Abstract
A free radical scavenger edaravone is clinically used in Japan for acute stroke, and several basic researches have carefully examined the mechanisms of edaravone's protective effects. However, its actions on pro-inflammatory responses under stroke are still understudied. In this study, we subjected adult male Sprague-Dawley rats to 90-min middle cerebral artery (MCA) occlusion followed by reperfusion. Edaravone was treated twice via tail vein; after MCA occlusion and after reperfusion. As expected, edaravone-treated group showed less infarct volume and edema formation compared with control group at 24-hour after ischemic onset. Furthermore, edaravone reduced the levels of plasma interleukin (IL)-1β and matrix metalloproteinase-9 at 3-hour after ischemic onset. Several molecules besides IL-1β and MMP-9 are involved in inflammatory responses under stroke conditions. Therefore, we also examined whether edaravone treatment could decrease a wide range of pro-inflammatory cytokines/chemokines by testing rat plasma samples with a rat cytokine array. MCAO rats showed elevations in plasma levels of CINC-1, Fractalkine, IL-1α, IL-1ra, IL-6, IL-10, IP-10, MIG, MIP-1α, and MIP-3α, and all these increases were reduced by edaravone treatment. These data suggest that free radical scavengers may reduce systemic inflammatory responses under acute stroke conditions, and therefore, oxidative stress can be still a viable target for acute stroke therapy.
Keywords: stroke, edaravone, oxidative stress, pro-inflammatory cytokine, middle cerebral artery occlusion, protein array
1. Introduction
Over the past decade, the basic molecular mechanisms of brain cell death have been extensively studied. Among the mechanisms, oxidative stress is thought as one of the major implicators for several CNS diseases, including stroke [4, 23]. Under cerebral ischemic conditions, free radicals (e.g. reactive oxygen and nitrogen species) are generated in the so-called ischemic penumbra [8, 34]. However, the Phase III clinical trial of the radical spin trap NXY-059 for acute stroke patients was unsuccessful [35]. Post-hoc analyses pointed out the efficacy issues related to the compound itself as well as the difficulty in drug delivery of that compound [14, 30, 33]. Nevertheless, accumulating evidence still supports the basic idea that free radical scavengers can be considered as a therapeutic approach for acute stroke therapy [27].
A radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is widely used in Japan for acute ischemic stroke [1, 25]. Past studies using in vivo rodent stroke models confirmed the neuroprotective effects of edaravone, especially in penumbra regions where free radicals are generated [3, 5, 17, 47]. Furthermore, edaravone can reduce brain edema formation by quenching hydroxyl radicals and inhibiting lipid peroxidation [2, 28, 39, 40, 42]. Also in in-vitro cell culture systems, edaravone has been demonstrated to show protective effects for many types of brain cells [9, 18, 22, 44, 45].
Several lines of experiments have shown that during the acute stroke phase, deleterious inflammatory cascades are also activated [6, 15, 21]. While the brain- and cyto-protective properties of edaravone are conclusive, its actions on the systemic inflammatory responses in animal models of stroke are still mostly unknown. Therefore, we used a rat stroke model to test the efficacy of edaravone on pro-inflammatory cytokines, chemokines, and proteases in plasma. First, we confirmed the protective effects of edaravone on cerebral infarction and brain swelling due to ischemic stress in our system. Then, we measured levels of interleukin-1β (IL-1β) and matrix metalloproteinase-9 (MMP-9) in rat plasma samples from the acute phase of stroke, and examined effects of edaravone on their upregulation. Finally, we tested rat plasma samples with a rat cytokine array to check if edaravone could broadly reduce inflammatory responses under the acute phase of stroke.
2. Material and Methods
Animal Model
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were anesthetized with isoflurane (5% induction, 1–1.2% maintenance) in a 30%-oxygen/70%-nitrous oxide mix. Temperature was maintained at 37 ± 0.5°C with a heating pad. Right femoral arteries were cannulated to monitor pressure, pH and gases. The intraluminal filament stroke model was prepared as described previously [13]. Briefly, a silicone-coated 4.0 nylon monofilament was inserted from the external carotid artery and advanced into the internal carotid artery until the tip occluded the proximal part of the middle cerebral artery (MCA). Ninety minutes after MCA occlusion (MCAO), reperfusion was achieved under anesthesia by withdrawal of the filament. Before MCAO, animals were randomly allocated to one of two treatment groups: (i) intravenous vehicle (saline) injected at 0 and 90 min after MCAO and (ii) intravenous edaravone treatment (3 mg/kg) at 0 and 90 min after MCAO. The dosage of edaravone was chosen based on previous papers [24, 41]. Consistent with STAIR recommendations [10], all the experiments were performed in a blinded fashion.
