Abstract
Objective:
We recently described the CHA2DS2-VASc-HS score as a novel predictor of coronary artery disease (CAD) severity in stable CAD patients. We aimed to assess the accuracy of the CHA2DS2-VASc-HS score in the determination of CAD severity and complexity and its availability in the risk stratification of in-hospital major adverse cardiovascular events (MACE) in non-ST elevation acute coronary syndrome (NSTE-ACS) patients.
Methods:
We prospectively analyzed the clinical and angiographic data of consecutive NSTE-ACS patients in our clinic. Patients were classified into three tertiles according to their SYNTAX score (SS): tertile 1 had an SS of 0–22; tertile 2 had an SS of 23–32; and tertile 3 had an SS of >32. There were no specific exclusion criteria except for previous coronary artery bypass grafting (CABG) because SS was validated for only native coronary arteries for this study. We used the following analyses: χ2 or Fisher’s exact tests, one-way analysis of variance or Kruskal–Wallis tests, Pearson’s or Spearman’s tests, the receiver operating characteristics (ROC) curve analysis, the area under the curve (AUC) or C-statistic, and pairwise comparisons of the ROC curves.
Results:
A total of 252 patients were enrolled. There were 131 patients in tertile 1, 79 in tertile 2, and 42 in tertile 3. The number of diseased vessels was correlated with the Global Registry for Acute Coronary Events (GRACE) (p<0.001), Thrombolysis in Myocardial Infarction (TIMI) (p<0.001), and CHA2DS2-VASc-HS (p<0.001) scores. In the ROC curve analyses, the cut-off value of the CHA2DS2-VASc-HS score in the prediction of in-hospital MACE was >5 with a sensitivity of 69.6% and specificity of 90.3% (AUC: 0.804, 95%: CI 0.750–0.851, p<0.001). We also compared the diagnostic accuracy of the CHA2DS2-VASc-HS score with the TIMI and GRACE risk scores in the determination of the in-hospital MACE and found no differences.
Conclusion:
The CHA2DS2-VASc-HS score was positively correlated with the severity and complexity of CAD. We also found that CHA2DS2-VASc-HS was comparable with other risk scores for the risk stratification of the in-hospital MACE of NSTE-ACS patients. Therefore, it may play an important role as a predictive model of NSTE-ACS patients in clinical practice. (Anatol J Cardiol 2016; 16: 742-8)
Keywords: non-ST elevation acute coronary syndrome, CHA2DS2-VASc-HS score, TIMI score, GRACE score
Introduction
Coronary artery disease (CAD) remains the most common cause of death worldwide (1). Most accepted risk factors for CAD, including hypertension (HT), smoking, hyperlipidemia (HL), and diabetes mellitus (DM), can be modified by changes in lifestyle and the use of medications (1). However, prospective risk stratification is essential to estimate early hospital outcomes, in patient prognosis, and to assist in clinical and treatment decisions.
Risk evaluation is important for the management of patients with non-ST elevation acute coronary syndrome (NSTE-ACS). The risk of morbidity and mortality in NSTE-ACS patients varies according to the presence of baseline risk factors, clinical syndrome features, and the management strategy. Current guidelines for the risk stratification of patients with NSTE-ACS (2, 3) recommend the use of the Thrombolysis in Myocardial Infarction (TIMI) risk score (4) or the Global Registry for Acute Coronary Events (GRACE) score (5). Additional tools for CAD prognosis have been developed, including synergy between percutaneous coronary intervention (PCI) with TAXUS and cardiac surgery (SYNTAX) score (SS) (6). Our group recently described the CHA2DS2-VASc-HS score as a novel predictor of CAD severity in stable CAD patients (7). The CHA2DS2-VASc-HS nomenclature represents congestive heart failure (C), HT (H), age ≥75 years (A2), DM (D), and history of stroke or TIA (S2), vascular disease (V), age 65–74 years (A), and male gender (as the sex category), hyperlipidemia (HL), and smoking (S). This scoring system includes HL and smoking as other major risk factors for CAD, in addition to using males rather than females.
In this study, we assessed the accuracy of the CHA2DS2-VASc-HS scoring system in the determination of CAD severity and complexity in NSTE-ACS patients who were at a high risk of severe CAD. We also compared the CHA2DS2-VASc-HS score with the TIMI and GRACE scores in the risk stratification of in-hospital major adverse cardiovascular events (MACE) in patients with NSTE-ACS undergoing coronary angiography (CAG).
