Abstract
Aging and vascular comorbidities such as hypertension comprise critical co-factors that influence how the brain responds to stroke. Ischemic stress induces neurogenesis and oligodendrogenesis in younger brains. However, it remains unclear whether these compensatory mechanisms can be maintained even under pathologic hypertensive and aged states. To clarify the age-related remodeling capacity after stroke under hypertensive conditions, we assessed infarct volume, behavioral outcomes, and surrogate markers of neurogenesis and oligodendrogenesis in acute and sub-acute phases after transient focal cerebral ischemia in 3 month old and 12 month old spontaneously hypertensive rats (SHRs). Hematoxylin and eosin staining showed that 3 and 12 month old SHRs exhibited similar infarction volumes at both 3 and 14 days after focal cerebral ischemia. However, recovery of behavioral deficits (neurological score assessment and adhesive removal test) were significantly less in 12-month-old SHRs compared to 3-month-old SHRs. Concomitantly, numbers of nestin-positive neural stem/progenitor cells (NSPCs) near the infarct border area or subventricular zone in 12-moth-old SHRs were lower than 3-month-old SHRs at day 3. Similarly, numbers of PDGF-R-α-positive oligodendrocyte precursor cells (OPCs) in corpus callosum was lower in 12-month-old SHRs at day 3. Lower levels of NSPC and OPC numbers were accompanied by lower expression levels of CREB phosphorylation. By day 14 post-ischemia, NSPC and OPC numbers in 12-month-old SHRs recovered to similar levels as in 3-month-old SHRs. But the numbers of proliferating NSPCs (Ki67+nestin+ cells) and proliferating OPCs (Ki67+PDGF-R-α+ cells) remained lower in the older brains even at day 14. Taken together, these findings suggest that aging may also decrease post-stroke compensatory responses for neurogenesis and oligodendrogenesis even under hypertensive conditions.
Keywords: neural stem cells, oligodendrocyte precursor cells, middle cerebral artery occlusion, stroke, spontaneously hypertensive rat, aging
Introduction
Brain pathophysiology is influenced by a dynamic balance between deleterious and beneficial responses to the initial insult (22). Stroke and brain injury trigger a wide spectrum of neurovascular perturbations, glial activation, and secondary neuroinflammation that may all amplify neuronal cell death cascades. But at the same time, many endogenous neuroprotective responses may also be activated. These beneficial processes include compensatory neurogenesis and oligodendrogenesis (25).
Although the adult brain retains capabilities for regeneration and recovery, aging may significantly dampen these endogenous protective mechanisms. In particular, the capacity for neurogenesis appears to diminish with age. This is primarily due to a general reduction of neuronal precursor cell proliferation because of age-related alterations in the cellular microenvironment (2,18,21,35). Similarly, oligodendrogenesis and white matter homeostasis may also be affected by white matter senescence. In healthy young adult brains, myelin-forming mature oligodendrocytes in white matter can be newly generated from their precursor cells (oligodendrocyte precursor cells: OPCs). After white matter injury, OPCs rapidly proliferate and migrate to fill the demyelinated area, differentiate into mature oligodendrocytes and restore myelin sheaths (15,24,31). However, myelin density, along with cognitive function, spontaneously declines with increasing age both in human and rodents (9,30), indicating that the capacity for oligodendrogenesis may be compromised by white matter senescence.
Aging is a major risk factor in stroke and other forms of cerebrovascular disease (16,19). The risk of stroke doubles every decade after age 55 (23), and older patients show less functional recovery after stroke when compared to younger patients (4). In rodent models of stroke, it has been confirmed that aging worsens brain damage and suppresses brain remodeling/repairing after ischemic damage (28,32). However, aging is not the only cofactor involved. Vascular comorbidities such as hypertension are also known to be major contributors to stroke risk and stroke injury (8,34,37), but it is unknown how aging may affect stroke-induced compensatory responses under hypertensive conditions. In this study, therefore, we asked how aging may affect NSPC and OPC responses after stroke in spontaneously hypertensive rats (SHRs).
Material and methods
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and NIH Guidelines on Ethical Animal Use and Rigor. All experiments and procedures were conducted following a Massachusetts General Hospital (MGH) IACUC-approved protocol. The MGH Subcommittee on Research Animal Care (institutional IACUC) reviewed and approved all experiments conducted here. These IACUC approval protocols and studies were all in compliance with the MGH ethics review procedure, and conducted in compliance with GlaxoSmithKline policy on care, welfare and treatment laboratory animals. All experimental animals were sacrificed by decapitation under deep isoflurane anesthesia, according to IACUC-approved protocols. All procedures and analyses were performed following standard requirements for experimental design including allocation concealment, randomization, blinding and statistical powering (www.ninds.nih.gov/funding/transparency_in_reporting_guidance.pdf).
