Abstract
While sarcopenia has been associated with decreased overall survival in diffuse large B-cell (DLBCL) patients, the impact of sarcopenia on treatment tolerance has not been well-studied. We evaluated the association of sarcopenia with febrile neutropenia hospitalization, treatment-related mortality, and ability to complete standard number of cycles in a retrospective cohort of United States veterans diagnosed with DLBCL between 1998 and 2008 and treated with cyclophosphamide, doxorubicin, vincristine, and prednisone, with or without rituximab. Baseline body composition parameters were evaluated using computed tomography analysis. In total, 522 patients were included in the study, of whom 245 (47%) had baseline sarcopenia. After controlling for other variables, baseline sarcopenia was independently associated with increased risk of febrile neutropenia hospitalization (adjusted Odds Ratio (aOR) 1.64, 95% Confidence Interval (CI) 1.01 to 2.65) and inability to complete standard number of treatment cycles (aOR 1.49, 95% CI 1.02 to 2.16) compared with no baseline sarcopenia. There was a non-statistically significant trend towards higher treatment-related mortality in sarcopenic patients than non-sarcopenic patients (aOR 1.77, 95% CI 0.92 to 3.41). Sarcopenia is associated with increased risk of treatment intolerance and may be useful in guiding treatment planning and supportive care measures.
Keywords: sarcopenia, non-Hodgkin’s lymphoma, chemotherapy, febrile neutropenia
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma (NHL), accounting for 30% of all NHL cases [1,2]. Sarcopenia, or the presence of low muscle mass, has been associated with decreased overall survival in patients with DLBCL [3–5]. However, the impact of sarcopenia on other outcomes, including treatment toxicity, treatment-related mortality, and ability to complete standard therapy, has not been well-studied in the DLBCL population.
Understanding the relationship of low muscle mass with treatment tolerance may be useful for guiding individual treatment decisions and supportive care measures. Treatment with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) is the current standard of care, front-line therapy for DLBCL [6]. In patients receiving CHOP-based therapy, maintaining dose intensity >70–90% has been associated with improved overall survival [7–9]. However, the benefits of standard, full-dose therapy must be weighed against the risks of treatment-related complications. There is evidence from solid tumor cancers that body composition parameters could be used to assess risk for chemotherapy toxicity. For example, sarcopenia has been associated with increased dose-limiting toxicities in patients undergoing treatment for colon, breast, and renal cell cancer [10–12].
In this study, we evaluated a cohort of United States veterans with DLBCL diagnosed within the United States Veterans Health Administration (VHA) system and treated with cyclophosphamide, doxorubicin, vincristine, and prednisone, with or without rituximab (CHOP +/− R). The primary objective of this study is to evaluate the association of sarcopenia with hospitalizations for febrile neutropenia, one of the major dose-limiting toxicities associated with CHOP-based therapy. Secondary outcomes of interest include treatment-related mortality and completion of standard number of treatment cycles.
Methods
Study Cohort
A retrospective cohort of patients with a new diagnosis of DLBCL between October 1, 1998 and September 30, 2008 was assembled from the Veteran’s Health Administration Central Cancer Registry (VACCR) based on the InterLymph classification system (International Classification of Diseases (ICD) -O3 codes 9680/3 and 9684/3 for DLBCL) [13]. Data were obtained from all 21 VHA regions throughout the United States. Patients were excluded for the following reasons: missing vital status, central nervous system involvement, tested positive for human immunodeficiency virus, primary cutaneous DLBCL, inadequate histologic confirmation, treatment with regimens other than CHOP +/− R, no treatment, treatment outside of the VHA, or no baseline computed tomography (CT) scan. The study was approved by the Veterans Affairs St. Louis Health Care System and Washington University institutional review boards prior to cohort assembly.
Clinical Data Collection
Data on histologic diagnosis, date of birth, date of diagnosis, race, sex, disease stage, and the presence of systemic B-symptoms (fever 100.4°F, weight loss >10% of body weight in 6 months, and night sweats) were provided by VACCR. Patient records were also linked to additional VHA administrative datasets to obtain vital sign data including height and weight, ICD-9 codes for comorbid conditions, and date of death. Focused data abstraction was performed using the VHA Compensation and Pension Records Interchange software system to collect data on lactate dehydrogenase (LDH) levels; chemotherapy drugs, doses, and dates of administration; myeloid growth factor doses and dates of administration; and hospitalization dates and admission diagnoses. CT scans were accessed through the VistA Imaging System’s Advanced Web Image Viewer (AWIV).
