Abstract
Background
In general, Ras proteins are thought to promote cardiac hypertrophy, an important risk factor for cardiovascular disease and heart failure. However, the contribution of different Ras isoforms has not been investigated. The objective of this study was to define the role of H- and K-Ras in modulating stress-induced myocardial hypertrophy and failure.
Methods and Results
We utilized H- and K-Ras gene knockout mice and subjected them to pressure overload to induce cardiac hypertrophy and dysfunction. We observed a worsened cardiac phenotype in Hras−/− mice while outcomes were improved in Kras+/− mice. We also used a neonatal rat cardiomyocyte culture system to elucidate the mechanisms underlying these observations. Our findings demonstrate that H-Ras, but not K-Ras, promotes cardiomyocyte hypertrophy both in vivo and in vitro. This response was mediated in part through the PI3K-AKT signaling pathway. Adeno-associated virus-mediated increase in AKT activation improved the cardiac function in pressure overloaded Hras null mice in vivo. These findings further support engagement of the PI3K-AKT signaling axis by H-Ras.
Conclusions
Taken together, these findings indicate that H- and K-Ras have divergent effects on cardiac hypertrophy and heart failure in response to pressure overload stress.
Keywords: hypertension, hypertrophy, heart failure, cardiomyocytes, Ras proteins
Journal Subject Terms: Hemodynamics, Cell Signaling/Signal Transduction, Heart Failure
The Ras family of small GTPases consists of ubiquitously expressed signal transducers that relay extracellular cues inside the cell, thereby regulating a host of signaling pathways and cellular responses1. Activation of Ras proteins is modulated through engagement of transmembrane receptors, both receptor tyrosine kinases (RTKs) and G-protein coupled receptors (GPCRs), and through mechanical forces, i.e. cell stretch/strain. These signals lead to guanine nucleotide exchange factor (GEF) activation and Ras GTP-loading. GTP-bound Ras is active and able to bind to and signal through downstream effectors, the most established being Raf2–5, PI3K6, RalGDS7–9, PLCε10 and Tiam111. Signal pathways elicited via Ras can modulate a variety of responses including gene expression, growth, survival, proliferation, endocytosis and cell motility.
Ras proteins are highly relevant to human disease and myriad studies have demonstrated Ras mutations in many types of cancers12. Human germline mutations in Ras proteins, and known modulators of Ras signaling pathways, have been linked to the developmental disorders Neurofibromatosis type 1, cardio-facio-cutaneous13, 14, Noonan15–17, Costello18 and LEOPARD syndromes. Collectively referred to as RASopathies, these genetic disorders share dysregulation of Ras/MAPK signaling and phenotypic overlap including craniofacial abnormalities, cardiac malformations, impaired cognitive ability and increased cancer risk. Specifically, these patients present with cardiovascular abnormalities including hypertrophic cardiomyopathy, atrial-septal defects, pulmonic stenosis and tachycardia. While the nature of these cardiac defects is heterogeneous and the cause of these differences remains unclear, these syndromes provide strong evidence of the importance of Ras proteins in human myocardial pathophysiology.
To date, studies investigating the role of Ras signaling in a cardiac context have almost exclusively focused on the H-Ras isoform. Early work demonstrated that agonists and interventions that promote cardiomyocyte hypertrophy, characterized by increased cell size and activation of embryonic gene expression, also elicit activation of Ras19, 20. This growth response was demonstrated to require Ras activity, and later, the expression of activated H-Ras was shown to be sufficient to promote cardiomyocyte hypertrophy19, 21, 22. Myocardial expression of activated H-Ras12V driven by the myosin light chain 2v (MLC2v) promoter was shown to cause increased left ventricular mass, hypertrophic gene expression and functional decompensation in transgenic animals23, further supporting the hypothesis that H-Ras promotes cardiomyocyte hypertrophy, as well as suggesting a maladaptive response to increased cardiac H-Ras activity. Additional studies suggested that this response was reversible and may involve altered SERCA2a function and calcium handling 24.