Animal Model
Twenty-three 3-month old and twenty-four 12-month old male SHRs (Charles River Laboratories Wilmington, MA) were used in this study. Rats were anesthetized with isoflurane (1–1.5%) in a 30/70% mixture of oxygen/nitrous-oxide mixture, with the anesthetic dose titrated to maintain spontaneous respiration. Rectal temperatures were monitored and maintained at 37 ± 0.5°C with a thermostat-controlled heating pad. All rats underwent middle cerebral artery occlusion (MCAO). The standard intraluminal method was used (26). Briefly, a ventral midline incision was performed on the neck. The common carotid artery (CCA), extra carotid artery (ECA) and internal carotid artery (ICA) were exposed. The superior thyroid artery and occipital artery branching from the ECA was coagulated and cut. The pterygopalatine artery (PPA) branching from the ICA was ligated using 4-0 silk. The ECA was ligated using 4-0 silk, and the CCA and ICA were closed temporarily. The ECA was cut and a filament was placed through the ICA to the bifurcation of the ICA and the middle cerebral artery (MCA), thus inducing focal cerebral ischemia. At 75 min after induction of ischemia, the filament was withdrawn for reperfusion, and the ECA was ligated using 4-0 silk. Cerebral blood flow (CBF) was monitored by laser Doppler flowmetry (LDF) during surgery to evaluate the severity of ischemia. As expected, LDF dropped below 30% of baseline (100%) for the duration of the focal cerebral ischemia. At 3 or 14 days after MCAO, rats were sacrificed while deeply anesthetized by 5% isoflurane, using intra-cardiac perfusion with ice-cold saline. Brains were removed and snap frozen. Coronal sections of 20 μM thickness were cut on a cryostat (Microm HM 505 E) at −20°C and collected on glass slides, then stored at −80°C for future use.
Behavioral tests
Two behavioral tests were performed at 28 days after MCAO by a blinded investigator, who also trained the animals before surgery. The modified Neurological Severity Score (NSS) reflects neurological deficits after stroke using a graded scale from 0 to 18 (normal: 0, maximal neurological deficit: 18). The NSS test is a comprehensive test that assesses motor and sensory function, stability using a beam balance test, abnormal reflexes, and abnormal movements (6). The adhesive removal test also assesses somatosensory function (5). In this test, a rat is first accommodated to a testing box and then removed gently from the box in order to apply adhesive tape to the wrist of each forelimb with equal pressure. The rat is then placed back in the box, and the time required for the rat to react to the adhesive tape is recorded. The maximal time was set to 120 seconds.
Hematoxylin and Eosin Staining, and Evaluation of infarct
Coronal sections were selected and processed for hematoxylin and eosin (HE) staining. Briefly, sections were removed from −80°C and thawed. The fresh frozen sections were fixed with 4% paraformaldehyde (PFA) for 15 min, then rinsed with warm running water for 1 min. Sections were stained with hematoxylin for 5 min to stain nuclei and rinsed for 3 min with water. Next, sections were stained with eosin for 3 min and followed by rinsing for 1 min. Afterwards, the sections were dehydrated with 70% ethanol, 100% ethanol, and 100% ethanol (in respective order) for 10–15 seconds each. Following that, the sections were cleared using xylene. Sections were then mounted with a cover slide with permount. Infarction volume was calculated as previously reported (12). Briefly, the sections were scanned, and the infarction area in each section was calculated by subtracting the non-infarcted area of the ipsilateral side from the area of the contralateral side with ImageJ analysis software. Infarction areas on each section were summed and multiplied by section thickness to give the total infarction volume.
Immunohistochemistry
Coronal sections (2 mm anterior to bregma) were selected and processed for immunohistochemistry staining. Staining was done as previously described (12). Briefly, sections were removed from −80°C and thawed. The fresh frozen sections were fixed with 4% PFA for 15 min. After being further washed three times in PBS containing 0.1% Triton X-100, they were incubated with 10% Block ACE (AbD Serotec) in PBS for 1 h at room temperature. Then sections were incubated in PBS/0.3% BSA solution containing primary antibodies anti-Ki67 (proliferative cell marker, 1:100, Abcam) and anti-PDGFRα (OPC marker, 1:100, Santa Cruz) at 4°C overnight. After washing with PBS three times, they were incubated with secondary antibodies (1:200; Jackson Immunoresearch Laboratories) for 1 h at room temperature. Similarly, sections were stained with anti-Ki67 and anti-nestin antibodies (NSPC marker, 1:100, Abcam). Then the sections were washed three times and covered with VECTASHIELD mounting medium with DAPI (Vector Laboratories). Immunostaining was analyzed with a fluorescence microscope (Nikon Eclipse Ti-5).
Western blot analysis
Tissue samples of brain sections were prepared in Pro-PREPTM Protein Extraction Solution (Boca Scientific). Samples were heated with equal volumes of sample buffer containing 2× SDS sample buffer and 2-mercaptoethanol (2-ME) (Sigma) at 95°C for 5 min. Then each sample was loaded onto 4–20% Tris-glycine gels. After electrophoresis and transferring to polyvinylidene difluoride membranes (Novex), the membranes were blocked in 0.2% I-Block™ regent (Applied Biosystems) for 1 h at room temperature. Then they were incubated overnight with primary antibodies anti-nestin (1:1000, Abcam), anti-PDGFRα (1:1000, Santa Cruz), anti-phosphorylated cAMP response element-binding (pCREB, 1:1000, Millipore), CREB antibody (1:1000, Millopore), BDNF antibody (1:1000, Applied Biological Materials) and anti- β-actin (1:5000, Sigma) at 4°C, followed by incubation with peroxidase-conjugated secondary antibodies. Signals were visualized by enhanced chemiluminescence (GE Healthcare). Optical density was assessed using ImageJ analysis software.
Measurements of cell area and Cell counting
Nestin signal in the of peri-infarct area, ipsi-lateral subventricular zone (SVZ) area, contra lateral cortex and SVZ was analyzed by quantifying the staining areas using ImageJ analysis software. To obtain data as to cell numbers from immunostained brain sections, an investigator blinded to the experimental groups counted the number of PDGFRα, double positive stained cells in peri-infarct area for Ki67 and nestin, and double positive stained cells in the ipsilateral corpus callosum for Ki67 and PDGFRα.