Outcome Definitions
The primary outcome of interest was hospitalization for febrile neutropenia, defined as any hospitalization with febrile neutropenia as the admission diagnosis.
Secondary outcomes of interest included treatment-related mortality and completion of standard number of chemotherapy cycles. Treatment-related mortality was defined as death <=30 days after last chemotherapy treatment. Standard number of cycles was defined as a minimum of 3 treatment cycles in patients with stage I or II disease who received radiotherapy and a minimum of 6 treatment cycles in all other patients [14].
Study Independent Variables and Definitions
The Romano adaptation of the Charlson co-morbidity index was calculated for patients included in the study cohort using ICD-9 codes for co-morbid conditions present at the time of diagnosis [15]. Age at diagnosis was dichotomized to ≥ 65 years of age and <65 years of age for analyses involving febrile neutropenia risk in concordance with current guidelines for prophylactic growth factor use [16]; or to >60 years of age and ≤60 years of age in analyses involving treatment-related mortality or completion of standard therapy in concordance with the International Prognostic Index for aggressive non-Hodgkin’s lymphoma [17]. Age was used as a continuous variable in other analyses. LDH was dichotomized as elevated or not elevated at time of diagnosis based on local reference ranges.
BMI was calculated as weight measured in kilograms divided by the square of height measured in meters (kg/m2) and was categorized in accordance with World Health Organization guidelines [18]. Body Surface Area (BSA) was calculated according to the DuBois formula [19] using weight measured within one month of treatment initiation and consistently recorded height data. Early granulocyte-colony stimulating factor (G-CSF) use was defined as any administration of G-CSF during the first 5 days of the first cycle of chemotherapy.
Agent-specific relative dose intensity was defined as the ratio of dose actually delivered over time to the standard dose intensity [20]. The standard doxorubicin and cyclophosphamide dose was 50 mg/m2 and 750 mg/m2, respectively, administered at twenty-one day intervals. The average relative dose intensity (ARDI) for each patient was obtained by averaging the agent-specific relative dose intensities of doxorubicin and cyclophosphamide. Consistent with previous literature, ARDI of ≥ 85% was considered full dose intensity, while < 85% was considered reduced dose intensity [21].
Body composition analysis was performed on computed tomography (CT) scans obtained within three months prior to treatment initiation. Analysis was performed by a single trained reviewer (DYX) who was blinded to the primary outcome. An axial image at the third lumbar vertebral level (L3) was identified [22] and this image was thresholded based on standard Hounsfield unit (HU) ranges for skeletal muscle (−29 to +150). The thresholded images were then copied into the National Institute of Health’s ImageJ Program, and skeletal muscle area was computed for each image in cm2 (Figure 1). Measurements were normalized to patient’s height and expressed as lumbar skeletal muscle index (cm2/m2). Sarcopenia was defined as lumbar skeletal muscle index <53 cm2/m2 in men and <41 cm2/m2 in women, based on previously published values [23]. No other threshold values were tested.
Figure 1.
Example of CT muscle area assessment. (A) Example of abdominal CT image at the third lumbar vertebral level. (B) CT image is thresholded to −29 to 150 HU. CT numbers above this range are shown as white pixels and CT numbers below this range are shown as black pixels. (C) Thresholded CT image is copied into ImageJ and black and white values are removed, leaving only areas corresponding to skeletal muscle density. Skeletal muscle is manually selected and area is calculated in cm2.
Abbreviations: CT=computed tomography; HU=Hounsfield unit
Statistical Analyses
Chi-square, Mann-Whitney U, and student’s t-test were used for analyses, where appropriate. Univariate logistic regression explored factors associated with febrile neutropenia hospitalization, treatment-related mortality, and inability to complete standard number of treatment cycles. Variables with p-value <0.05 on univariate analysis were then entered simultaneously into a multivariable logistic regression model. A two-tailed α significance level of 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS version 20.