Methods
Study design
This was a prospective cross-sectional study performed at the Adıyaman University Training and Research Hospital. The study protocol conformed to the principles of the Declaration of Helsinki and was approved by the Local Ethics Committee. Informed consent was obtained from all participants.
Study population
This study involved 252 consecutive patients with NSTE-ACS admitted to our clinic between October 2012 and October 2014. NSTE-ACS was defined as presenting with typical chest discomfort at rest for the past 48 h along with at least one of the following characteristics: 1) ST or T wave changes on the electrocardiogram suggestive of myocardial ischemia, 2) positive cardiac enzymes, and 3) new documentation of CAD or prior existence of CAD (2). We used cardiac troponin I as a positive biomarker with a threshold of 0.04 ng/mL measured by the Alere Triage MeterPro device (Alere San Diego, Inc, CA, USA). In addition, we selected all patients who underwent invasive coronary angiography during hospitalization. Because SS had been validated for only native coronary arteries at the time of our analysis (8), patients with previous CABG were excluded. There were no other specific exclusion criteria. Lesions with in-stent restenosis were scored as de novo lesions.
Study protocol
Demographic characteristics including age, gender, DM, HT, HL, current cigarette smoking, family history of premature CAD, chronic heart failure, previous ischemic stroke or transient ischemic attack (TIA), peripheral artery disease (PAD), medical history (prior PCI and/or CABG), presenting symptoms, biochemical and electrocardiography findings, echocardiographic examination, and digital and/or non-digital hospital outcome data were obtained.
DM was diagnosed as a fasting blood glucose ≥126 mg/dL or the current use of anti-diabetic medications. HT was diagnosed if repeated measurements of systolic and diastolic blood pressure were ≥140 mm Hg and ≥90 mm Hg, respectively, or if the patient received chronic anti-hypertensive medication treatment. A level of low-density lipoprotein cholesterol above 160 mg/dL according to the National Cholesterol Education Program Adult Treatment Panel III recommendations (9) or the usage of lipid-lowering medications was defined as HL. Cigarette smoking was defined as smoking a minimum of 10 cigarettes per day for at least 1 year in patients who had never stopped smoking before the day of evaluation. Family history was defined as the presence of heart disease or sudden cardiac death in a male first-degree relative aged <55 years or in a female first-degree relative aged <65 years. Chronic heart failure was defined as reduced left ventricular ejection fraction (<40%). Simpson’s method (10) was used to measure left ventricular ejection fraction in the 2-dimensional echocardiographic apical 4-chamber view. Vascular disease was considered to be the presence of PAD. PAD was defined as at least 50% stenosis diagnosed by Dupplex-sonography of the non-coronary artery circulation. When the stroke or TIA was due to thromboembolism in the carotid or vertebral arteries, they were included in the scoring. Patients who had a stroke and TIA were characterized as having severe carotid artery disease.
CAG was performed using the standard Judkins technique via the femoral route. The severity of CAD was determined by the number of significantly diseased coronary arteries. Significant vessel disease was defined as the presence of ≥50% luminal diameter stenosis in at least one major coronary artery. Multivessel coronary disease was defined as the presence of ≥50% luminal diameter stenosis involving at least two major epicardial coronary arteries. Two-vessel disease was defined as ≥50% left main coronary artery narrowing. Two experienced cardiologists evaluated all of the angiograms and assessed SS of each coronary lesion producing ≥50% diameter stenosis in vessels ≥1.5 mm by visual estimation using the algorithm reported on the website (6). Thereafter, two experienced cardiologists, without knowledge of the patients’ CAD status, calculated the TIMI, GRACE, and CHA2DS2-VASc-HS scores. The CHA2DS2-VASc-HS score was calculated by assigning one point each for the presence of chronic heart failure, HT, DM, vascular disease, age 65–74 years, male gender (as a sex category), HL, and smoking (S), and by assigning two points for history of stroke or TIA and age ≥75 years (Table 1) (7). The maximum CHA2DS2-VASc-HS score was 11. The intra- and interobserver coefficients of variation for CHA2DS2-VASc-HS score were 3.5% and 3.4%, respectively.
Table 1.