Plasma sample preparation
At 0, 1.5, 3, 6, and 24 hours after MCAO, blood was collected from the right femoral artery under anesthesia, and was mixed with EDTA. Then, plasma was separated by centrifuging blood at 3000 rpm for 15 minutes. Plasma samples were stored at -80°C for later zymography/ELISA/array analysis.
Measurement of Infarction and Brain swelling
Rats were sacrificed at 24 hours after ischemic onset under deep pentobarbital anesthesia. Then the brains were perfused with saline, and seven coronal sections (2 mm thick) were stained with 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO) to quantify infarct volumes. Brain swelling was estimated as the volumetric ratio of the ischemic side divided by the contra-lateral side.
Measurement of plasma MMP-9 and IL-1β
The level of MMP-9 in plasma was measured by gelatin zymography according to previously described techniques [26]. The level of IL-1β in plasma was measured by using the Quantikine Rat IL-1β (R&D Systems) according to the manufacture's instructions.
Rat Cytokine/Chemokine Array
Cytokine/Chemokine levels in plasma were measured by using the Proteome Profiler Rat Cytokine Array Panel A Array Kit (R&D Systems) according to the manufacture's instructions. Optical density of each signal was measured using Image-J software and calculated based on the positive controls.
Statistical Methods
Unpaired t-test was done to compare differences between the two groups. Differences with P < 0.05 were considered statistically significant. Data are expressed as mean ± SD. Animal numbers for each figure were described in figure legends.
3. Results
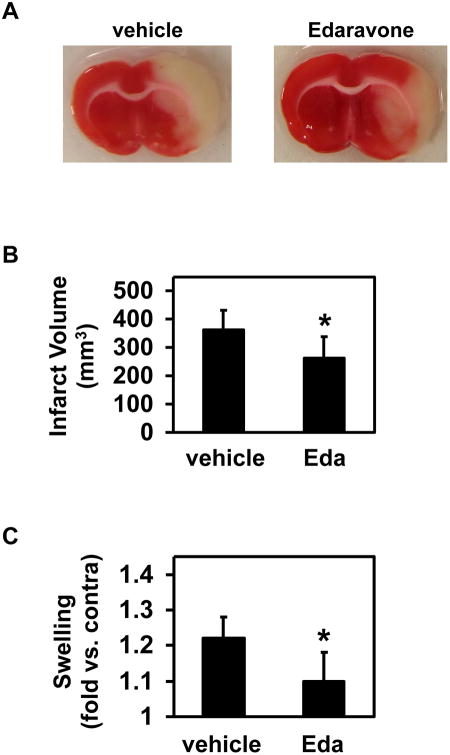
There were no large differences between vehicle- and edaravone-treated stroke animals in physiological parameters; body weight, rectal temperature, mean arterial blood pressure (MABP), heart rate, pO2, pCO2, and cerebral blood flow (CBF) (Supplementary Table). In both vehicle- and edaravone-treated groups, the physiological parameters remained within normal range (Supplementary Table). Using the rat stroke model, we first examined whether edaravone treatment reduced brain injury. Three mg/kg edaravone was injected twice through tail vein at 0 and 90 min after MCAO. Then, 24 hours later, cerebral infarct volume and brain swelling were measured. As previous papers reported [19, 29, 43], edaravone-treated rats showed less infarct volume and swelling (Figure 1).
Figure 1. Cerebral infarction volumes and Brain Swelling.
(A) Representative images of TTC-stained brain sections are shown. (B & C) Effects of edaravone on infarction volume and brain swelling at 24 hours after MCAO. Intravenous edaravone treatment (Eda) reduced both infarct volumes and brain swelling. Data expressed as mean ± SD. N=7 for Vehicle, and N=7 for Edaravone. *P<0.05.