Statistical Analysis
Pearson’s chi-squared test was used for mortality rate. For other data comparisons, Mann Whitney U test was used to determine the significant differences between 3-month-old and 12-month-old groups. All data are expressed as means ± SD. A p-value less than 0.05 was considered statistically significant.
Results
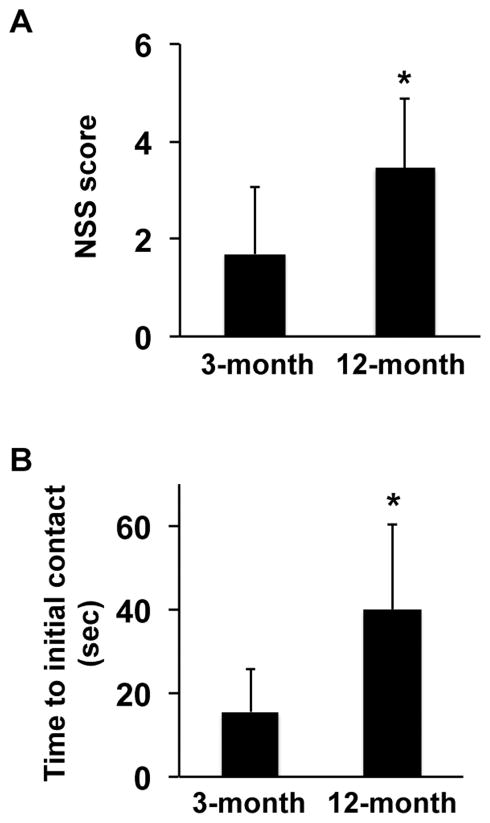
We subjected 3-month-old and 12-month-old SHRs to transient 75 min middle cerebral artery occlusion followed by reperfusion. We performed surgery on 23 rats in the 3-month old group and 24 rats in the 12-month old group. Within the 47 rats, no animals died in the 3-month old group during the recovery phase, but 3 rats died in the 12-month old group. However, there was no significant difference in the mortality rate between 3- and 12-month old groups (p=0.08). Laser Doppler measurement confirmed that there was no difference in regional cerebral blood flow before and after arterial occlusions (data not shown). Standard hematoxylin and eosin (HE) staining demonstrated that both 3-month-old and 12-month-old SHRs exhibited similar infarction volumes at days 3 and 14 post-ischemia (3-month-old at day 3: 173 ± 47 mm3 of N=5, 12-month-old at day 3: 182 ± 30 mm3 of N=5, 3-month-old at day 14: 222 ± 29 mm3 of N=5, 12-month-old at day 14: 228 ± 67 mm3 of N=5). In addition, up to day 14, there was no significant difference in cerebral hemorrhage transformation between 3- and 12-month old groups (1 out of 10 rats in 3-month old group, 2 out of 10 rats in 12-month old group). However, there were differences in terms of neurological recovery over time. At day 28 post-ischemia, NSS scores in 12-month-old SHRs were higher (i.e. worse behavioral deficits) compared with 3-month-old SHRs (Figure 1A). In addition, in the adhesive removal test, the time of initial contact to tape removal was also longer in 12-month-old SHRs (Figure 1B).
Figure 1. 3-month-old SHRs showed better neurological outcome at day 28 after MCAO.
(A) Although there was no difference in the NSS score before MCAO, 3-month-old SHRs exhibited lower NSS score (e.g. better neurological outcome) at day 28 after MCAO. Values are expressed as mean ± SD of N=13 for 3-month-old and N=11 for 12-month-old SHRs. *p<0.05. (B) Although there was no difference in the time to initial contact adhesive tape before MCAO, 3-month-old SHRs exhibited better score in the adhesive test at day 28 after MCAO. Values are expressed as mean ± SD of N=13 for 3-month-old and N=11 for 12-month-old SHRs. *p<0.05.
Since there were apparent differences in stroke recovery between the two groups, we next asked whether differences in cellular compensatory responses were also detectable. We assessed surrogate markers of stroke-induced neurogenesis and oligodendrogenesis by immunostaining for NSPCs and OPCs. Anti-nestin antibody was used as a marker for NSPCs and anti-PDGF-R-α for OPCs. At both acute and sub-acute times, nestin-positive areas in the contralateral side were similar between 3-month- and 12-month-old SHRs (3-month-old at day 3: 1.9 ± 1.1%, 12-month-old at day 3: 1.1 ± 0.6%, 3-month-old at day 14: 1.9 ± 1.0%, 12-month-old at day 14: 1.5 ± 0.7%). In addition, numbers of PDGF-R-α-positive cells in corpus callosum of contralateral side were also not different between 3-month-old and 12-month-old SHRs (3-month-old at day 3: 180 ± 21 cells/mm2, 12-month-old at day 3: 176 ± 23 cells/mm2, 3-month-old at day 14: 183 ± 17 cells/mm2, 12-month-old at day: 186 ± 18 cells/mm2).