Definition of CHA2DS2-VASc-HS score
| C | Congestive heart failure | 1 point |
| H | Hypertension | 1 point |
| A2 | Age >75 years | 2 point |
| D | Diabetes mellitus | 1 point |
| S2 | Previous stroke or TIA | 2 point |
| V | Vascular disease | 1 point |
| A | Age 65–74 years | 1 point |
| Sc | Sex category, male gender | 1 point |
| H | Hyperlipidemia | 1 point |
| S | Smoker | 1 point |
Maximum total score=11 points
In-hospital MACE were defined as non-fatal myocardial infarction (MI), stent thrombosis, and in-hospital mortality during the period of in-hospital follow-up. Nonfatal MI was defined as recurrent chest pain and/or development of new electrocardiogram changes together with a new rise of ≥20% in cardiac biomarkers measured following the recurrent pain. Stent thrombosis was defined according to the Academic Research (11). In-hospital mortality had to be verified as death due to MI, cardiac arrest, or other cardiac causes. Patients with more than one event have been assigned the highest rank event.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (version 20.0, SPSS Inc., Chicago, Illinois) and MedCalc Statistical Software (version 12.7.8, Mariakerke, Belgium). Continuous variables were presented as means±SD and/or medians (minimum to maximum). Categorical variables compared with the χ2 or Fisher’s exact tests were summarized as percentages. The Kolmogorov–Smirnov test was used to evaluate normal distribution of continuous variables. One-way analysis of variance or Kruskal–Wallis tests were used to compare the three groups. Correlation analysis was performed using Pearson’s or Spearman’s tests. The receiver operating characteristics (ROC) curve analysis was used to evaluate the sensitivity and specificity of the CHA2DS2-VASc-HS score and its cut-off value for predicting the severity and complexity of CAD. The area under the curve (AUC), or C-statistic, was used as a measure of the predictive accuracy of the risk scores. The AUC comparison of these scoring systems was performed using the Delong method (12). The relative performance of each test was evaluated by calculating the 95% confidence interval (CI) for the difference between two AUCs. To compare the in-hospital prognostic performance of the CHA2DS2-VASc-HS score with the GRACE and TIMI risk score, pairwise comparisons of the ROC curves were also performed. A p-value <0.05 was considered to indicate statistical significance.
Results
A total of 252 patients [mean age: 60.9 ± 11.9 years, 64 female (25.4%)] were included in this study. Patients were classified into three tertiles according to their SS: tertile 1 had an SS <22; tertile 2 had an SS of 22–32; and tertile 3 had an SS of >32. The baseline, clinical, and laboratory characteristics of the study population stratified according to SS tertile are presented in Table 2. The GRACE, TIMI, and CHA2DS2-VASc-HS scores were significantly increased from tertile 1 to tertile 3.
Table 2.
Baseline demographic and clinical parameters of the study population
| Parameters | Tertile 1 SS <23 (n=131) |
Tertile 2 SS 23–32 (n=79) |
Tertile 3 SS >32 (n=42) |
P (T 1-2) |
P (T 1–3) |
P (T 2-3) |
|---|---|---|---|---|---|---|
| Age, years | 59.2±12.0 | 60.6±12.0 | 66.9±9.6 | 0.656 | 0.001 | 0.015 |
| Sex: female, n % | 41 (31) | 18 (23) | 5 (12) | 0.351 | 0.032 | 0.386 |
| DM, n % | 51 (39) | 37 (47) | 24 (57) | 0.503 | 0.098 | 0.521 |
| Hypertension, n % | 73 (56) | 43 (54) | 31(74) | 0.981 | 0.097 | 0.099 |
| Hyperlipidemia, n % | 53(41) | 46 (58) | 28 (67) | 0.031 | 0.008 | 0.641 |
| Family history, n % | 13 (10) | 29 (37) | 14 (33) | <0.001 | 0.003 | 0.897 |
| Smoking, n % | 40 (31) | 45 (57) | 25 (60) | <0.001 | 0.002 | 0.958 |