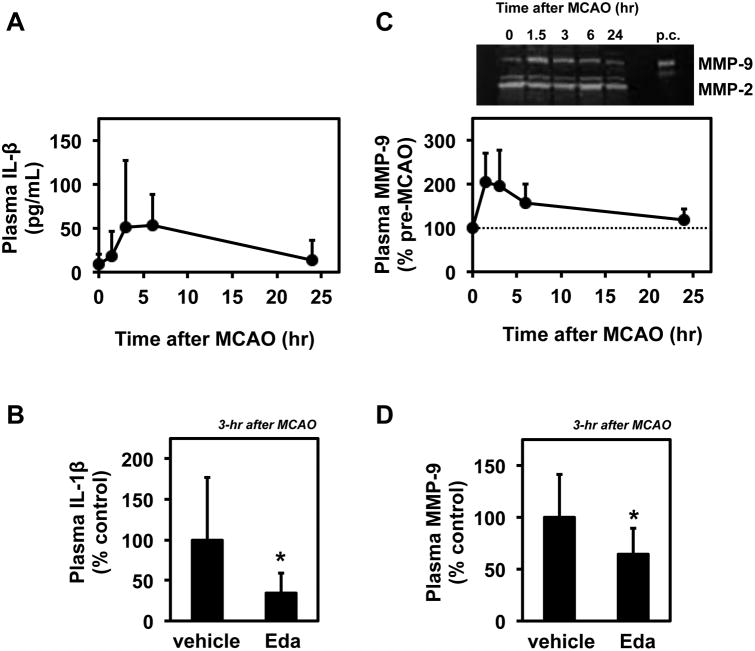
Several lines of experiments have shown that inflammatory cytokines, chemokines and proteases are upregulated under stroke conditions to worsen brain damage [21]. Although some molecular mechanisms of the protective effects of edaravone were elucidated [9, 18, 44, 45], its actions on the systemic inflammatory responses in animal models of stroke are not fully understood. To address this question, we measured levels of pro-inflammatory factors in rat plasma samples. Since interleukin-1β (IL-1β) and matrix metalloproteinase-9 (MMP-9) are associated with brain damage after stroke [21], these factors were examined as an initial experiment. We collected rat plasma samples at 0, 1.5, 3, 6, and 24 hours after MCAO. Data from IL-1β ELISA and MMP-9 gelatin zymography experiments showed that the levels of these factors were transiently increased (Figure 2A and 2C). Importantly, at the peak time point of 3 hours after MCAO (1.5-h MCAO + 1.5-h reperfusion), the edaravone-treated group showed lower levels in both IL-1β and MMP-9 compared with the control group (Figure 2B and 2D).
Figure 2. Effects of edaravone treatment on plasma IL-1β and MMP-9 levels.
(A) Time course of plasma IL-1β levels measured with IL-1β ELISA. Plasma samples were collected at 0, 1.5, 3, 6, and 24 hours after MCAO as described in Materials and Methods. Plasma IL-1β levels were transiently upregulated. Data expressed as mean ± SD. N=6 for each time point. (B) IL-1β ELISA showed that edaravone treatment (Eda) reduced plasma IL-1β levels at 3 hours after MCAO. Data expressed as mean ± SD. N=6 for Vehicle, and N=5 for Edaravone. *P<0.05. (C) Time course of plasma MMP-9 levels measured with gelatin zymography. Upper panel shows representative gelatin zymogram image. PC indicates positive controls loaded with MMP-2 and MMP-9 standards. Plasma samples were collected at 0, 1.5, 3, 6, and 24 hours after MCAO as described in Materials and Methods. Plasma MMP-9 levels were transiently upregulated. Data expressed as mean ± SD. N=6 for each time point. (D) Gelatin zymography showed that edaravone treatment (Eda) reduced plasma MMP-9 levels at 3 hours after MCAO. Data expressed as mean ± SD. N=6 for Vehicle, and N=5 for Edaravone. *P<0.05.
