar]
- 8.Özkan M, Gündüz S, Gürsoy OM, Karakoyun S, Astarcıoğlu MA, Kalçık M, et al. A novel strategy in the management of PROsthetic Mechanical valve Thrombosis and the prEdictors of outcomE: the Ultra-slow PROMETEE trial. Am Heart J. 2015;170:409–18. doi: 10.1016/j.ahj.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 9.Castilho FM, De Sousa MR, Mendonça AL, Ribeiro AL, Cáceres-Lóriga FM. Thrombolytic therapy or surgery for valve prosthesis thrombosis: systematic review and meta-analysis. J Thromb Haemost. 2014;12:1218–28. doi: 10.1111/jth.12577. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JM, Schoen EJ. Interaction of blood with artificial surfaces. In: Butchart E, Bodnar E, editors. Thrombosis Embolism and Bleeding. London: ICR Publishers; 1992. pp. 160–71. [Google Scholar]
- 11.Horstkotte D, Aul C, Seipel L, Körfer R, Budde T, Schulte HD, et al. Effect of valve type and valve function on chronic intravascular hemolysis following mitral and aortic valve replacement using alloprostheses. Z Kardiol. 1983;72:119–31. [PubMed] [Google Scholar]
- 12.Gürsoy OM, Karakoyun S, Kalçık M, Gökdeniz T, Yesin M, Gündüz S, et al. Usefulness of novel hematologic inflammatory parameters to predict prosthetic mitral valve thrombosis. Am J Cardiol. 2014;113:860–4. doi: 10.1016/j.amjcard.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Aykan AC, Gökdeniz T, Gündüz S, Astarcıoğlu MA, Gürsoy OM, Ertürk E, et al. Value of serum fibrinogen levels in the assessment of mechanical prosthetic valve thrombosis. J Heart Valve Dis. 2014;23:222–7. [PubMed] [Google Scholar]
- 14.Aykan AÇ, Gökdeniz T, Kalçık M, Astarcıoğlu MA, Gündüz S, Karakoyun S, et al. Role of anticardiolipin antibodies in the pathogenesis of prosthetic valve thrombosis: An observational study. Herz. 2015;40:528–33. doi: 10.1007/s00059-013-4038-1. [DOI] [PubMed] [Google Scholar]
- 15.Kalçık M, Gürsoy MO, Karakoyun S, Yesin M, Astarcıoğlu MA, Özkan M. Potential inherited causes of recurrent prosthetic mitral valve thrombosis in a pregnant patient suffering from recurrent miscarriage. Korean Circ J. 2014;44:268–70. doi: 10.4070/kcj.2014.44.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Özkan M, Kalçık M, Gürsoy MO, Öcal L, Griffini S, Karakoyun S, et al. Assessment of Anti-Tissue Type Plasminogen Activator Antibodies in Patients With Prosthetic Heart Valve Thrombosis: The ATA Trial. J Cardiovasc Pharmacol Ther. 2016;21:372–80. doi: 10.1177/1074248415615236. [DOI] [PubMed] [Google Scholar]
- 17.Özkan M, Oğuz AE, Gürsoy OM, Gündüz S, Aykan CA, Astarcıoğlu MA, et al. Management of heparin-induced thrombocytopenia during thrombolytic therapy for prosthetic valve thrombosis. J Heart Valve Dis. 2012;21:636–40. [PubMed] [Google Scholar]
- 18.Tong AT, Roudaut R, Özkan M, Sagie A, Shahid MS, Pontes Júnior SC, et al. Prosthetic Valve Thrombolysis-Role of Transesophageal Echocardiography (PRO-TEE) Registry Investigators. Transesophageal echocardiography improves risk assessment of thrombolysis of prosthetic valve thrombosis: results of the international PRO-TEE registry. J Am Coll Cardiol. 2004;43:77–84. doi: 10.1016/j.jacc.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Özkan M, Gürsoy OM, Astarcıoğlu MA, Gündüz S, Çakal B, Karakoyun S, et al. Real-time three-dimensional transesophageal echocardiography in the assessment of mechanical prosthetic mitral valve ring thrombosis. Am J Cardiol. 2013;112:977–83. doi: 10.1016/j.amjcard.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Karakoyun S, Gürsoy MO, Kalçık M, Yesin M, Özkan M. A case series of prosthetic heart valve thrombosis-derived coronary embolism. Türk Kardiyol Dern Ars. 2014;42:467–71. doi: 10.5543/tkda.2014.05031. [DOI] [PubMed] [Google Scholar]