| Previous history, n % | ||||||
| MI | 23 (18) | 26 (33) | 20 (48) | 0.036 | <0.001 | 0.180 |
| PCI | 8 (21) | 21 (27) | 19 (45) | 0.550 | 0.021 | 0.199 |
| Previous medication, n % | ||||||
| Platelet inhibitors | 23 (17) | 34 (43) | 21 (50) | <0.001 | <0.001 | 0.689 |
| Beta-blockers | 45 (34) | 33 (42) | 18 (43) | 0.534 | 0.587 | 0.993 |
| ACE inhibitors/ARBs | 83 (63) | 50 (63) | 25 (60) | 0.998 | 0.897 | 0.913 |
| Statins | 65 (50) | 38 (48) | 17 (41) | 0.975 | 0.560 | 0.706 |
| Stroke/TIA, n % | 0 (0) | 3 (4) | 7 (17) | 0.330 | <0.001 | 0.001 |
| PAD, n % | 3 (2) | 19 (24) | 17 (41) | <0.001 | <0.001 | 0.027 |
| LVEF, % | 52.5±7.8 | 43.9±8.1 | 42.3±8.1 | <0.001 | <0.001 | 0.527 |
| Glucose, mg/dL | 143.3±80.7 | 157.3±76.8 | 160.0±93.2 | 0.449 | 0.479 | 0.983 |
| Creatinine, mg/dL | 0.9±0.3 | 1.0±0.9 | 1.4±1.1 | 0.440 | <0.001 | 0.014 |
| LDL, mg/dL | 111.1±34.5 | 112.3±43.0 | 115.7±39.2 | 0.973 | 0.778 | 0.889 |
| HDL, mg/dL | 39.1±18.2 | 37.6±10.3 | 40.6±13.3 | 0.773 | 0.844 | 0.562 |
| TG, mg/dL | 177.1±108.4 | 156.0±95.1 | 134.2±82.7 | 0.306 | 0.044 | 0.493 |
| GRACE score | 133.4±45.9 | 167.7±63.6 | 204.1±69.1 | <0.001 | <0.001 | 0.002 |
| TIMI score | 2.21±1.32 | 3.46±1.41 | 4.31±1.54 | <0.001 | <0.001 | 0.004 |
| CHA2DS2-VASc-HS score | 2.89±1.31 | 4.19±1.60 | 5.55±2.28 | <0.001 | <0.001 | <0.001 |
Data are expressed as mean±SD and/or median (minimum–maximum) or count (percentage) for categorical variables
ACE - angiotensin converting enzyme; ARB - angiotensin receptor blockers; DM - diabetes mellitus; HDL - high-density lipoprotein; LDL - low-density lipoprotein; LVEF - left ventricular ejection fraction; MI - myocardial infarction; PAD - peripheral arterial disease; PCI - percutaneous coronary intervention; T - tertile; TG - triglycerides
A comparison of the tertiles revealed that as SS increased, higher values were observed for each scoring system. SS was positively correlated with the GRACE (r=0.431, p<0.001), TIMI (r=0.523, p<0.001), and CHA2DS2-VASc-HS (r=0.574, p<0.001) scores. The number of diseased vessels was correlated with the GRACE (r=0.343, p<0.001), TIMI (r=0.460, p<0.001), and CHA2DS2-VASc-HS (r=0.474, p<0.001) scores.
A comparison of the risk scores according to the number of diseased vessels is presented in Table 3. We also evaluated two models based on SS in the ROC curve analysis: Model 1=ROC curve analysis based on a low SS >22; Model 2=ROC curve analysis based on a high SS >32. In Model 1, the cut-off value of the CHA2DS2-VASc-HS score was ≥4 with a sensitivity of 70.3%, specificity 72.5%, PPV 70.2%, and NPV 72.5% (AUC: 0.766, 95% CI: 0.710–0.817, p<0.001). In Model 2, the cut-off value of the CHA2DS2-VASc-HS score was ≥5 with a sensitivity 61.9%, specificity 79.5%, PPV 37.7%, and NPV 91.3% (AUC: 0.781, 95% CI: 0.725–0.831, p<0.001).
Table 3.
Comparison of the risk scores according to the number of diseased vessels
| Vessel disease | P (for trend)* | ||||
|---|---|---|---|---|---|
| 0 (n=12) | 1 (n=76) | 2 (n=82) | 3 (n=82) | ||
| GRACE score | 134.5±62.5 | 127.9±50.4 | 159.9±55.6 | 181.0±66.5 | <0.001 |
| TIMI score | 1.6±1.3 | 2.1±1.4 | 3.0±1.5 | 3.9±1.5 | <0.001 |
| CHA2DS2-VASc-HS score | 2.0±1.1 | 2.8±1.2 | 3.7±1.5 | 4.9±2.1 | <0.001 |
One-way analysis of variance or Kruskal–Wallis tests were used to compare the three groups
Pairwise comparisons of the ROC curves of these scoring systems were performed according to the presence of multivessel disease and SS (Tables 4, 5). In these models, no difference was observed in the AUC between the CHA2DS2-VASc-HS score and the other scoring systems in the prediction of severity and complexity of CAD in NSTE-ACS patients.