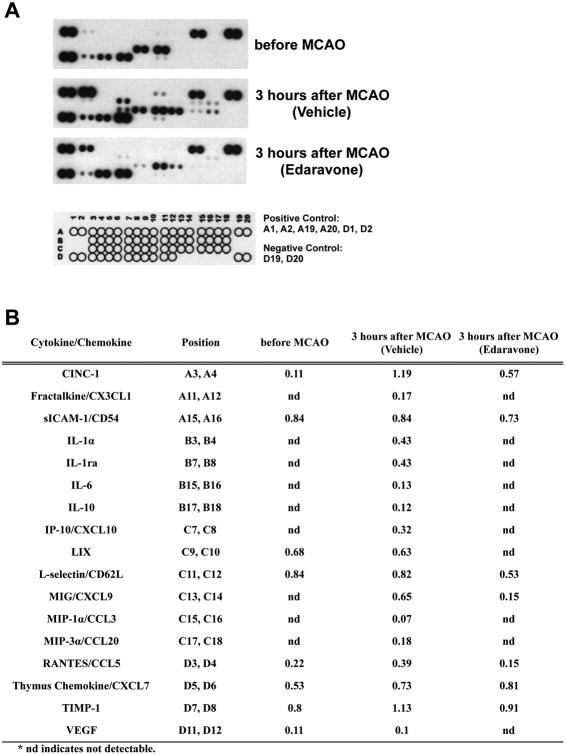
These initial experiments suggested that some pro-inflammatory factors could be upregulated under the acute phase of stroke, and treatment with edaravone may suppress inflammatory responses. Therefore, we tested rat plasma samples at 3 hours after MCAO with the Rat Cytokine Array Kit (Promega #ARY008), consisting of 29-plex antibody arrays (CINC-1, CINC-2α/β, CINC-3, CNTF, Fractalkine, GM-CSF, soluble intercellular adhesion molecule-1 (sICAM-1), IFN-γ, IL-1α, IL-1β, IL-1 receptor antagonist (IL-1ra), IL-2, IL-3, IL4, IL-6, IL-10, IL-13, IL-17, IP-10, C-X-C motif chemokine 5 (LIX), L-Selectin, MIG, MIP-1α, MIP-3α, RANTES, Thymus Chemokine, tissue inhibitor matrix metalloproteinase-1 (TIMP-1), TNF-α, and vascular endothelial growth factor (VEGF)). Under normal conditions (e.g. before MCAO), eight factors were detected by this kit; CINC-1, sICAM-1, LIX, L-Selectin, RANTES, Thymus Chemokine, TIMP-1, and VEGF (Figure 3). Among these factors, the CINC-1 level was upregulated more than 2-fold after MCAO, but was reduced by edaravone treatment (Figure 3B). Also, there were nine factors which were not detected under normal conditions but increased after MCAO; Fractalkine, IL-1α, IL-1ra, IL-6, IL-10, IP-10, MIG, MIP-1α, and MIP-3α (Figure 3). These upregulations were all blocked by edaravone treatment (Figure 3B), indicating that edaravone might play an important role in suppressing systemic inflammatory responses in the acute stroke phase. Twelve factors of 29 could not be detected by this rat cytokine protein array kit both in normal and MCAO conditions; CINC-2α/β, CINC-3, CNTF, GM-CSF, IFN-γ, IL-1β, IL-2, IL-3, IL-4, IL-13, IL-17, and TNF-α. A small caveat here is that we could detect plasma IL-1β with the IL-1β ELISA kit, but not with the array kit. The reason for this might be due to a difference in sensitivity for IL-1β detection between the ELISA and the chemiluminescent-based array systems.
Figure 3. A cytokine/chemokine array analysis in rat plasma.
(A) Images of chemiluminiscent intensity from the cytokine/chemokine array. Schematic image indicates the layout of cytokines and chemokines being measured. Three rat plasma samples were tested with the cytokine/chemokine array - before MCAO, 3 hours after MCAO (vehicle-treated), and 3 hours after MCAO (edaravone-treated). These samples were confirmed to contain average levels of IL-1β (tested by IL-1β ELISA) and MMP-9 (tested by gelatin zymography) before the protein array experiments. (B) Quantitative readouts from the array measurements. Optical density of each signal was measured by Image-J, and each value was calculated based on signals of the positive controls in the same membrane.
3. Discussion
In our current study, we demonstrate that a free radical scavenger edaravone reduced ischemia-induced cerebral infarction and swelling. Furthermore, in our rat stroke model, plasma levels of IL-1β and MMP-9 were transiently upregulated, and edaravone treatment significantly blocked these increases. Importantly, a protein array system demonstrated that at least ten more cytokines/chemokines were upregulated in plasma after stroke, and these changes were all suppressed by edaravone treatment. Since the systemic inflammatory response is one of the major deleterious cascades that occur in the acute stroke phase, our data suggest that oxidative stress could still be considered as a viable target for stroke therapy.