- 21.Gürsoy OM, Karakoyun S, Kalçık M, Özkan M. The incremental value of RT three-dimensional TEE in the evaluation of prosthetic mitral valve ring thrombosis complicated with thromboembolism. Echocardiography. 2013;30:E198–201. doi: 10.1111/echo.12246. [DOI] [PubMed] [Google Scholar]
- 22.Aykan AÇ, Gürsoy OM, Özkan M, Yıldız M, Kahveci G, Uslu Z. Successful treatment of renal artery thromboembolism with low-dose prolonged infusion of tissue-typed plasminogen activator in a patient with mitral mechanical heart valve thrombosis under the guidance of multimodality imaging. Blood Coagul Fibrinolysis. 2012;23:663–5. doi: 10.1097/MBC.0b013e328355e86d. [DOI] [PubMed] [Google Scholar]
- 23.Gürsoy MO, Kalçık M, Karakoyun S, Özkan M. The current status of fluoroscopy and echocardiography in the diagnosis of prosthetic valve thrombosis-a review article. Echocardiography. 2015;32:156–64. doi: 10.1111/echo.12721. [DOI] [PubMed] [Google Scholar]
- 24.Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocardiogr. 2009;22:975–1014. doi: 10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Ben Zekry S, Saad RM, Ozkan M, Al Shahid MS, Pepi M, Muratori M, et al. Flow acceleration time and ratio of acceleration time to ejection time for prosthetic aortic valve function. JACC Cardiovasc Imaging. 2011;4:1161–70. doi: 10.1016/j.jcmg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Muratori M, Montorsi P, Teruzzi G, Celeste F, Doria E, Alamanni F, et al. Feasibility and diagnostic accuracy of quantitative assessment of mechanical prostheses leaflet motion by transthoracic and transesophageal echocardiography in suspected prosthetic valve dysfunction. Am J Cardiol. 2006;97:94–100. doi: 10.1016/j.amjcard.2005.07.112. [DOI] [PubMed] [Google Scholar]
- 27.Gürsoy OM, Özkan M. The role of real-time 3-dimensional transesophageal echocardiography in depiction of the concealed base of the iceberg. Anadolu Kardiyol Derg. 2012;12:E22–3. doi: 10.5152/akd.2012.142. [DOI] [PubMed] [Google Scholar]
- 28.Reddy VK, Faulkner M, Bandarupalli N, Nanda NC, Singh P, Dutta R, et al. Incremental value of live/real time three-dimensional transthoracic echocardiography in the assessment of right ventricular masses. Echocardiography. 2009;26:598–609. doi: 10.1111/j.1540-8175.2009.00952.x. [DOI] [PubMed] [Google Scholar]
- 29.Sugeng L, Shernan SK, Weinert L, Shook D, Raman J, Jeevanandam V, et al. Real-time three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr. 2008;21:1347–54. doi: 10.1016/j.echo.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Kalçık M, Gürsoy OM, Astarcıoğlu MA, Özkan M. A serial fluoroscopy-guided thrombolytic therapy of a mechanical tricuspid prosthetic valve thrombosis with low-dose and ultra-slow infusion of tissue-type plasminogen activator. Turk Kardiyol Dern Ars. 2014;42:478–81. doi: 10.5543/tkda.2014.09804. [DOI] [PubMed] [Google Scholar]
- 31.Montorsi P, Cavoretto D, Alimento M, Muratori M, Pepi M. Prosthetic mitral valve thrombosis: Can fluoroscopy predict the efficacy of thrombolytic treatment? Circulation. 2003;108:II79–84. doi: 10.1161/01.cir.0000087900.45365.45. [DOI] [PubMed] [Google Scholar]
- 32.Gündüz S, Özkan M, Kalçık M, Gürsoy OM, Astarcıoğlu MA, Karakoyun S, et al. Sixty-Four–Section Cardiac Computed Tomography in Mechanical Prosthetic Heart Valve Dysfunction Thrombus or Pannus. Circ Cardiovasc Imaging. 2015;8:e003246. doi: 10.1161/CIRCIMAGING.115.003246. [DOI] [PubMed] [Google Scholar]
- 33.Pibarot P, Dumesnil JG. Prosthesis-patient mismatch:definition, clinical impact, and prevention. Heart. 2006;92:1022–9. doi: 10.1136/hrt.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Özkan M, Gündüz S, Yıldız M, Duran NE. Diagnosis of the prosthetic heart valve pannus formation with real-time three-dimensional transoesophageal echocardiography. Eur J Echocardiogr. 2010;11:E17. doi: 10.1093/ejechocard/jep206. [DOI] [PubMed] [Google Scholar]