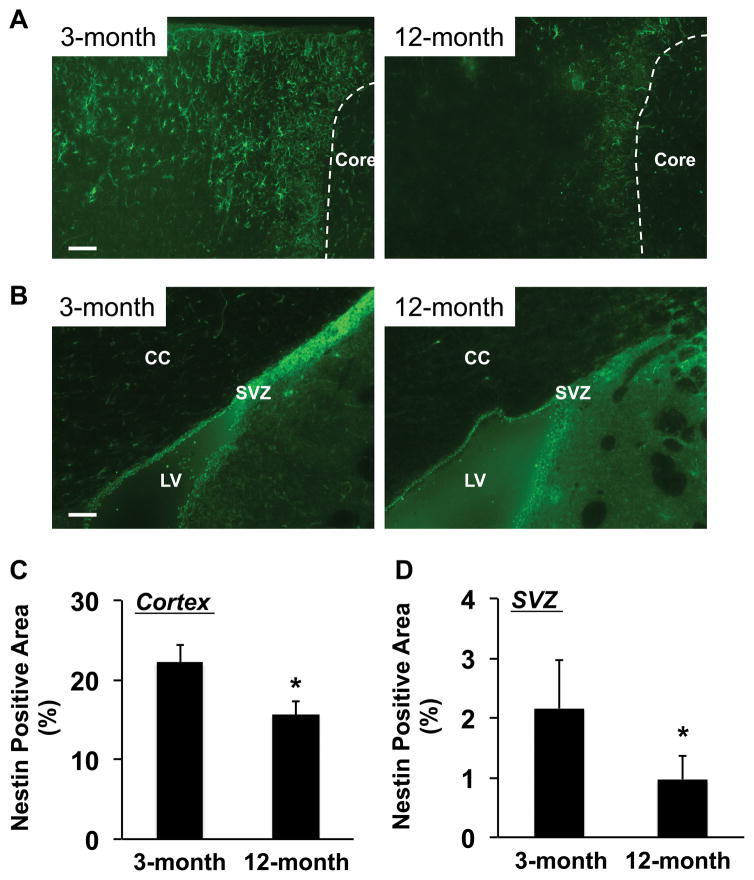
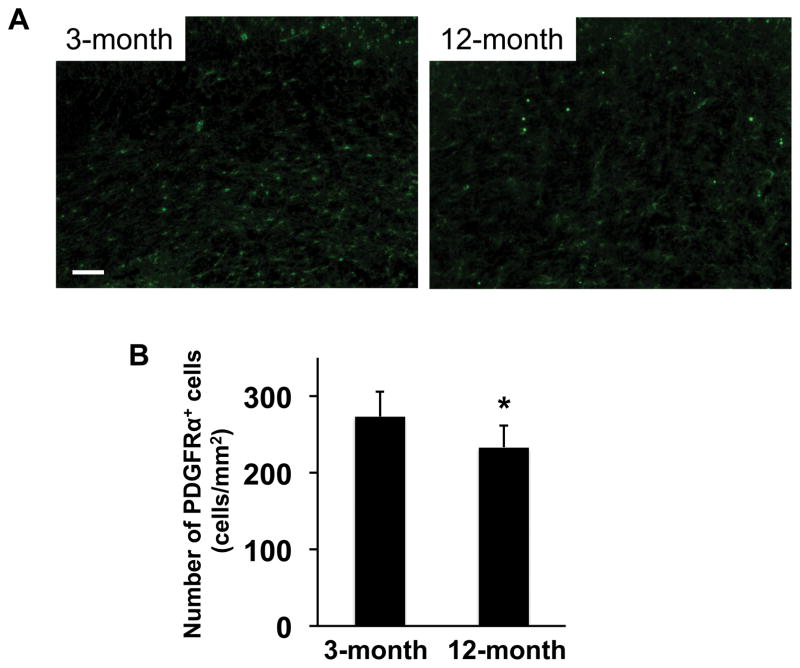
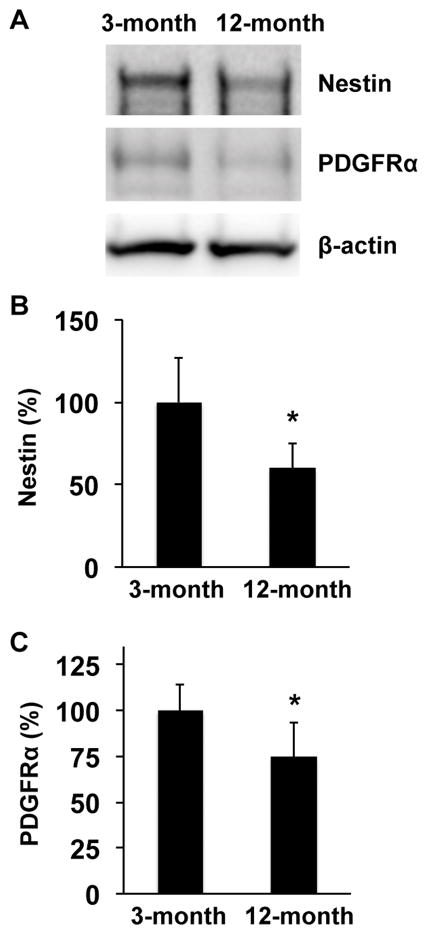
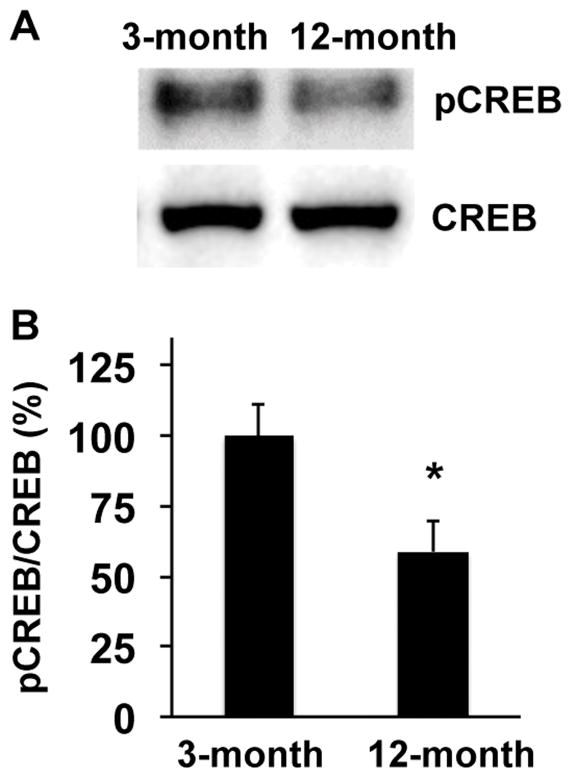
However, ipsilateral responses were different. At day 3 post-ischemia, the areas of nestin-positive NSPCs in peri-infarct cortex and around the SVZ region were larger in 3-month-old SHRs compared to 12-month-old SHRs (Figure 2). Similarly, 3-month-old SHRs showed larger number of PDGF-R-α-positive OPCs in corpus callosum of ipsilateral ischemic side (Figure 3). Western blot analysis of dissected brain homogenates demonstrated that expression levels of nestin and PDGF-R-α were higher in brain samples from 3-month-old SHRs (Figure 4), although there were no significant differences in expressions of nestin and PDGF-R-α in contralateral sides (Supplementary Figure S1). Notably, levels of phospho-CREB expression were also higher in the 3-month-old SHR brains (Figure 5 and Supplementary Figure S1), indicating that age-related decline in acute post-ischemic neurogenesis and oligodendrogenesis may be, at least partly, related to the downregulation of CREB signaling.