Results
Patient demographics and clinical characteristics
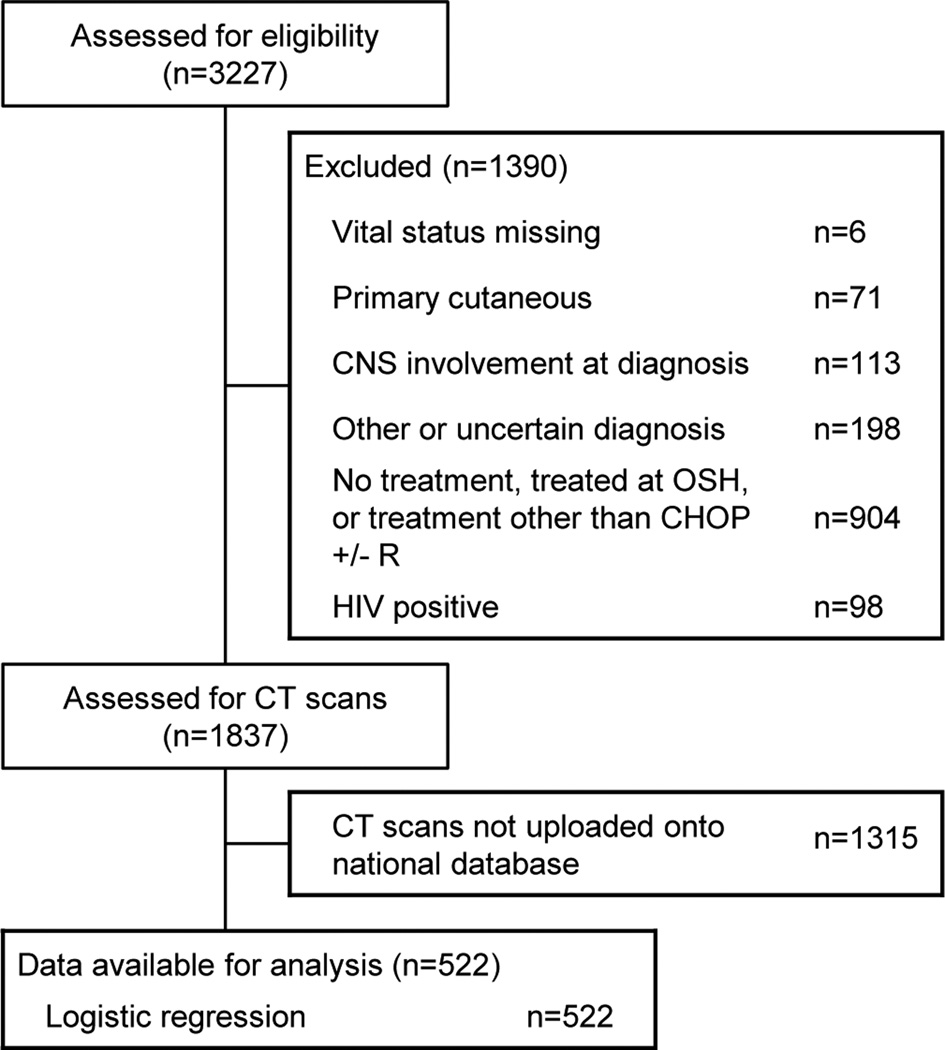
Of the 3,227 patients with DLBCL initially identified, 1837 patients remained after applying exclusion criteria (Figure 2). Of these patients, 1315 did not have baseline CT scans uploaded to the national database, leaving a final cohort of 522 patients with baseline body composition information. Baseline characteristics of those who did and did not have baseline CT scans were similar (Supplementary Table S1).
Figure 2.
STROBE Diagram
Abbreviations: CNS=Central nervous system; OSH=outside hospital; CT=computed tomography; CHOP +/− R=cyclophosphamide, doxorubicin, vincristine, and prednisone, with or without rituximab; HIV=human immunodeficiency virus
Patient characteristics by sarcopenia status are summarized in Table I. Forty-seven percent of patients were classified as sarcopenic. Compared to non-sarcopenic patients, sarcopenic patients were more likely to be older (mean age 68.1 years versus 61.2 years, p<0.001), white (93.1% versus 83.8%, p=0.005), have a higher comorbidity index (mean score 2.3 versus 1.9, p=0.018), have B-symptoms (60.0% versus 52.7%, p=0.004), and have a lower mean BMI (24.6 versus 29.2, p<0.001).