- 35.Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374–82. doi: 10.1016/j.jacc.2013.01.092. [DOI] [PubMed] [Google Scholar]
- 36.Bemurat LR, Laffort PR, Deville CJ, Roques XG, Baudet EM, Roudaut RP. Management of Nonobstructive Thrombosis of Prosthetic Mitral Valve in Asymptomatic Patients in the Early Postoperative Period: A Study in 20 Patients. Echocardiography. 1999;16:339–46. doi: 10.1111/j.1540-8175.1999.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 37.Lengyel M, Vegh G, Vandor L. Thrombolysis is superior to heparin for non-obstructive mitral mechanical valve thrombosis. J Heart Valve Dis. 1999;8:167–73. [PubMed] [Google Scholar]
- 38.Laurent M, Lelong B, Verhoye JP, Khattar C, de Place C, Matali P, et al. Prolonged heparin and vitamin K antagonist regimen for early non-obstructive thrombosis of mechanical mitral valve prostheses. J Heart Valve Dis. 2008;17:533–41. [PubMed] [Google Scholar]
- 39.Yesin M, Kalçık M, Cerşit S, Gürsoy MO, Kılıçgedik A, Börekçi A, et al. Monitorization of patients with thrombotic prosthetic valves with infusion of unfractionated heparin under the guidance of serial transesophageal echocardiographic examinations. Anadolu Kardiyol Derg. 2014;14(Suppl 1):1–165. [Google Scholar]
- 40.Lengyel M, Horstkotte D, Völler H, Mistiaen WP. Working Group Infection, Thrombosis, Embolism and Bleeding of the Society for Heart Valve Disease. Recommendations for the management of prosthetic valve thrombosis. J Heart Valve Dis. 2005;14:567–75. [PubMed] [Google Scholar]
- 41.Biteker M, Altun I, Başaran O, Doğan V, Yıldırım B, Ergun G. Treatment of prosthetic valve thrombosis: Current evidence and future directions. J Clin Med Res. 2015;7:932–6. doi: 10.14740/jocmr2392w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–96. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 44.Karthikeyan G, Senguttuvan NB, Joseph J, Devasenapathy N, Bahl VK, Airan B. Urgent surgery compared with fibrinolytic therapy for the treatment of left-sided prosthetic heart valve thrombosis: a systematic review and meta-analysis of observational studies. Eur Heart J. 2013;34:1557–66. doi: 10.1093/eurheartj/ehs486. [DOI] [PubMed] [Google Scholar]
- 45.Bonou M, Lampropoulos K, Barbetseas J. Prosthetic heart valve obstruction: thrombolysis or surgical treatment? Eur Heart J Acute Cardiovasc Care. 2012;1:122–7. doi: 10.1177/2048872612451169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang G, Schaff HV, Sundt TM, Rahimtoola SH. Treatment of obstructive thrombosed prosthetic heart valve. J Am Coll Cardiol. 2013;62:1731–6. doi: 10.1016/j.jacc.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 47.Caceres-Loriga FM, Perez-Lopez H, Morlans-Hernandez K, Facundo-Sánchez H, Santos-Gracia J, Valiente-Mustelier J, et al. Thrombolysis as first choice therapy in prosthetic heart valve thrombosis. A study of 68 patients. J Thromb Thrombolysis. 2006;21:185–90. doi: 10.1007/s11239-006-4969-y. [DOI] [PubMed] [Google Scholar]
- 48.Özkan M, Gürsoy OM, Atasoy B, Uslu Z. Management of acute ischemic stroke occurred during thrombolytic treatment of a patient with prosthetic mitral valve thrombosis: continuing thrombolysis on top of thrombolysis. Anadolu Kardiyol Derg. 2012;12:689–90. doi: 10.5152/akd.2012.222. [DOI] [PubMed] [Google Scholar]
- 49.Weiss BM, von Segesser LK, Alon E, Seifert B, Turina MI. Outcome of cardiovascular surgery and pregnancy: a systematic review of the period 1984-1996. Am J Obstet Gynecol. 1998;179:1643–53. doi: 10.1016/s0002-9378(98)70039-0. [DOI] [PubMed] [Google Scholar]
- 50.Gündüz S, Özkan M, Yesin M, Kalçık M, Gürsoy MO, Karakoyun S, et al. Prolonged Infusions of Low-Dose Thrombolytics in Elderly Patients With Prosthetic Heart Valve Thrombosis. Clin Appl Thromb Hemost. 2015 Oct 6; doi: 10.1177/1076029615609698. Epub ahead of print. [DOI] [PubMed] [Google Scholar]