Figure 2. Number of NSPCs was larger in 3-month-old SHRs at day 3 after MCAO.
(A) Representative pictures for staining of anti-Nestin antibody (a marker for NSPCs) in the peri-infarct area in cortex. SHR brains were removed at day 3 after MCAO. (B) Representative pictures for staining of anti-Nestin antibody around SVZ region. SHR brains were removed at day 3 after MCAO. CC stands for corpus callosum, LV for left ventricle, and SVZ for subventricular zone. (C–D) At day 3 after MCAO, Nestin-positive areas were significantly smaller in 12-month-old SHRs in both peri-infarct region and SVZ area. Values are expressed as mean ± SD of N=5. *p<0.05. Scale bar = 100μm.
Figure 3. Number of OPC was larger in 3-moth-old SHRs at day 3 after MCAO.
(A) Representative pictures for staining of anti-PDGFRα antibody (a marker for OPCs) in corpus callosum. SHR brains were removed at day 3 after MCAO. (B) At day 3 after MCAO, PDGFRα-positive cells were significantly smaller in 12-month-old SHRs in corpus callosum. Values are expressed as mean ± SD of N=5. *p<0.05. Scale bar = 100μm.
Figure 4. Nestin and PDGFRα expression were higher in 3-month-old SHRs at day 3 after MCAO.
(A) Representative images for western blotting of anti-Nestin, anti-PDGFRα, or anti-β-actin antibodies. Brain samples from the ischemic side at day 3 after MCAO were subjected to western blot analysis. β-actin was used as a loading control. (B–C) Levels of Nestin and PDGFRα expression in 3-month-old SHRs were higher at day 3 after MCAO. Values are expressed as mean ± SD of N=5. *p<0.05.
Figure 5. Phosphorylation level of CREB was higher in 3-month-old SHRs at day 3 after MCAO.
(A) Representative images for western blotting of anti-pCREB antibody. Brain samples from ischemic side at day 3 after MCAO were subjected to western blot analysis. Total CREB was used a baseline control. (B–C) Level of pCREB in 3-month-old SHRs was higher at day 3 after MCAO. Values are expressed as mean ± SD of N=5. *p<0.05.
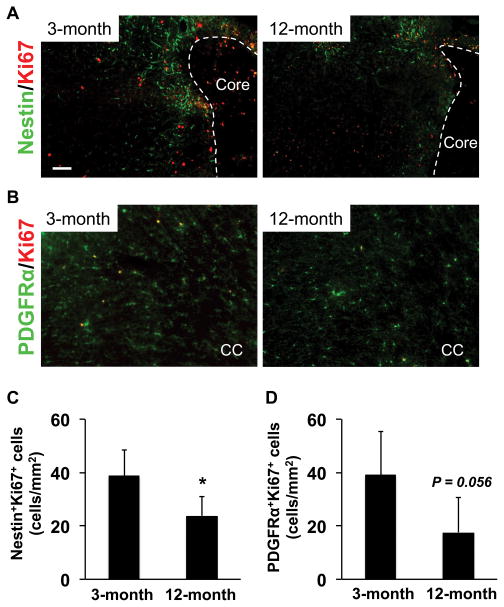
Next we investigated the sub-acute phase of neurogenesis and oligodendrogenesis at day 14 after cerebral ischemia. There were no significant differences between 3-month-old versus 12-month-old SHRs in term of NSPC and OPC numbers (NSPCs in peri-infarct area: 3-month-old - 4.2 ± 0.9%, 12-month-old - 3.3 ± 1.4%, OPCs in corpus callosum: 3-month-old - 271 ± 36 cells/mm2, 12-month-old - 262 ± 20 cells/mm2). However, the number of proliferating NSPCs (Ki67/nestin-double positive cells) and proliferating OPCs (Ki67/PDGF-R-α positive cells) were higher in 3-month-old SHR brains (Figure 6), suggesting that even in the sub-acute phase after stroke, NSPCs/OPCs may tend to proliferate as a compensatory response and younger SHRs may continue to maintain a higher proliferation of NSPCs and OPCs during prolonged recovery post-ischemia.
Figure 6. Numbers of proliferating NSPCs and OPCs were larger in 3-month-old SHRs at day 14 after MCAO.