Table I.
Demographic characteristics, dose characteristics, and toxicity outcomes of US veterans diagnosed with DLBCL from 1998 to 2008 according to sarcopenia status
| Clinical and demographic characteristics |
No sarcopenia n=277 | Sarcopenia n=245 | p value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (mean years, STD, IQR) |
61.2 (11.4, 15) | - | 68.1 (10.7, 17) | - | <0.001‡ |
| Male | 270 | 97.5 | 240 | 98.0 | 0.711* |
| Race | 0.005* | ||||
| White | 232 | 83.8 | 228 | 93.1 | |
| Black | 42 | 15.2 | 16 | 6.5 | |
| Other | 3 | 1.1 | 1 | 0.4 | |
| Comorbidity score (mean, STD, IQR) |
1.9 (1.9, 3.0) | - | 2.3 (2.0, 3.0) | - | 0.018‡ |
| Stage | 0.172* | ||||
| Stage I/II | 116 | 41.9 | 94 | 38.4 | |
| Stage III/IV | 158 | 57.0 | 151 | 61.6 | |
| Unknown | 3 | 1.1 | 0 | 0.0 | |
| LDH | 0.168* | ||||
| Elevated | 136 | 49.1 | 140 | 57.1 | |
| Not Elevated | 121 | 43.7 | 88 | 35.9 | |
| Unknown | 20 | 7.2 | 17 | 6.9 | |
| B-symptoms | 0.004* | ||||
| Yes | 129 | 52.7 | 147 | 60.0 | |
| No | 146 | 46.6 | 94 | 38.4 | |
| Unknown | 2 | 0.7 | 4 | 1.6 | |
| Type of treatment | 0.618* | ||||
| CHOP | 38 | 13.7 | 30 | 12.2 | |
| R-CHOP | 239 | 86.3 | 215 | 87.8 | |
| Year of diagnosis (median) |
2005 | - | 2005 | - | 0.631§ |
| BMI category at diagnosis (kg/m2) |
<0.001* | ||||
| <18.5 | 0 | 0.0 | 9 | 3.7 | |
| 18.5 to <25 | 56 | 20.2 | 128 | 52.2 | |
| 25 to <30 | 114 | 41.2 | 90 | 36.7 | |
| ≥30 | 107 | 38.6 | 18 | 7.3 | |
| Number of cycles (mean, STD, IQR) |
5.4 (1.9, 2.0) | - | 4.9 (2.1, 2.5) | - | 0.007‡ |
| First-cycle average relative dose-intensity |
0.143* | ||||
| >=85% | 237 | 85.6 | 220 | 89.8 | |
| <85% | 40 | 14.4 | 25 | 10.2 | |
| Average relative dose- intensity across all cycles |
0.762* | ||||
| >=85% | 166 | 59.9 | 150 | 61.2 | |
| <85% | 111 | 40.1 | 95 | 38.8 | |
| Early G-CSF use | 105 | 37.9 | 115 | 46.9 | 0.037* |
| Hospitalization for febrile neutropenia |
47 | 17.0 | 69 | 28.2 | 0.002* |
| Treatment-related mortality |
16 | 5.8 | 28 | 11.4 | 0.020* |
Footnotes:
T test,
Chi-square test,
Mann-Whitney U-test
Abbreviations: DLBCL=diffuse large B-cell lymphoma; US=United States; STD=standard deviation; IQR=interquartile range; LDH=lactate dehydrogenase; CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone, without rituximab; R-CHOP=cyclophosphamide, doxorubicin, vincristine, and prednisone, with rituximab; BMI=body mass index; G-CSF=granulocyte-colony stimulating factor
Hospitalizations for febrile neutropenia
There were a total of 435 unplanned hospitalizations across all treatment cycles, and of these 150 had an admission diagnosis of febrile neutropenia. Overall, 116 patients (22.2%) had at least one hospitalization for febrile neutropenia. In the entire cohort, 28.2% of sarcopenic patients had at least one hospitalization for febrile neutropenia, compared to 17.0% of non-sarcopenic patients (p=0.002). In the R-CHOP subgroup, 26.9% of sarcopenic patients had at least one hospitalization for febrile neutropenia, compared to 15.9% of non-sarcopenic patients (p=0.008).
