Abstract
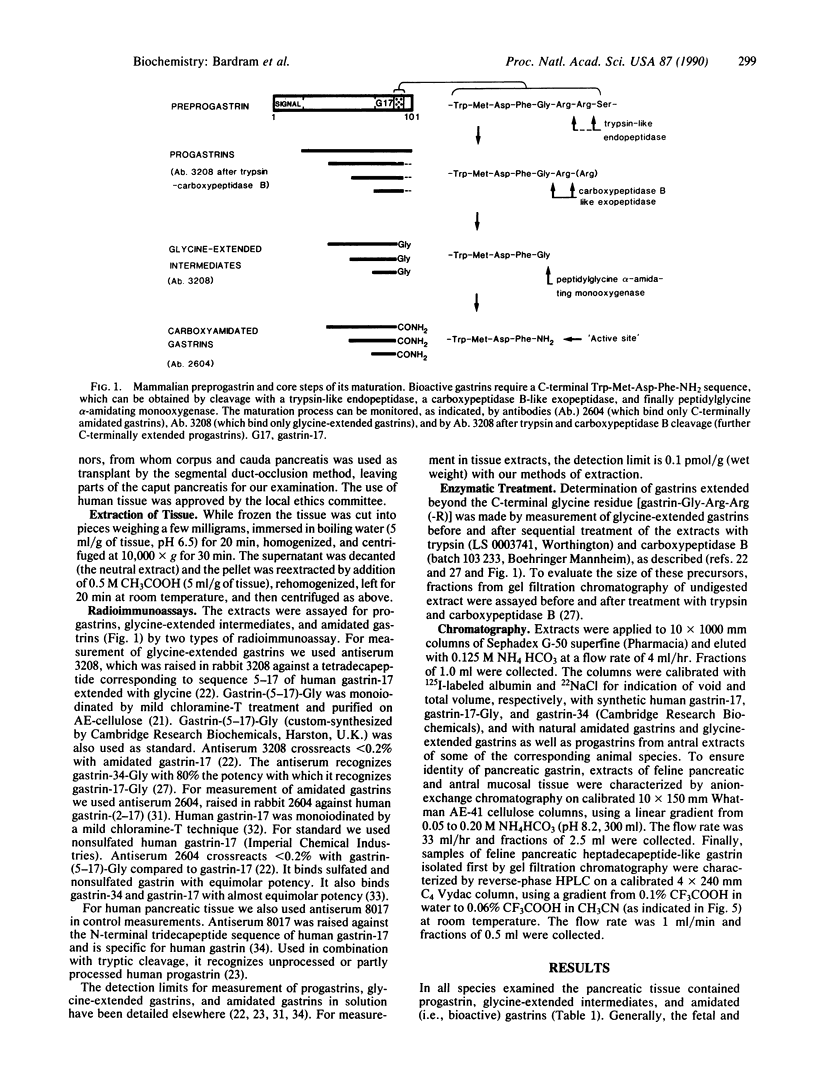
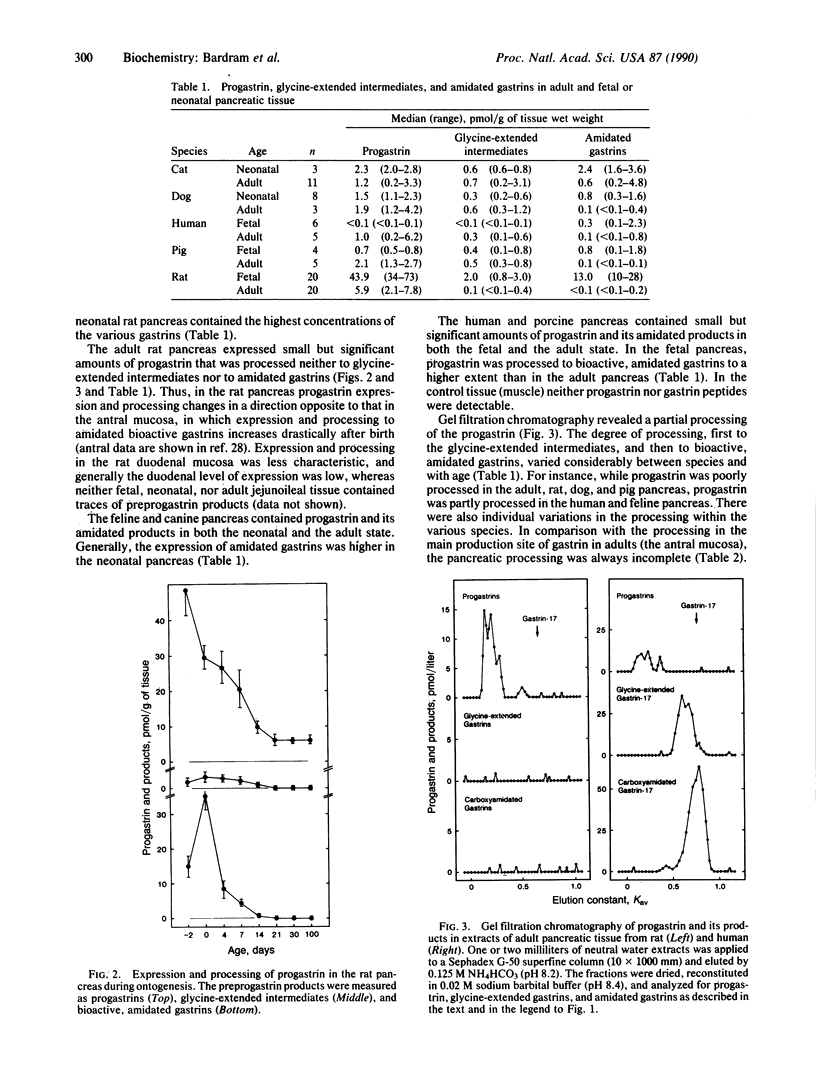
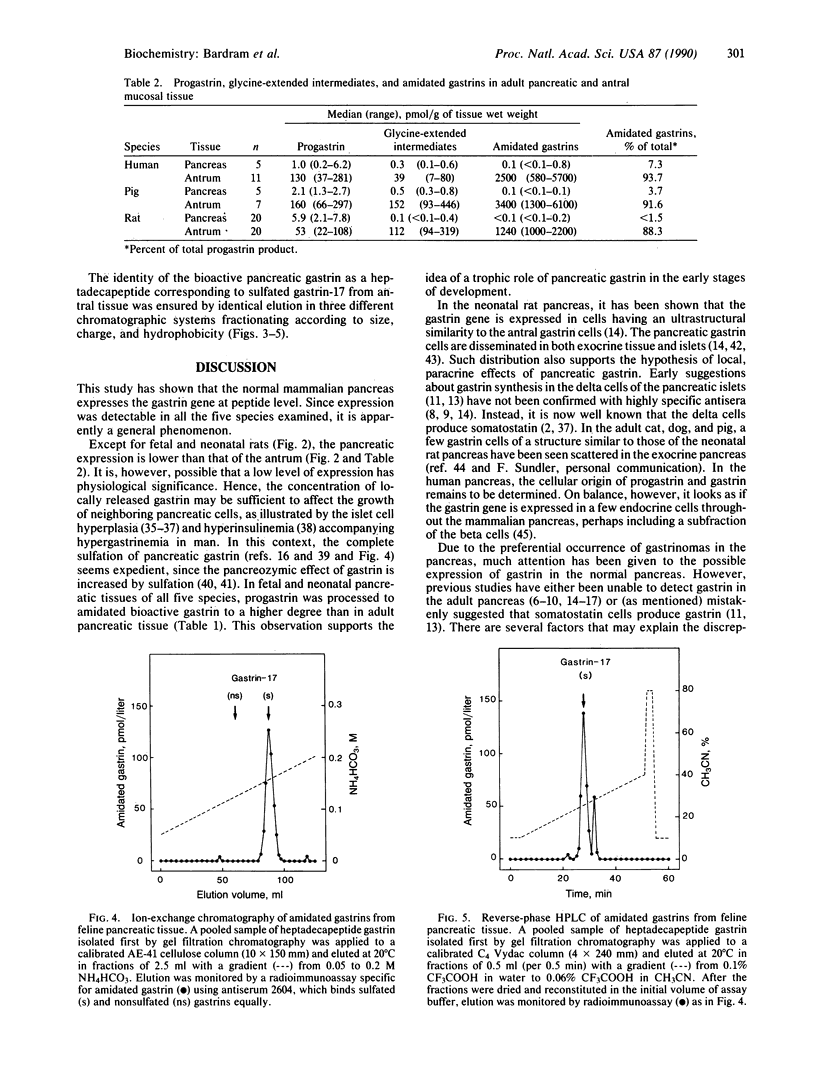
Expression and processing of progastrin were examined in fetal, neonatal, and adult pancreatic tissue from five mammalian species (cat, dog, man, pig, and rat). A library of sensitive, sequence-specific immunoassays for progastrin and its products was used to monitor extractions and chromatography before and after cleavage with processing-like enzymes. The results showed that progastrin and its products are expressed in the pancreas of all species in total concentrations varying from 0.3 to 58.9 pmol/g of tissue (medians). The degree of processing was age- and species-dependent. In comparison with adult pancreatic tissue the fetal or neonatal pancreas processed a higher fraction to bioactive, C-terminally amidated gastrin. Nevertheless, the pancreatic processing was always less complete than that of the adult antral mucosa. The moderate level of expression and the attenuated processing in the adult pancreas contribute to explain previous failures to detect gastrin in normal pancreatic tissue. Our results indicate that gastrin-producing tumors in the pancreas are not ectopic, but arise from cells that normally express the gastrin gene.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. N., Abramovich D., Brand S. J., Petersen B., Rehfeld J. F. Complete sulfation of jejunal gastrin in the human fetus. Regul Pept. 1985 Apr;10(4):329–338. doi: 10.1016/0167-0115(85)90045-x. [DOI] [PubMed] [Google Scholar]
- Bardram L., Rehfeld J. F. Processing-independent radioimmunoanalysis: a general analytical principle applied to progastrin and its products. Anal Biochem. 1988 Dec;175(2):537–543. doi: 10.1016/0003-2697(88)90580-5. [DOI] [PubMed] [Google Scholar]
- Bardram L., Rehfeld J. F. Production and evaluation of monospecific antibodies for a processing-independent sequence of human progastrin. Scand J Clin Lab Invest. 1989 Apr;49(2):173–182. doi: 10.3109/00365518909105418. [DOI] [PubMed] [Google Scholar]
- Boel E., Vuust J., Norris F., Norris K., Wind A., Rehfeld J. F., Marcker K. A. Molecular cloning of human gastrin cDNA: evidence for evolution of gastrin by gene duplication. Proc Natl Acad Sci U S A. 1983 May;80(10):2866–2869. doi: 10.1073/pnas.80.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S. J., Andersen B. N., Rehfeld J. F. Complete tyrosine-O-sulphation of gastrin in neonatal rat pancreas. 1984 May 31-Jun 6Nature. 309(5967):456–458. doi: 10.1038/309456a0. [DOI] [PubMed] [Google Scholar]