A major novelty of this study is that edaravone broadly suppressed systemic inflammatory responses under stroke conditions. A recent study by Yuan et al. [46] demonstrated anti-inflammatory effects of edaravone in stroke animals, focusing on inflammatory cytokines from activated microglia in the brain. Our current study may expand the previous finding from novel aspects. While the Yuan's study examined the inflammatory cytokines (IL-1β and TNF-α) in microglia in the brain at relatively late stage after brain injury (e.g. 3 and 7 days after MCAO), we focused on the inflammatory responses in plasma at the acute phase (3 hours after MCAO). Since there was no detectable infarction in the TTC staining at 3 hours after MCAO in our system (data not shown), the edaravone's systemic effects against inflammation after stroke in this study may not heavily depend on its brain-protective effects. Furthermore, with the usage of a protein array approach, we revealed that at least ten pro-inflammatory factors were upregulated in plasma after MCAO, which all were suppressed by edaravone treatment. Among those inflammatory factors, IL-1α and IL-6 are well-known cytokines that are related to brain inflammation under stroke [7, 21]. Also, Fractakline, MIP-1α, and MIP3-α showed deleterious effects through leukocyte infiltration under pathological conditions [20, 38]. In clinical studies, soluble intercellular adhesion molecule-1 (sICAM-1) levels were higher in acute stroke patients who died compared to those who survived [31]. Altogether, our findings indicate that the edaravone's systemic effects against inflammation after stroke might be one of the mechanisms of edaravone's protective effects for stroke patients.
Our current study shows that edaravone broadly suppressed systemic inflammatory responses under acute ischemic conditions. However, there are still some important caveats in our experimental design and data analysis. First, we targeted only one time point (i.e. 3 hours after MCAO) for the cytokine array system. To be more rigorous, edaravone's effects on inflammatory responses must be tested at several time points after stroke onset (e.g. 1.5, 6, 12, and 24 hours after MCAO). Second, some factors such as LIX, L-selectin, and VEGF were not robustly changed by ischemic stress at 3-hour after MCAO, but those factors were downregulated by edaravone. It might be possible that the levels of those factors would increase at the different time points after MCAO. Therefore, once again, we may need to examine the effects of edaravone on those factors at different time points to fully understand the anti-inflammatory effects of edaravone. Third, our experiments did not intend to examine the mechanisms of edaravone for inflammatory responses during ischemic conditions. While the anti-inflammatory properties of edaravone were implicated in this study, it would be important to examine how edaravone decreased the upregulation of pro-inflammatory cytokines/chemokines. Forth, edaravone suppressed the upregulation of IL-1ra and IL-10 in our study. Both IL-1ra and IL-10 are known to have anti-inflammatory properties by suppressing cytokine receptor expression/activation [12, 32, 36, 37]. While edaravone is protective for stroke patients and in the animal model, our study suggests that edaravone might also suppress the beneficial cascades as well. Therefore, future studies for screening drug candidates may need to evaluate the effects of compounds on the beneficial cascades as well for better efficacy. Lastly, our experiments are essentially an in vivo proof-of-concept study, which is sometimes difficult to translate into clinical application [11, 16]. Thus, clinical studies testing plasma samples from edaravone-treated stroke patients would be warranted.
In summary, we have demonstrated that the free radical scavenger edaravone is effective in our rat stroke model at least partly via its anti-inflammatory properties. Although the radical spin trap NXY-059 failed in Phase III clinical trial, our findings suggest that oxidative stress may be still a relevant target for acute stroke therapy.
Acknowledgments
Funding: This study was funded by National Institutes of Health (grant number NS037-74, NS055104, NS065089).
Footnotes
Conflict of Interest: All authors declare no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. And, this article does not contain any studies with human participants performed by any of the authors.