(A) Representative pictures for double staining of anti-Nestin and anti-Ki67 (a marker for proliferating cells) antibodies in the peri-infarct area in cortex. SHR brains were removed at day 14 after MCAO. (B) Representative pictures for double staining of anti-PDGFRα and anti-Ki67 antibodies in corpus callosum. SHR brains were removed at day 14 after MCAO. (C–D) At day 14 after MCAO, the number of proliferating NSPCs (Nestin/Ki67-double positive cells) was significantly larger in 3-month-old SHRs. As for proliferating OPCs (PDGFRα/Ki67-double positive cells), although the p-value was not less than 0.05, there was a trend that 3-month-old SHRs showed a larger number of proliferating OPCs at day 14 after MCAO. Values are expressed as mean ± SD of N=5. *p<0.05. Scale bar = 100 μm.
Discussion
Neuronal and oligodendrocyte loss is one of the most important and common features in neurological diseases such as stroke. But it is now recognized that in parallel with ongoing pathophysiology, endogenous mechanisms are also triggered to compensate for lost brain cells. In the context of compensatory brain repair mechanisms, NSPCs and OPCs play critical roles because they constitute the major types of immature cells in adult brain and are activated after injury to differentiate into neurons or oligodendrocytes. However, aged brains tend to slowly lose these endogenous repair systems. Past studies extensively examined the mechanisms of age-related decline in neurogenesis and oligodendrogenesis, but most of those studies used normal undiseased animal models. In contrast, stroke tends to occur in patients with ongoing vascular comorbidities such as hypertension. Our current study confirmed that in SHRs, aging dampens stroke-induced neurogenesis and oligodendrogenesis. Therefore, our findings provide an additional insight into how aging affects brain remodeling after injury within the context of a common ischemic injury.
In most CNS diseases, the extent of loss of neurons and oligodendrocytes will likely impact the disease phenotype. Therefore, enhancement of endogenous neurogenesis and oligodendrogenesis could potentially boost functional recovery by replacing damaged neurons and oligodendrocytes (38). Although several difficult hurdles remain in this approach, stem cell therapy may provide an avenue to enhance endogenous compensative neurogenesis and oligodendrogenesis. Cell-based therapy is now relatively well accepted as a valid therapeutic strategy for repairing damaged organs, including brain. In preclinical studies using CNS disease models, stem cell treatment was shown to be protective for damaged brain. Notably, transplanted cells may not support brain only by a direct way in cell replacement but also protect brain cells indirectly by secreting trophic factors (3,14,27). In addition, recent studies suggest that transplanted cells can create a biobridge that fills the gap between the neurogenic niche and the site of the brain injury(11,33). These studies support the idea that the efficacy of cell-based therapy depends in part on the rate of compensatory neurogenesis and oligodendrogenesis. In this regard, our current study may be useful in describing the age-related decline in stroke-induced neurogenesis and oligodendrogenesis under hypertensive conditions.
In this study, we used anti-nestin antibody to identify NSPCs and anti-PDGFRα antibody to identify OPCs. Although nestin and PDGFRα are widely used as markers for NSPCs and OPCs respectively, many cell marker proteins are shared between several kinds of mature/immature cells in the central nervous system. For example, after ischemic stroke, activated astrocytes also expressed nestin (10,20,29). In addition, PDGFRα-positive NSPCs can generate both neurons and oligodendrocytes (17). In fact, our pilot experiments for triple staining of nestin/GFAP/Ki67 detected several nestin/GFAP-double positive cells in cortex (Supplementary Figure S2). But in the penumbral region (e.g. near the core edge) on day 3 after MCAO, most nestin-positive were negative for GFAP (Supplementary Figure S2). And more importantly, 3-month old SHRs exhibited a larger number of nestin+/Ki67+/GFAP− cells compared to 12-month old animals (Supplementary Figure S2), further supporting our conclusion that aging may dampen neurogenesis in SHRs after stroke. Another pilot experiment of nestin/PDGFRα/Ki67 triple staining showed that most PDGFRα signals did not overlapp with nestin signals in the corpus callopus region (Supplementary Figure S3), indicating that PDGFRα can be a reasonable marker for OPCs rather than NSPCs, at least in corpus callosum. Nevertheless, we should be careful with these cell markers since cells may change their protein production profiles in unexpected ways, especially after injury. Furthermore, since NSPCs may express GFAP under some conditions (7), future studies need to carefully identify NSPCs (and OPCs) with other cell maker antibodies.
Our current study demonstrates that similar to normotensive animals, aging may suppress regenerative responses after brain injury in hypertensive rats. However, we did not examine how hypertensive conditions affect the cerebrovascular system and neurological outcomes after stroke in SHRs. SHRs have normal systolic blood pressure by 4–8 weeks of age, after which hypertension rapidly develops over 12–14 weeks (1). After that, there may be no substantial differences in blood pressure between 3- and 12-month old SHRs (1). However, it still remains to be elucidated how chronic hypertension affects the cerebrovascular system. In general, despite elevation of total peripheral resistance in hypertensive conditions, resting cerebrovascular resistance and cerebral blood flow (CBF) would remain at normal levels due to cerebral autoregulation (36). But chronic hypertension induces an upward shift in the autoregulation curve, and therefore, CBF would be more susceptible to pressure-associated fluctuations in aged SHRs than younger animals (13). Our current study did not detect clear differences in regional CBF during MCAO between 3- and 12-month old. Nonetheless, it is possible that during the recovery phase, CBF (and the cerebrovascular system itself) in the 12-month old group may be abnormal and affect neurological outcomes in a deleterious way after stroke. Therefore, future studies need to carefully examine the cerebrovascular systems of young and aged SHRs to understand pathophysiological mechanisms of stroke under hypertensive conditions.