Table 4.
Pairwise comparison of ROC curves according to the multi-vessel disease
| Difference between areas | SE | 95% CI | Z statistic | P | |
|---|---|---|---|---|---|
| TIMI vs. GRACE | 0.0404 | 0.0328 | -0.0238 to 0.105 | 1.233 | 0.2174 |
| CHA2DS2-VASc-HS vs. TIMI | 0.0103 | 0.0322 | -0.0528 to 0.0734 | 0.320 | 0.7492 |
| CHA2DS2-VASc-HS vs. GRACE | 0.0507 | 0.0386 | -0.0249 to 0.126 | 1.314 | 0.1889 |
CI - confidence interval; SE - standard error
Table 5.
Pairwise comparison of ROC curves according to the SYNTAX score
| Scores | Difference between areas | SE | 95% CI | Z statistic | P |
|---|---|---|---|---|---|
| Model 1. According to SYNTAX score of >22 or not | |||||
| TIMI vs. GRACE | 0.0623 | 0.0347 | -0.00578 to 0.130 | 1.793 | 0.0729 |
| CHA2DS2-VASc-HS vs. TIMI | 0.00609 | 0.0320 | -0.0567 to 0.0689 | 0.190 | 0.8493 |
| CHA2DS2-VASc-HS vs. GRACE | 0.0562 | 0.0399 | -0.0221 to 0.134 | 1.406 | 0.1596 |
| Model 2. According to SYNTAX score of >32 or not | |||||
| TIMI vs. GRACE | 0.0197 | 0.0446 | -0.0678 to 0.107 | 0.441 | 0.6593 |
| CHA2DS2-VASc-HS vs. TIMI | 0.0111 | 0.0401 | -0.0675 to 0.0896 | 0.276 | 0.7827 |
| CHA2DS2-VASc-HS vs. GRACE | 0.0307 | 0.0488 | -0.0649 to 0.126 | 0.630 | 0.5289 |
CI - confidence interval; ROC - receiver operating characteristic; SE - standard error
In-hospital adverse events are reported in Table 6. The overall in-hospital MACE (p<0.001), non-fatal MI (p=0.002), in-hospital mortality (p=0.001), and stent thrombosis (p=0.051) were significantly greater in patients with high CHA2DS2-VASc-HS scores. We also compared the diagnostic accuracy of the CHA2DS2-VASc-HS score in the determination of the in-hospital MACE with the TIMI and GRACE risk scores by comparison of the pairwise ROC curve analysis and found no differences between them (Fig. 1). The cut-off value of the CHA2DS2-VASc-HS score in the prediction of in-hospital MACE was >5 with a sensitivity 69.6% (95% CI: 47.1–86.8), specificity 90.3% (95% CI: 85.8–93.9), PPV 42.1% (95% CI: 26.3–59.2), and NPV 96.7% (95% CI: 93.4–98.7) (AUC: 0.804, 95% CI: 0.750–0.851, p<0.001).
Table 6.
In-hospital adverse events
| Variable | Overall (n=252) |
CHA2DS2-VASc-HS Score ≤4 (n=183) |
CHA2DS2-VASc-HS Score >4 (n=69) |
P* |
|---|---|---|---|---|
| MACE, n % | 23 (9.1) | 7 (3.8) | 16 (23.2) | <0.001 |
| In hospital mortality, n % | 4 (1.6%) | 0 | 4 (5.8%) | 0.001 |
| Non-fatal MI, n % | 8 (3.2%) | 2 (1.1%) | 6 (8.7%) | 0.002 |
| Stent thrombosis, n % | 14 (5.6%) | 7 (3.8%) | 7 (10.1%) | 0.051 |
Categorical variables were compared with the χ2 or Fisher’s exact tests. MACE - major adverse cardiovascular events; MI - myocardial infarction
Figure 1.