References
- 1.Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 2.Abe K, Yuki S, Kogure K. Strong attenuation of ischemic and postischemic brain edema in rats by a novel free radical scavenger. Stroke; a journal of cerebral circulation. 1988;19:480–485. doi: 10.1161/01.str.19.4.480. [DOI] [PubMed] [Google Scholar]
- 3.Ahmad A, Khan MM, Javed H, Raza SS, Ishrat T, Khan MB, Safhi MM, Islam F. Edaravone ameliorates oxidative stress associated cholinergic dysfunction and limits apoptotic response following focal cerebral ischemia in rat. Mol Cell Biochem. 2012;367:215–225. doi: 10.1007/s11010-012-1335-6. [DOI] [PubMed] [Google Scholar]
- 4.Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- 5.Amemiya S, Kamiya T, Nito C, Inaba T, Kato K, Ueda M, Shimazaki K, Katayama Y. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol. 2005;516:125–130. doi: 10.1016/j.ejphar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Becker KJ. Strain-Related Differences in the Immune Response: Relevance to Human Stroke. Transl Stroke Res. 2016;7:303–312. doi: 10.1007/s12975-016-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke; a journal of cerebral circulation. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Wang S, Ding JH, Hu G. Edaravone protects against MPP+ -induced cytotoxicity in rat primary cultured astrocytes via inhibition of mitochondrial apoptotic pathway. J Neurochem. 2008;106:2345–2352. doi: 10.1111/j.1471-4159.2008.05573.x. [DOI] [PubMed] [Google Scholar]
- 10.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke; a journal of cerebral circulation. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher M, Henninger N. Translational research in stroke: taking advances in the pathophysiology and treatment of stroke from the experimental setting to clinical trials. Current neurology and neuroscience reports. 2007;7:35–41. doi: 10.1007/s11910-007-0019-1. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. Journal of the neurological sciences. 2005;233:125–132. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 13.Fujiwara N, Murata Y, Arai K, Egi Y, Lu J, Wu O, Singhal A, Lo E. Combination therapy with normobaric oxygen (NBO) plus thrombolysis in experimental ischemic stroke. BMC neuroscience. 2009;10:79. doi: 10.1186/1471-2202-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg MD. Life after cerovive: a personal perspective on ischemic neuroprotection in the post-NXY-059 era. Stroke; a journal of cerebral circulation. 2007;38:1967–1972. doi: 10.1161/STROKEAHA.106.479170. [DOI] [PubMed] [Google Scholar]
- 15.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolkkonen J, Kwakkel G. Translational Hurdles in Stroke Recovery Studies. Transl Stroke Res. 2016;7:331–342. doi: 10.1007/s12975-016-0461-y. [DOI] [PubMed] [Google Scholar]
- 17.Kawai H, Nakai H, Suga M, Yuki S, Watanabe T, Saito KI. Effects of a novel free radical scavenger, MCl-186, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J Pharmacol Exp Ther. 1997;281:921–927. [PubMed] [Google Scholar]
- 18.Kawasaki T, Kitao T, Nakagawa K, Fujisaki H, Takegawa Y, Koda K, Ago Y, Baba A, Matsuda T. Nitric oxide-induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger. Glia. 2007;55:1325–1333. doi: 10.1002/glia.20541. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi K, Tancharoen S, Matsuda F, Biswas KK, Ito T, Morimoto Y, Oyama Y, Takenouchi K, Miura N, Arimura N, Nawa Y, Meng X, Shrestha B, Arimura S, Iwata M, Mera K, Sameshima H, Ohno Y, Maenosono R, Tajima Y, Uchikado H, Kuramoto T, Nakayama K, Shigemori M, Yoshida Y, Hashiguchi T, Maruyama I, Kawahara K. Edaravone attenuates cerebral ischemic injury by suppressing aquaporin-4. Biochemical and biophysical research communications. 2009;390:1121–1125. doi: 10.1016/j.bbrc.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KM. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. Journal of neuroimmunology. 1995;56:127–134. doi: 10.1016/0165-5728(94)00138-e. [DOI] [PubMed] [Google Scholar]
- 21.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. Journal of translational medicine. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BJ, Egi Y, van Leyen K, Lo EH, Arai K. Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain research. 1307:22–27. doi: 10.1016/j.brainres.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, Mandeville JB, Kim KW, Lo EH, Arai K. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morihara R, Kono S, Sato K, Hishikawa N, Ohta Y, Yamashita T, Deguchi K, Manabe Y, Takao Y, Kashihara K, Inoue S, Kiriyama H, Abe K. Thrombolysis with Low-Dose Tissue Plasminogen Activator 3-4.5 h After Acute Ischemic Stroke in Five Hospital Groups in Japan. Transl Stroke Res. 2016;7:111–119. doi: 10.1007/s12975-016-0448-8. [DOI] [PubMed] [Google Scholar]