Taken together, our initial findings here suggest that in SHRs, aging dampens the compensative responses in neuronal and oligodendrocyte repair after injury, which may result in worse neurological recovery. However, there are several important caveats and limitations to keep in mind. First, we did not compare the rate of stroke-induced neurogenesis/oligodendrogenesis in SHRs with that in normotensive rats. Whether hypertensive conditions confound the age-related decline in compensative mechanism should be examined in future studies. Second, we examined histological outcomes on days 3 and 14, and assessed neurological outcomes on day 28. Therefore we could not analyze correlations between neurological and histological outcomes. In future studies, we need to carefully investigate how histological changes would affect neurological behaviors. Third, we assessed only proliferation of NSPCs and OPCs as an index for neurogenesis and oligodendrogenesis. But for successful brain repairing, proliferated NSPCs and OPCs must differentiate into neurons and oligodendrocytes (25). Further studies into this subject are required to understand the effects of aging in stroke-induced neurogenesis and oligodendrogenesis. Fourth, although we showed that downregulation of CREB activation in 12-month-old SHRs may be associated with age-related decline in the proliferation of NSPCs and OPCs, the precise underlying mechanisms are yet mostly unknown. Our pilot study indicated that most cells with p-CREB signal did not overlap with nestin or PDGFRα signals (Supplementary Figure S4), raising the possibility that CREB activity in their neighboring cells may upregulate trophic factor production, such as BDNF, to support NSPC and OPC function. In fact, BDNF expression level in 3-month old SHRs was higher than that in 12-month old animals after stroke (Supplementary Figure S4). How CREB signaling mediates neurogenesis and oligodendrogenesis after stroke would be an important research topic in this field. And finally, our study did not examine the mechanisms through which compensatory neurogenesis/oligodendrogenesis can help neurological recovery. In our model systems, although infarct volumes assessed by HE staining at days 3 and 14 were the same between 3-month-old and 12-month-old SHRs, stroke-induced proliferation in NSPCs/OPCs was larger in younger animals. And at the later time point, younger animals showed better neurological scores in NSS and adhesive removal tests. However, we only assessed neurological function by two behavioral tests at one time point. Multiple behavioral tests at multiple time points would be needed to further investigate the relationship between compensatory neurogenesis/oligodendrogenesis and neurological/cognitive recovery.
In summary, our current study described an age-related decline in stroke-induced regenerative responses in NSPC and OPC in a hypertensive animal model of focal cerebral ischemia. In addition to aging, hypertension is also a major risk factor for stroke. Further investigations into the mechanisms that mediate the interaction between aging and hypertension is warranted in order to pursue therapeutic approaches for boosting endogenous repair mechanisms in stroke-damaged brains.
Acknowledgments
Funding Statement: Supported in part by grants from NIH (P01-NS55104, R01-NS065089) and the Glaxo-Smith-Kline-Harvard-Stem-Cell-Institute consortium.
Footnotes
Disclosures: Salaries for authors with primary appointments with Glaxo-Smith-Kline (TTC, JDM, JCH) were paid by Glaxo-Smith-Kline. These authors (TTC, JDM, JCH) participated in study design and data analysis.
Please see supplementary figures at http://www.nmr.mgh.harvard.edu/~etm/supplement/liang-et-al-2016/
References
- 1.Adams MA, Bobik A, Korner PI. Differential development of vascular and cardiac hypertrophy in genetic hypertension. Relation to sympathetic function. Hypertension. 1989;14(2):191–202. doi: 10.1161/01.hyp.14.2.191. [DOI] [PubMed] [Google Scholar]
- 2.Asanuma M, Nishibayashi S, Iwata E, Kondo Y, Nakanishi T, Vargas MG, Ogawa N. Alterations of cAMP response element-binding activity in the aged rat brain in response to administration of rolipram, a cAMP-specific phosphodiesterase inhibitor. Brain Res Mol Brain Res. 1996;41(1–2):210–215. doi: 10.1016/0169-328x(96)00098-8. [DOI] [PubMed] [Google Scholar]
- 3.Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15(6):862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bejot Y, Rouaud O, Jacquin A, Osseby GV, Durier J, Manckoundia P, Pfitzenmeyer P, Moreau T, Giroud M. Stroke in the very old: incidence, risk factors, clinical features, outcomes and access to resources--a 22-year population-based study. Cerebrovasc Dis. 2010;29(2):111–121. doi: 10.1159/000262306. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Li Y, Chopp M. Intracerebral transplantation of bone marrow with BDNF after MCAo in rat. Neuropharmacology. 2000;39(5):711–716. doi: 10.1016/s0028-3908(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 7.Chojnacki AK, Mak GK, Weiss S. Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both? Nat Rev Neurosci. 2009;10(2):153–163. doi: 10.1038/nrn2571. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey RJ, Sailor KA, Bowen KK, Tureyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87(3):586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- 9.Doucette JR, Jiao R, Nazarali AJ. Age-related and cuprizone-induced changes in myelin and transcription factor gene expression and in oligodendrocyte cell densities in the rostral corpus callosum of mice. Cell Mol Neurobiol. 2010;30(4):607–629. doi: 10.1007/s10571-009-9486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duggal N, Schmidt-Kastner R, Hakim AM. Nestin expression in reactive astrocytes following focal cerebral ischemia in rats. Brain Res. 1997;768(1–2):1–9. doi: 10.1016/s0006-8993(97)00588-x. [DOI] [PubMed] [Google Scholar]