In addition to baseline sarcopenia, other factors associated with febrile neutropenia hospitalization on univariate analysis included: age ≥ 65 years, comorbidity score, stage III/IV, presence of B-symptoms, first-cycle ARDI ≥ 85%, and underweight BMI (Table II). Variables with a p-value <0.05 identified by univariate analysis were further tested in a multiple logistic regression model (n=522). In the multivariable model, baseline sarcopenia was an independent predictor of febrile neutropenia (Odds Ratio (OR) 1.64, 95% Confidence Interval (CI) 1.01 to 2.65). Other factors independently associated with higher febrile neutropenia risk included comorbidity score (OR 1.23, 95% CI 1.09 to 1.39), stage III/IV (OR 1.66, 95% CI 1.04 to 2.65), and first-cycle ARDI ≥ 85% (OR 3.21, 95% CI 1.40 to 7.40).
Table II.
Univariate and multivariable logistic regression analysis of factors associated with hospitalization for febrile neutropenia
| Univariate regression analysis |
Multivariable regression analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age >=65 years | 1.82 (1.20 to 2.77) | 0.005 | 1.37 (0.85 to 2.20) | 0.195 |
| Comorbidity score | 1.22 (1.10 to 1.35) | <0.001 | 1.23 (1.09 to 1.39) | <0.001 |
| Stage III/IV | 1.63 (1.05 to 2.54) | 0.03 | 1.66 (1.04 to 2.65) | 0.034 |
| B-symptoms, yes | 1.71 (1.11 to 2.63) | 0.014 | 1.53 (0.97 to 2.44) | 0.070 |
| LDH, elevated | 1.11 (0.73 to 1.71) | 0.622 | - | |
| First-cycle ARDI >=85% |
2.21 (1.02 to 4.77) | 0.044 | 3.21 (1.40 to 7.40) | 0.006 |
| Early G-CSF use | 1.47 (0.97 to 2.23) | 0.073 | - | |
| BMI | ||||
| <18.5 | 4.36 (1.12 to 16.98) | 0.033 | 2.89 (0.71 to 11.82) | 0.138 |
| 18.5 to <25 | Reference | Reference | ||
| >=25 | 0.96 (0.62 to 1.48) | 0.853 | 1.23 (0.75 to 2.00) | 0.415 |
| Baseline sarcopenia | 1.92 (1.26 to 2.92) | 0.002 | 1.64 (1.01 to 2.65) | 0.046 |
Abbreviations: OR=odds ratio; CI=confidence interval; LDH=lactate dehydrogenase; ARDI=average relative dose intensity; G-CSF=granulocyte-colony stimulating factor; BMI=body mass index
Treatment-related mortality
In the entire cohort, 44 patients (8.4%) died within 30 days of receiving chemotherapy. The majority of deaths occurred during the first cycle of chemotherapy, with 21 occurring in the first cycle, 8 in the second cycle, 4 in the third, fourth, and sixth cycles, and 3 in the fifth cycle. In the entire cohort, treatment-related mortality occurred in 11.4% of sarcopenic patients, compared to 5.8% of non-sarcopenic patients (p=0.020). In the R-CHOP subgroup, treatment-related mortality occurred in 11.2% of sarcopenic patients, compared to 5.4% of non-sarcopenic patients (p=0.029).
In addition to baseline sarcopenia, age > 60 years was also associated with treatment-related mortality on univariate analysis (Supplementary Table S2). After adjusting for age, there was a non-statistically significant trend towards increased treatment-related mortality in sarcopenic patients (OR 1.77, 95% CI 0.92 to 3.41) compared to non-sarcopenic patients.
Completion of standard therapy
There was no difference in ARDI across all delivered cycles between sarcopenic and non-sarcopenic patients, with 61% of sarcopenic patients receiving ARDI ≥ 85% across all delivered cycles compared to 60% of nonsarcopenic patients (p=0.762). However, sarcopenic patients were less likely to complete the standard number of cycles, with only 59.2% of sarcopenic patients completing the standard number of treatment cycles compared to 70.4% of non-sarcopenic patients (p=0.007). In the R-CHOP subgroup, 60.4% of sarcopenic patients versus 70.3% of non-sarcopenic patients completed standard treatment (p=0.028). In the CHOP subgroup, 50.0% of sarcopenic patients versus 71.1% of non-sarcopenic patients completed standard treatment (p=0.08).