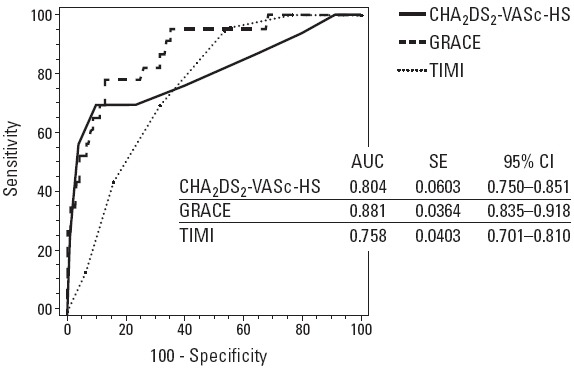
Comparison of ROC curves of the CHA2DS2-VASc-HS, TIMI, and GRACE scores in the risk stratification of the in-hospital MACE in NSTE-ACS patients.
AUC - area under the curve; CI - confidence interval; MACE - major adverse cardiovascular events; SE - standard error
Discussion
The main findings of our study are (i) the TIMI, GRACE, and CHA2DS2-VASc-HS risk scores were increased in patients with NSTE-ACS; (ii) the TIMI, GRACE, and CHA2DS2-VASc-HS scores significantly correlated with the number of diseased vessels and SS, and the strongest correlations were observed with the CHA2DS2-VASc-HS score; and (iii) ROC comparisons indicated that the CHA2DS2-VASc-HS score was equal and comparable to other risk scores in the prediction of severity and complexity of CAD and risk stratification of in-hospital MACE in the NSTE-ACS patients, a CHA2DS2-VASc-HS score of ≥4 may also predict the severity and complexity of CAD, and a score of >5 may predict the in-hospital MACE.
Most CAD patients have at least one coronary risk factor; the presence of more than one of these risk factors increases the risk of CAD (13, 14). It is of great importance to assess the risk of CAD to provide appropriate medical treatment and account for morbidity and mortality. Thus, several risk prediction algorithms, including major CAD risk factors, have been developed. Clinicians need simple, reliable, reproducible, and quantitative tools to identify patients’ risks and recommend prevention strategies. The TIMI and GRACE scoring systems used for the risk stratification of NSTE-ACS patients are primarily based on multivariable models that include components of the medical history, admission electrocardiogram, and cardiac biomarker variables (4, 5). SS has many potential applications and represents a powerful stratification tool for the standardized assessment of CAD extent and severity (15). Moreover, studies of multivessel or left main disease have shown that SS independently predicts mortality and MACE (16-18). Although the TIMI and GRACE scores were used in the risk stratification of patients with NSTE-ACS, they do not include angiographic variables, which might contribute to independent prognosis (19). Based on this deficiency, patients were grouped according to SS and were compared with those using other scoring systems, including the CHA2DS2-VASc-HS score. The number of diseased vessels and SS were significantly correlated with the TIMI, GRACE, and CHA2DS2-VASc-HS scores; the strongest correlation was with the CHA2DS2-VASc-HS score.
We also investigated the accuracy and comparative usage of these scores in patients with NSTE-ACS to identify those at a high risk of severe CAD. The results indicate that the CHA2DS2-VASc-HS score was comparable with the other risk scores (TIMI and GRACE) and that a score of ≥4 may predict the severity and complexity of CAD in the ROC analysis. Goncalves et al. (20) found that the GRACE risk model was slightly better than the TIMI risk score for the estimation of prognosis at 1 year, likely because signs of heart failure were not included and age was included only as a simple categorical variable. Increasing age, HL, smoking, and male gender increase the cardiovascular risk and are fixed features in the evaluation of risk classifications (21-23). In the CHA2DS2-VASc-HS scoring system, age of ≥75 years is assigned 2 points, and male gender, smoking, HL, and congestive heart failure are included in the equation to improve the reliability of the scoring system. In previous studies (24, 25), the relationships between the TIMI or GRACE scores and the extent of CAD in patients with NSTE-ACS were evaluated. The TIMI and GRACE scores are useful for the initial stratification of NSTE-ACS patients but are not optimized for patients undergoing PCI. Although the CHA2DS2-VASc-HS score may also have poorer discriminatory accuracy than that of the scoring systems that incorporate angiographic variables, it is important to note that the CHA2DS2-VASc-HS score, which evaluates only clinical variables, is comparable with the other risk scores and was developed using databases from large clinical trials including TIMI and GRACE scores. The outcomes of high-risk patients can be predicted with comorbidity findings using this scoring system without the need for information regarding vital signs at admission, which is convenient for the rapid screening of high-risk patients in clinics. The CHA2DS2-VASc-HS scoring system is simple to use, time-saving, and does not require any software to calculate the total risk assessment of NSTE-ACS patients in clinical practice.