- Brand S. J., Fuller P. J. Differential gastrin gene expression in rat gastrointestinal tract and pancreas during neonatal development. J Biol Chem. 1988 Apr 15;263(11):5341–5347. [PubMed] [Google Scholar]
- Brand S. J., Wang T. C. Gastrin gene expression and regulation in rat islet cell lines. J Biol Chem. 1988 Nov 15;263(32):16597–16603. [PubMed] [Google Scholar]
- Cantor P., Andersen B. N., Rehfeld J. F. Complete tyrosine O-sulfation of gastrin in adult and neonatal cat pancreas. FEBS Lett. 1986 Jan 20;195(1-2):272–274. doi: 10.1016/0014-5793(86)80175-2. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Arnold R., Creutzfeldt C., Feurle G., Ketterer H. Gastrin and G-cells in the antral mucosa of patients with pernicious anaemia, acromegaly and hyperparathyroidism and in a Zollinger-Ellison tumour of the pancreas. Eur J Clin Invest. 1971 Sep;1(6):461–479. doi: 10.1111/j.1365-2362.1971.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W., Arnold R., Creutzfeldt C., Track N. S. Pathomorphologic, biochemical, and diagnostic aspects of gastrinomas (Zollinger-Ellison syndrome). Hum Pathol. 1975 Jan;6(1):47–76. doi: 10.1016/s0046-8177(75)80109-2. [DOI] [PubMed] [Google Scholar]
- Del Valle J., Sugano K., Yamada T. Progastrin and its glycine-extended posttranslational processing intermediates in human gastrointestinal tissues. Gastroenterology. 1987 Jun;92(6):1908–1912. doi: 10.1016/0016-5085(87)90623-8. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. The action of gastrin and cholecystokinin-related peptides on pancreatic secretion in the rat. Q J Exp Physiol Cogn Med Sci. 1973 Apr;58(2):163–169. [PubMed] [Google Scholar]
- Erlandsen S. L., Hegre O. D., Parsons J. A., McEvoy R. C., Elde R. P. Pancreatic islet cell hormones distribution of cell types in the islet and evidence for the presence of somatostatin and gastrin within the D cell. J Histochem Cytochem. 1976 Jul;24(7):883–897. doi: 10.1177/24.7.60437. [DOI] [PubMed] [Google Scholar]
- Fujii S. Development of pancreatic endocrine cells in the rat fetus. Arch Histol Jpn. 1979 Oct;42(4):467–479. doi: 10.1679/aohc1950.42.467. [DOI] [PubMed] [Google Scholar]