In addition to baseline sarcopenia, other factors associated with inability to complete the standard number of treatment cycles included age > 60 years and higher comorbidity score (Supplementary Table S3). After controlling for age and comorbidity score, baseline sarcopenia was an independent predictor of inability to complete standard number of treatment cycles (OR 1.49, 95% CI 1.02 to 2.16).
Discussion
This study evaluated the association of sarcopenia with treatment tolerability and feasibility in a largely white male cohort of patients with DLBCL. Compared with non-sarcopenic patients, sarcopenic patients had increased risk of febrile neutropenia hospitalization and a trend towards increased risk of treatment-related mortality after controlling for other variables. Sarcopenic patients were also less likely to complete the standard number of treatment cycles compared to non-sarcopenic patients.
These results are consistent with previous literature evaluating sarcopenia in patients with hematologic malignancies. In a cohort of 82 elderly patients with DLBCL, Lanic et al. found that 60% of sarcopenic patients with DLBCL completed therapy compared to 84% of non-sarcopenic patients [3]. Similarly, in a cohort of 187 Korean patients with DLBCL, Go et al. reported that both treatment-related mortality (21.7% vs. 5.0%) and early treatment discontinuation (32.6% vs 14.9%) were higher in sarcopenic patients compared to non-sarcopenic patients. Finally, Caram et al. found that sarcopenia, as measured by psoas muscle index, was associated with higher complication rates and longer hospital stays in men undergoing autologous transplant for non-Hodgkin or Hodgkin lymphoma [24].
We found that sarcopenia was an independent predictor of febrile neutropenia hospitalization. Consistent with previously published studies, other variables associated with febrile neutropenia hospitalization in this study included higher comorbidity index, advanced stage, and first-cycle ARDI ≥85% [25]. Lower BMI was also associated with increased risk of febrile neutropenia [26], but the association was no longer significant after controlling for sarcopenia. We observed a trend towards increased risk for febrile neutropenia hospitalization in patients with early G-CSF use compared with those without early G-CSF use. This is in contrast to previously published randomized controlled trials which demonstrate protective effects with primary G-CSF prophylaxis [27]. This is likely explained by confounding by indication, as patients at higher risk for febrile neutropenia may have been more likely to be prescribed primary G-CSF prophylaxis.
We observed a trend towards increased risk of treatment-related mortality in sarcopenic patients compared to non-sarcopenic patients, and consistent with previous studies, observed that the majority of treatment-related deaths occurred in the first cycle [28]. While no study has specifically evaluated sarcopenia as a predictor of early death, Peyrade et al. found that low albumin level was associated with poorer overall survival in a cohort of elderly patients with DLBCL, and Soubeyran et al. found that the Mini Nutritional Assessment (MNA) and the Timed Get Up and Go (GUG) were associated with early death in a cohort of elderly cancer patients [28,29]. Sarcopenia has been associated with decreased MNA scores in previous studies and may reflect both poor nutritional status and decreased functional mobility [30]. Taken together, these results lend evidence that nutritional parameters, along with muscle mass and strength, may be important for predicting treatment-related mortality.
The mechanism for increased chemotherapy toxicity in sarcopenic patients has not been well-studied. One potential hypothesis is that altered body composition may affect the distribution, metabolism, and clearance of chemotherapy drugs [31]. Patients with low lean body mass may therefore be exposed to higher concentrations of cytotoxic drugs than those with higher lean body mass. Supporting this, a previous study of epirubicin pharmacokinetics demonstrated an association between epirubicin clearance and lean body mass [32].