- 26.Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH. Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke; a journal of cerebral circulation. 2008;39:3372–3377. doi: 10.1161/STROKEAHA.108.514026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niizuma K, Endo H, Chan PH. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. Journal of neurochemistry. 2009;109(1):133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishi H, Watanabe T, Sakurai H, Yuki S, Ishibashi A. Effect of MCI-186 on brain edema in rats. Stroke; a journal of cerebral circulation. 1989;20:1236–1240. doi: 10.1161/01.str.20.9.1236. [DOI] [PubMed] [Google Scholar]
- 29.Niyaz M, Numakawa T, Matsuki Y, Kumamaru E, Adachi N, Kitazawa H, Kunugi H, Kudo M. MCI-186 prevents brain tissue from neuronal damage in cerebral infarction through the activation of intracellular signaling. Journal of neuroscience research. 2007;85:2933–2942. doi: 10.1002/jnr.21412. [DOI] [PubMed] [Google Scholar]
- 30.Proctor PH, Tamborello LP. SAINT-I worked, but the neuroprotectant is not NXY-059. Stroke. 2007;38:e109. doi: 10.1161/STROKEAHA.107.489161. author reply e110. [DOI] [PubMed] [Google Scholar]
- 31.Rallidis LS, Zolindaki MG, Vikelis M, Kaliva K, Papadopoulos C, Kremastinos DT. Elevated soluble intercellular adhesion molecule-1 levels are associated with poor short-term prognosis in middle-aged patients with acute ischaemic stroke. International journal of cardiology. 2009;132:216–220. doi: 10.1016/j.ijcard.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 32.Relton JK, Martin D, Thompson RC, Russell DA. Peripheral administration of Interleukin-1 Receptor antagonist inhibits brain damage after focal cerebral ischemia in the rat. Experimental neurology. 1996;138:206–213. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- 33.Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Annals of neurology. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- 34.Shi H, Liu KJ. Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front Biosci. 2007;12:1318–1328. doi: 10.2741/2150. [DOI] [PubMed] [Google Scholar]
- 35.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T, Wasiewski WW, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. The New England journal of medicine. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 36.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neuroscience letters. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 37.Stroemer RP, Rothwell NJ. Cortical protection by localized striatal injection of IL-1ra following cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:597–604. doi: 10.1097/00004647-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Terao Y, Ohta H, Oda A, Nakagaito Y, Kiyota Y, Shintani Y. Macrophage inflammatory protein-3alpha plays a key role in the inflammatory cascade in rat focal cerebral ischemia. Neuroscience research. 2009;64:75–82. doi: 10.1016/j.neures.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Toyoda K, Fujii K, Kamouchi M, Nakane H, Arihiro S, Okada Y, Ibayashi S, Iida M. Free radical scavenger, edaravone, in stroke with internal carotid artery occlusion. J Neurol Sci. 2004;221:11–17. doi: 10.1016/j.jns.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597–1604. [PubMed] [Google Scholar]
- 41.Yagi K, Kitazato KT, Uno M, Tada Y, Kinouchi T, Shimada K, Nagahiro S. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40:626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito KI, Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–242. doi: 10.1016/s0006-8993(97)00490-3. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto Y, Yanagisawa M, Tak NW, Watanabe K, Takahashi C, Fujisawa A, Kashiba M, Tanaka M. Repeated edaravone treatment reduces oxidative cell damage in rat brain induced by middle cerebral artery occlusion. Redox Rep. 2009;14:251–258. doi: 10.1179/135100009X12525712409779. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12:9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan WJ, Yasuhara T, Shingo T, Muraoka K, Agari T, Kameda M, Uozumi T, Tajiri N, Morimoto T, Jing M, Baba T, Wang F, Leung H, Matsui T, Miyoshi Y, Date I. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008;9:75. doi: 10.1186/1471-2202-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan Y, Zha H, Rangarajan P, Ling EA, Wu C. Anti-inflammatory effects of Edaravone and Scutellarin in activated microglia in experimentally induced ischemia injury in rats and in BV-2 microglia. BMC neuroscience. 2014;15:125. doi: 10.1186/s12868-014-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang P, Li W, Li L, Wang N, Li X, Gao M, Zheng J, Lei S, Chen X, Lu H, Liu Y. Treatment with edaravone attenuates ischemic brain injury and inhibits neurogenesis in the subventricular zone of adult rats after focal cerebral ischemia and reperfusion injury. Neuroscience. 2012;201:297–306. doi: 10.1016/j.neuroscience.2011.11.005. [DOI] [PubMed] [Google Scholar]