- Fuller P. J., Stone D. L., Brand S. J. Molecular cloning and sequencing of a rat preprogastrin complementary deoxyribonucleic acid. Mol Endocrinol. 1987 Apr;1(4):306–311. doi: 10.1210/mend-1-4-306. [DOI] [PubMed] [Google Scholar]
- Greider M. H., McGuigan J. E. Cellular localization of gastrin in the human pancreas. Diabetes. 1971 Jun;20(6):387–396. [PubMed] [Google Scholar]
- HALLENBECK G. A., CODE C. F., McILRATH C. Absence of demonstrable gastric secretagogue in normal pancreatic tissue. Gastroenterology. 1963 May;44:627–630. [PubMed] [Google Scholar]
- Happé R. P., van der Gaag I., Lamers C. B., van Toorenburg J., Rehfeld J. F., Larsson L. I. Zollinger-Ellison syndrome in three dogs. Vet Pathol. 1980 Mar;17(2):177–186. doi: 10.1177/030098588001700206. [DOI] [PubMed] [Google Scholar]
- Hilsted L., Bardram L., Rehfeld J. F. Progastrin maturation during ontogenesis. Accumulation of glycine-extended gastrins in rat antrum at weaning. Biochem J. 1988 Oct 15;255(2):397–402. doi: 10.1042/bj2550397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F. Alpha-carboxyamidation of antral progastrin. Relation to other post-translational modifications. J Biol Chem. 1987 Dec 15;262(35):16953–16957. [PubMed] [Google Scholar]
- Hilsted L., Rehfeld J. F. Measurement of precursors for alpha-amidated hormones by radioimmunoassay of glycine-extended peptides after trypsin-carboxypeptidase B cleavage. Anal Biochem. 1986 Jan;152(1):119–126. doi: 10.1016/0003-2697(86)90129-6. [DOI] [PubMed] [Google Scholar]
- Jacobsen O., Bardram L., Rehfeld J. F. The requirement for gastrin measurements. Scand J Clin Lab Invest. 1986 Sep;46(5):423–426. doi: 10.3109/00365518609083693. [DOI] [PubMed] [Google Scholar]
- Jensen S. L., Rehfeld J. F., Holst J. J., Fahrenkrug J., Nielsen O. V., Schaffalitzky de Muckadell O. B. Secretory effects of gastrins on isolated perfused porcine pancreas. Am J Physiol. 1980 Feb;238(2):E186–E192. doi: 10.1152/ajpendo.1980.238.2.E186. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Ljungberg O., Sundler F., Håkanson R., Svensson S. O., Rehfeld J., Stadil R., Holst J. Antor-pyloric gastrinoma associated with pancreatic nesidioblastosis and proliferation of islets. Virchows Arch A Pathol Pathol Anat. 1973;360(4):305–314. doi: 10.1007/BF00548351. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Rehfeld J. F., Sundler F., Håkanson R. Pancreatic gastrin in foetal and neonatal rats. Nature. 1976 Aug 12;262(5569):609–610. doi: 10.1038/262609a0. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Two distinct types of islet abnormalities associated with endocrine pancreatic tumours. Virchows Arch A Pathol Anat Histol. 1977 Nov 25;376(3):209–219. doi: 10.1007/BF00432397. [DOI] [PubMed] [Google Scholar]
- Lomský R., Langer F., Vortel V. Immunohistochemical demonstration of gastrin in mammalian islets of Langerhans. Nature. 1969 Aug 9;223(5206):618–619. doi: 10.1038/223618a0. [DOI] [PubMed] [Google Scholar]
- Lotstra F., van der Loo W., Gepts W. Are gastrin-cells present in mammalian pancreatic islets? Diabetologia. 1974 Aug;10(4):291–302. doi: 10.1007/BF02627730. [DOI] [PubMed] [Google Scholar]
- Majumdar A. P., Rehfeld J. F. Effect of hydrocortisone on gastrin cell function in various tissues of suckling rats. Digestion. 1983;27(3):165–173. doi: 10.1159/000198947. [DOI] [PubMed] [Google Scholar]
- Onolfo J. P., Lehy T. Comparative development of gastrin and somatostatin cell populations in the pancreas, stomach, and duodenum of the rat during the perinatal period. Anat Rec. 1987 Aug;218(4):416–425. doi: 10.1002/ar.1092180409. [DOI] [PubMed] [Google Scholar]
- Pauwels S., Desmond H., Dimaline R., Dockray G. J. Identification of progastrin in gastrinomas, antrum, and duodenum by a novel radioimmunoassay. J Clin Invest. 1986 Feb;77(2):376–381. doi: 10.1172/JCI112315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C. T., Ney C., Aran P., Agarwal K. A gastrin gene is expressed in both porcine pituitary and antral mucosal tissues. Nucleic Acids Res. 1985 Oct 25;13(20):7299–7305. doi: 10.1093/nar/13.20.7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F. Accumulation of nonamidated preprogastrin and preprocholecystokinin products in porcine pituitary corticotrophs. Evidence of post-translational control of cell differentiation. J Biol Chem. 1986 May 5;261(13):5841–5847. [PubMed] [Google Scholar]