Our findings that sarcopenia is associated with decreased treatment tolerance may have important clinical implications. First, these results may guide supportive care measures in the DLBCL population. Current guidelines recommend use of primary G-CSF prophylaxis in patients with DLBCL >=65 years who are being treated with R-CHOP, although other considerations including comorbidities may be taken into account [16]. While this retrospective study must be confirmed by future studies, physicians could consider broader use of primary G-CSF prophylaxis in patients with sarcopenia who may not otherwise fulfill current criteria. Second, given that most treatment-related deaths occurred during the first cycle of chemotherapy, our findings may support the use of pre-phase treatment in elderly patients with sarcopenia. Pre-phase treatment involves corticosteroids for 7 days prior to initiation of chemotherapy, and was used in the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) trials in elderly patients [33,34]. A decrease in early treatment-related deaths and improvement in performance status was observed in elderly patients who underwent pre-phase treatment, although no statistical data was available to support this clinical experience [33]. Third, evaluation of sarcopenia could be used to guide treatment planning and dosing, particularly in the elderly. While maintaining high dose-intensity improves overall survival, identification of elderly patients fit for full-dose therapy is challenging. A dose-reduced R-CHOP regimen (R-miniCHOP) has been shown to be safe and effective in a cohort of patients with DLBCL greater than 80 years old [28]. Future prospective studies investigating the use of body composition parameters in guiding supportive care and treatment decisions are warranted.
There are multiple strengths to this study. First, the VHA provided a large study cohort with comprehensive clinical data drawn from patients diagnosed and treated throughout the United States. Second, patients had an equal opportunity for inclusion regardless of comorbidities or other factors that may introduce selection bias observed in studies of patients enrolled in clinical trials.
Limitations to this study should be noted. First, 70% of the study cohort did not have CT scans available for analysis, potentially introducing selection bias. To address these concerns, we compared baseline characteristics between those who did and did not have CT scans, and found no statistically significant differences. Second, the European Working Group on Sarcopenia in Older People released a consensus definition on sarcopenia, which recommends using the presence of both low muscle mass and low muscle function to diagnosis sarcopenia [35]. Because of the retrospective nature of our study, we were unable to measure muscle function. We were also unable to control for performance status, as this information was not available in many patients. Third, multiple threshold values used for defining sarcopenia exist in the literature. We chose to use the sex-specific threshold values reported by Martin et al. for overweight patients because it is the largest study of sarcopenia in cancer patients to date, and because 63% of our patients have a BMI of 25 or higher [23]. Fourth, the study cohort was comprised almost entirely of men, which may limit our ability to extrapolate these findings to women. Finally, the VHA largely serves individuals who served in the United States military, who at the time of their service met the physical requirements for military enlistment. As a result, it is possible that the veteran population may be more physically fit and have higher muscle mass than average, potentially influencing the generalizability of our findings to the general population.
In conclusion, baseline sarcopenia was associated with poor treatment tolerance in patients with DLBCL undergoing CHOP-based chemotherapy. After controlling for other variables, baseline sarcopenia was independently associated with increased risk for febrile neutropenia hospitalizations and inability to complete the standard number of treatment cycles compared to no baseline sarcopenia. There was also a non-statistically significant trend towards increased treatment-related mortality in sarcopenic patients compared to non-sarcopenic patients. Future prospective studies examining the utility of body composition parameters in guiding supportive care and treatment decisions in the DLBCL population are warranted.
Supplementary Material
Acknowledgments
This work was supported by: The NCCN Young Investigator Awards (YIA), The Barnes-Jewish Hospital Foundation, The American Cancer Society (MSRG-13-077-01-CPHPS), the National Cancer Institute at the National Institutes of Health (U54CA155496 and 5K12HL087107), and the Washington University Institute of Clinical and Translational Sciences grant UL1TR000448, sub-award TL1TR000449, from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of: The United States Department of Veterans Affairs, the National Cancer Institute, or the National Institutes of Health. This material is the result of work supported with resources and the use of facilities at the St. Louis Veterans Affairs Medical Center.
Footnotes
Author contributions
KRC and DYX designed the research study. DYX, SL, KO, AG, PR, KMS, RCL, and WL were involved in data acquisition. SL, DYX, and KRC analyzed and interpreted the data. DYX drafted the paper, and KRC, SL, KO, AG, PR, KMS, RCL and WL revised it critically. All authors approved the final manuscript.
Competing interests
The authors have no competing interests.