- 11.Duncan K, Gonzales-Portillo GS, Acosta SA, Kaneko Y, Borlongan CV, Tajiri N. Stem cell-paved biobridges facilitate stem transplant and host brain cell interactions for stroke therapy. Brain Res. 2015;1623:160–165. doi: 10.1016/j.brainres.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito E, Mandeville ET, Hayakawa K, Singhal AB, Lo EH. Effects of normobaric oxygen on the progression of focal cerebral ischemia in rats. Experimental neurology. 2013;249:33–38. doi: 10.1016/j.expneurol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujishima M, Sadoshima S, Ogata J, Yoshida F, Shiokawa O, Ibayashi S, Omae T. Autoregulation of cerebral blood flow in young and aged spontaneously hypertensive rats (SHR) Gerontology. 1984;30(1):30–36. doi: 10.1159/000212604. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara K, Date I, Shingo T, Yoshida H, Kobayashi K, Takeuchi A, Yano A, Tamiya T, Ohmoto T. Reduction of infarct volume and apoptosis by grafting of encapsulated basic fibroblast growth factor-secreting cells in a model of middle cerebral artery occlusion in rats. J Neurosurg. 2003;99(6):1053–1062. doi: 10.3171/jns.2003.99.6.1053. [DOI] [PubMed] [Google Scholar]
- 15.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19(1):197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 16.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, Roy M, Quinones-Hinojosa A, VandenBerg S, Alvarez-Buylla A. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51(2):187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan MS, Bell DH. Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci. 1984;4(6):1429–1441. doi: 10.1523/JNEUROSCI.04-06-01429.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, et al. Functional recovery after ischemic stroke--a matter of age: data from the Austrian Stroke Unit Registry. Neurology. 2012;78(4):279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- 20.Kronenberg G, Wang LP, Synowitz M, Gertz K, Katchanov J, Glass R, Harms C, Kempermann G, Kettenmann H, Endres M. Nestin-expressing cells divide and adopt a complex electrophysiologic phenotype after transient brain ischemia. J Cereb Blood Flow Metab. 2005;25(12):1613–1624. doi: 10.1038/sj.jcbfm.9600156. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16(6):2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 23.Marini C, Triggiani L, Cimini N, Ciancarelli I, De Santis F, Russo T, Baldassarre M, di Orio F, Carolei A. Proportion of older people in the community as a predictor of increasing stroke incidence. Neuroepidemiology. 2001;20(2):91–95. doi: 10.1159/000054766. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto N, Tanaka R, Shimura H, Watanabe T, Mori H, Onodera M, Mochizuki H, Hattori N, Urabe T. Phosphodiesterase III inhibition promotes differentiation and survival of oligodendrocyte progenitors and enhances regeneration of ischemic white matter lesions in the adult mammalian brain. J Cereb Blood Flow Metab. 2010;30(2):299–310. doi: 10.1038/jcbfm.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murata Y, Fujiwara N, Seo JH, Yan F, Liu X, Terasaki Y, Luo Y, Arai K, Ji X, Lo EH. Delayed inhibition of c-Jun N-terminal kinase worsens outcomes after focal cerebral ischemia. J Neurosci. 2012;32(24):8112–8115. doi: 10.1523/JNEUROSCI.0219-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Neill TJt, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97(10):1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 28.Rosenzweig S, Carmichael ST. Age-dependent exacerbation of white matter stroke outcomes: a role for oxidative damage and inflammatory mediators. Stroke. 2013;44(9):2579–2586. doi: 10.1161/STROKEAHA.113.001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin YJ, Kim HL, Park JM, Cho JM, Kim SY, Lee MY. Characterization of nestin expression and vessel association in the ischemic core following focal cerebral ischemia in rats. Cell and tissue research. 2013;351(3):383–395. doi: 10.1007/s00441-012-1538-x. [DOI] [PubMed] [Google Scholar]
- 30.Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22(7):2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skihar V, Silva C, Chojnacki A, Doring A, Stallcup WB, Weiss S, Yong VW. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc Natl Acad Sci U S A. 2009;106(42):17992–17997. doi: 10.1073/pnas.0909607106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, Leak RK, Stetler RA, Gao Y, Chen J. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol. 2015 doi: 10.1016/j.expneurol.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tajiri N, Kaneko Y, Shinozuka K, Ishikawa H, Yankee E, McGrogan M, Case C, Borlongan CV. Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One. 2013;8(9):e74857. doi: 10.1371/journal.pone.0074857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takaba H, Fukuda K, Yao H. Substrain differences, gender, and age of spontaneously hypertensive rats critically determine infarct size produced by distal middle cerebral artery occlusion. Cellular and molecular neurobiology. 2004;24(5):589–598. doi: 10.1023/B:CEMN.0000036400.55503.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter J, Keiner S, Witte OW, Redecker C. Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol Aging. 2011;32(10):1906–1914. doi: 10.1016/j.neurobiolaging.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto J, Nakai M, Natsume T. Cardiovascular responses to acute stress in young-to-old spontaneously hypertensive rats. Hypertension. 1987;9(4):362–370. doi: 10.1161/01.hyp.9.4.362. [DOI] [PubMed] [Google Scholar]
- 37.Yao H, Sadoshima S, Ooboshi H, Sato Y, Uchimura H, Fujishima M. Age-related vulnerability to cerebral ischemia in spontaneously hypertensive rats. Stroke. 1991;22(11):1414–1418. doi: 10.1161/01.str.22.11.1414. [DOI] [PubMed] [Google Scholar]
- 38.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8(5):491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]