- Rehfeld J. F. Disturbed islet-cell function related to endogenous gastrin release. Studies on insulin secretion and glucose tolerance in pernicious anemia. J Clin Invest. 1976 Jul;58(1):41–49. doi: 10.1172/JCI108457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J. F., Larsson L. I., Goltermann N. R., Schwartz T. W., Holst J. J., Jensen S. L., Morley J. S. Neural regulation of pancreatic hormone secretion by the C-terminal tetrapeptide of CCK. Nature. 1980 Mar 6;284(5751):33–38. doi: 10.1038/284033a0. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Lindholm J., Andersen B. N., Bardram L., Cantor P., Fenger M., Lüdecke D. K. Pituitary tumors containing cholecystokinin. N Engl J Med. 1987 May 14;316(20):1244–1247. doi: 10.1056/NEJM198705143162004. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Stadil F., Rubin B. Production and evaluation of antibodies for the radioimmunoassay of gastrin. Scand J Clin Lab Invest. 1972 Oct;30(2):221–232. doi: 10.3109/00365517209081114. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. The expression of progastrin, procholecystokinin and their hormonal products in pituitary cells. J Mol Endocrinol. 1988 Sep;1(2):87–94. doi: 10.1677/jme.0.0010087. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., de Magistris L., Andersen B. N. Sulfation of gastrin: effect on immunoreactivity. Regul Pept. 1981 Sep;2(5):333–342. doi: 10.1016/0167-0115(81)90037-9. [DOI] [PubMed] [Google Scholar]
- Stadil F., Rehfeld J. F. Preparation of 125 I-labelled synthetic human gastrin I for radioimmunoanalysis. Scand J Clin Lab Invest. 1972 Dec;30(4):361–368. doi: 10.3109/00365517209080271. [DOI] [PubMed] [Google Scholar]
- Straus E., Johnson G. F., Yalow R. S. Canine Zollinger-Ellison syndrome. Gastroenterology. 1977 Feb;72(2):380–381. [PubMed] [Google Scholar]
- Sugano K., Aponte G. W., Yamada T. Identification and characterization of glycine-extended post-translational processing intermediates of progastrin in porcine stomach. J Biol Chem. 1985 Sep 25;260(21):11724–11729. [PubMed] [Google Scholar]
- Track N. S., Creutzfeldt C., Litzenberger J., Neuhoff C., Arnold R., Creutzfeldt W. Appearance of gastrin and somatostatin in the human fetal stomach, duodenum and pancreas. Digestion. 1979;19(5):292–306. doi: 10.1159/000198374. [DOI] [PubMed] [Google Scholar]
- Yoo O. J., Powell C. T., Agarwal K. L. Molecular cloning and nucleotide sequence of full-length of cDNA coding for porcine gastrin. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1049–1053. doi: 10.1073/pnas.79.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]