References
- 1.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swerdlow S, Campo E, Harris N, et al. World Health Organization classification of tumours of haematopoietic and lymphoid tissues. 4th. Lyon: IARC Press; 2008. [Google Scholar]
- 3.Lanic H, Kraut-tauzia J, Modzelewski R, et al. Sarcopenia is an independent prognostic factor in elderly patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Leuk Lymphoma. 2014;55:817–823. doi: 10.3109/10428194.2013.816421. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura N, Hara T, Shibata Y, et al. Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Ann Hematol. 2015;94:2043–2053. doi: 10.1007/s00277-015-2499-4. [DOI] [PubMed] [Google Scholar]
- 5.Go S, Park MJ, Song H, et al. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Cachexia Sarcopenia Muscle. 2016 doi: 10.1002/jcsm.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 7.Epelbaum R, Haim N, Ben-Shahar M, et al. Dose-intensity analysis for CHOP chemotherapy in diffuse aggressive large cell lymphoma. Isr J Med Sci. 1988;24:533–538. [PubMed] [Google Scholar]
- 8.Kwak LW, Halpern J, Olshen RA, et al. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma : results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–977. doi: 10.1200/JCO.1990.8.6.963. [DOI] [PubMed] [Google Scholar]
- 9.Lepage E, Gisselbrecht C, Haioun C, et al. Prognostic significance of received relative dose intensity in non-Hodgkin’s lymphoma patients: Application to LNH-87 protocol. Ann Oncol. 1993;4:651–656. doi: 10.1093/oxfordjournals.annonc.a058619. [DOI] [PubMed] [Google Scholar]
- 10.Prado CMM, Baracos VE, Mccargar LJ, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–3269. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]
- 11.Prado CMM, Baracos VE, Mccargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 12.Antoun S, Baracos VE, Birdsell L, et al. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21:1594–1598. doi: 10.1093/annonc/mdp605. [DOI] [PubMed] [Google Scholar]
- 13.Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph) Blood. 2007;110:695–708. doi: 10.1182/blood-2006-11-051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelenetz A. Non-Hodgkin lymphomas. Accessed from: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf. [Google Scholar]
- 15.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases: differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 16.Smith TJ, Bolke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 17.The International non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 18.WHO Expert Commitee. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 19.Dubois D, Dubois E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 20.Hryniuk W, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8:1935–1937. doi: 10.1200/JCO.1990.8.12.1935. [DOI] [PubMed] [Google Scholar]
- 21.Lyman GH, Dale DC, Friedberg J, et al. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol. 2004;22:4302–4311. doi: 10.1200/JCO.2004.03.213. [DOI] [PubMed] [Google Scholar]
- 22.Mourtzakis M, Prado CMM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 23.Martin L, Birdsell L, MacDonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 24.Caram MV, Bellile EL, Englesbe MJ, et al. Sarcopenia is associated with autologous transplant-related outcomes in patients with lymphoma. Leuk Lymphoma. 2015;56:2855–2862. doi: 10.3109/10428194.2015.1014359. [DOI] [PubMed] [Google Scholar]
- 25.Lyman G, Lyman C, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10:427–437. doi: 10.1634/theoncologist.10-6-427. [DOI] [PubMed] [Google Scholar]
- 26.Ganti A, Liu W, Luo Su, et al. Impact of BMI on incidence of febrile neutropenia and treatment-related mortality in United States veterans with diffuse large B-cell lymphoma receiving R-CHOP. Br J Haematol. 2013;167:699–702. doi: 10.1111/bjh.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 28.Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12 doi: 10.1016/S1470-2045(11)70069-9. [DOI] [PubMed] [Google Scholar]
- 29.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829–1834. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 30.Bahat G, Saka B, Tufan F, et al. Prevalence of sarcopenia and its association with functional and nutritional status among male residents in a nursing home in Turkey. Aging Male. 2010;13:211–214. doi: 10.3109/13685538.2010.489130. [DOI] [PubMed] [Google Scholar]
- 31.Prado CMM, Maia YLM, Ormsbee M, et al. Assessment of nutritional status in cancer - the relationship between body composition and pharmacokinetics. Anticancer Agents Med Chem. 2013;13:1197–1203. doi: 10.2174/18715206113139990322. [DOI] [PubMed] [Google Scholar]
- 32.Cosolo W, Morgan D, Seeman E, et al. Lean body mass, body surface area and epirubicin kinetics. Anticancer Drugs. 1994;3:293–297. doi: 10.1097/00001813-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Pfreundschuh M, Trumper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104:634–641. doi: 10.1182/blood-2003-06-2095. [DOI] [PubMed] [Google Scholar]
- 34.Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20 + B-cell lymphomas : a randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 35.Cruz-Jentoft A, Baeyens J, Bauer J, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.