Study limitations
The main limitations of our study were its cross-sectional design, relatively small sample size, and the lack of follow-up data. For these reasons, prospective studies with long-term follow-up are necessary to obtain more data regarding the relationship between the CHA2DS2-VASc-HS score and long-term morbidity and mortality. Further, although this study included patients diagnosed with NSTE-ACS, those with previous CABG were excluded because the SS algorithm was developed for patients with native CAD. Therefore, our results need to be supported by population-based studies. Finally, SS was visually calculated, rather than by intravascular ultrasound; online quantitative coronary angiography measurement might overcome this issue.
Conclusion
In the current study, the CHA2DS2-VASc-HS score was correlated with the severity and complexity of CAD and was found to be comparable with other risk scores for the risk stratification of in-hospital MACE of NSTE-ACS patients. Because the CHA2DS2-VASc-HS score is simple to use, time-saving, does not require any software to calculate the total risk assessment, and does not entail additional costs in a real-life situation, it may play an important role as a predictive model of NSTE-ACS patients in clinical practice. Moreover, the addition of SS to the CHA2DS2-VASc-HS score may improve its ability to stratify risk in patients with NSTE-ACS. To clarify this issue, further prospective large-scale studies are needed.
Acknowledgements
The English in this document has been checked by at least two professional editors, both of whom are native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/IHLJgd.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – H.T., M.Ç., M.B., A.B., Y.Ö.O., S.T., E.A.; Design – H.T., M.Ç., M.B., S.T., E.A.; Supervision – H.T., M.Ç., M.B., A.B., Y.Ö.O.; Materials – H.T., M.Ç., M.B., A.B., Y.Ö.O.; Data collection&/or processing – H.T., M.Ç., M.B., A.B., Y.Ö.O., E.A., S.T.; Analysis and/or interpretation – H.T., M.Ç., M.B.; Literature search – H.T., M.Ç., M.B.; Writing – H.T., M.Ç., M.B., A.B., Y.Ö.O.; Critical review – H.T., M.Ç., M.B., A.B., Y.Ö.O., S.T., E.A.
References
- 1.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Association for Cardiovascular Prevention & Rehabilitation (EACPR);ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version. 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33:1635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 2.Wright RS, Anderson JL, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, et al. 2011 ACCF/AHA focused update of the Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction (updating the 2007 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American College of Emergency Physicians, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;57:1920–59. doi: 10.1016/j.jacc.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010;31:2501–55. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 4.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 5.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6- month postdischarge death in an international registry. JAMA. 2004;291:2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 6.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–27. [PubMed] [Google Scholar]
- 7.Çetin M, Çakıcı M, Zencir C, Taşolar H, Baysal E, Ballı M, et al. Prediction of coronary artery disease severity using CHADS2and CHA2DS2-VASc scores and a newly defined CHA2DS2-VASc-HS score. Am J Cardiol. 2014;113:950–6. doi: 10.1016/j.amjcard.2013.11.056. [DOI] [PubMed] [Google Scholar]
- 8.Farooq V, Girasis C, Magro M, Onuma Y, Morel MA, Heo JH, et al. The CABG SYNTAX Score - an angiographic tool to grade the complexity of coronary disease following coronary artery bypass graft surgery: from the SYNTAX Left Main Angiographic (SYNTAX-LE MANS) substudy. EuroIntervention. 2013;8:1277–85. doi: 10.4244/EIJV8I11A196. [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. National Heart, Lung, and Blood Institute;American College of Cardiology Foundation;American Heart Association. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 10.Duncan RF, Dundon BK, Nelson AJ, Pemberton J, Williams K, Worthley MI, et al. A study of the 16-Segment Regional Wall Motion Scoring Index and biplane Simpson’s rule for the calculation of left ventricular ejection fraction: a comparison with cardiac magnetic resonance imaging. Echocardiography. 2011;28:597–604. doi: 10.1111/j.1540-8175.2011.01394.x. [DOI] [PubMed] [Google Scholar]
- 11.Valenti R, Marcucci R, Comito V, Marrani M, Cantini G, Migliorini A, et al. Prasugrel in Clopidogrel Nonresponders Undergoing Percutaneous Coronary Intervention: The RECLOSE (REsponsiveness to CLOpidogrel and StEnt Thrombosis) 3 Study. JACC Cardiovasc Interv. 2015;8:1563–70. doi: 10.1016/j.jcin.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 12.De Long ER, De Long DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 13.Ford ES, Giles WH, Mokdad AH. The distribution of 10-year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol. 2004;43:1791–6. doi: 10.1016/j.jacc.2003.11.061. [DOI] [PubMed] [Google Scholar]
- 14.Eberly LE, Neaton JD, Thomas AJ, Yu D Multiple Risk Factor Intervention Trial Research Group. Multiple-stage screening and mortality in the Multiple Risk Factor Intervention Trial. Clin Trials. 2004;1:148–61. doi: 10.1191/1740774504cn018oa. [DOI] [PubMed] [Google Scholar]
- 15.Yadav M, Palmerini T, Caixeta A, Madhavan MV, Sanidas E, Kirtane AJ, et al. Prediction of coronary risk by SYNTAX and derived scores: synergy between percutaneous coronary intervention with taxus and cardiac surgery. J Am Coll Cardiol. 2013;62:1219–30. doi: 10.1016/j.jacc.2013.06.047. [DOI] [PubMed] [Google Scholar]
- 16.Capodanno D, Di Salvo ME, Cincotta G, Miano M, Tamburino C, Tamburino C. Usefulness of the SYNTAX score for predicting clinical outcome after percutaneous coronary intervention of unprotected left main coronary artery disease. Circ Cardiovasc Interv. 2009;2:302–8. doi: 10.1161/CIRCINTERVENTIONS.108.847137. [DOI] [PubMed] [Google Scholar]
- 17.Palmerini T, Genereux P, Caixeta A, Cristea E, Lansky A, Mehran R, et al. Prognostic value of the SYNTAX score in patients with acute coronary syndromes undergoing percutaneous coronary intervention analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage StrategY) Trial. J Am Coll Cardiol. 2011;57:2389–97. doi: 10.1016/j.jacc.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Wykrzykowska JJ, Garg S, Girasis C, de Vries T, Morel MA, van Es GA, et al. Value of the SYNTAX score for risk assessment in the all-comers population of the randomized multicenter LEADERS (Limus Eluted from A Durable versus ERodable Stent coating) trial. J Am Coll Cardiol. 2010;56:272–7. doi: 10.1016/j.jacc.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Lansky AJ, Goto K, Cristea E, Fahy M, Parise H, Feit F, et al. Clinical and angiographic predictors of short- and long-term ischemic events in acute coronary syndromes: results from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial. Circ Cardiovasc Interv. 2010;3:308–16. doi: 10.1161/CIRCINTERVENTIONS.109.887604. [DOI] [PubMed] [Google Scholar]
- 20.de Araújo Gonçalves P, Ferreira J, Aguiar C, Seabra-Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005;26:865–72. doi: 10.1093/eurheartj/ehi187. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg RJ, McCormick D, Gurwitz JH, Yarzebski J, Lessard D, Gore JM. Age-related trends in short- and long-term survival after acute myocardial infarction: a 20- year population-based perspective (1975–1995) Am J Cardiol. 1998;82:1311–7. doi: 10.1016/s0002-9149(98)00633-x. [DOI] [PubMed] [Google Scholar]
- 22.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Jimenez F, Batsis JA, Roger VL, Brekke L, Ting HH, Somers VK. Trends in 10-year predicted risk of cardiovascular disease in the United States 1976 to 2004. Circ Cardiovasc Qual Outcomes. 2009;2:443–50. doi: 10.1161/CIRCOUTCOMES.108.847202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmood M, Achakzai AS, Akhtar P, Zaman KS. Comparison of the TIMI and the GRACE risk scores with the extent of coronary artery disease in patients with non-ST-elevation acute coronary syndrome. J Pak Med Assoc. 2013;63:691–5. [PubMed] [Google Scholar]
- 25.Barbosa CE, Viana M, Brito M, Sabino M, Garcia G, Maraux M, et al. Accuracy of the GRACE and TIMI scores in predicting the angiographic severity of acute coronary syndrome. Arq Bras Cardiol. 2012;99:818–24. doi: 10.1590/s0066-782x2012005000080. [DOI] [PubMed] [Google Scholar]