The objective of the current study was to determine the role of endogenous H- and K-Ras in pressure overload-induced cardiac hypertrophy and heart failure. We employed genetic loss-of-function mouse models and cultured neonatal rat cardiomyocytes (NRCMs) to interrogate each isoform in vivo and in vitro. Our findings indicate that H-Ras promotes cardiomyocyte hypertrophy and is cardioprotective during chronic pressure overload, whereas K-Ras does not promote growth and serves a deleterious function. We observed markedly less phosphorylated AKT and ERK in pressure overloaded Hras null hearts. Inhibition of AKT activation attenuated H-Ras-induced cardiomyocyte hypertrophy in vitro, and restoration of AKT signaling in vivo was able to rescue cardiac function in pressure overloaded Hras null hearts. These results suggest that endogenous H-Ras mediates hemodynamic stress-induced cardiac hypertrophy and affords a cardioprotective function in the murine heart in part through activation of AKT.
METHODS
An expanded Methods section is available in the Data Supplement.
Animals
Kras+/− and Hras+/− mice have been described previously 25, 26. All protocols concerning the use of animals were approved by the Institutional Animal Care and Use Committee at Rutgers, New Jersey Medical School.
Transverse aortic constriction
Mice were anesthetized and pressure overload induced by ligation of the transverse thoracic aorta. Sham operation was performed without aortic constriction.
Histology
Hearts were analyzed for interstitial fibrosis using Masson’s Trichrome, myocyte cross-sectional area using wheat germ agglutinin, and apoptosis by TUNEL.
RBD Pulldown Assay
Homogenates were incubated with Raf-1 RBD agarose (Upstate, Millipore) for 40 minutes at 4°C to precipitate GTP-bound Ras according to manufacturer’s instructions.
Statistical Analysis
All data are reported as mean ± standard error of the mean. Student t test was used to evaluate the difference in means between two groups. Multiple groups were analyzed using Levene’s test to determine heterogeneity of variances, followed by Welch’s ANOVA. Post hoc multiple comparisons were performed using Tukey’s test. Statistical analyses were performed using SPSS v24 and Graph Pad Prism 6.0. A p value less than 0.05 was considered significant.
Results
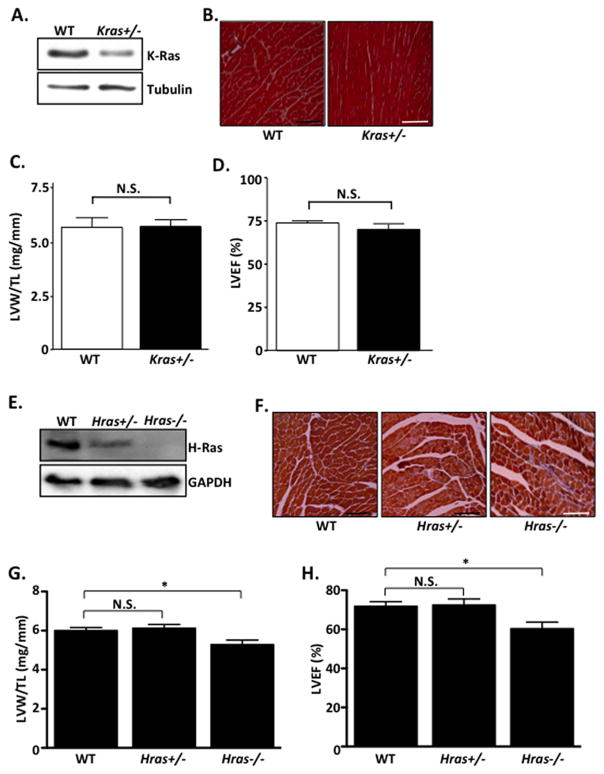
To determine the role of endogenous Ras isoforms on basal cardiac structure and function, we utilized H- and K-Ras loss-of-function mouse lines. Homozygous deletion of Kras is embryonic lethal25; therefore, we used Kras+/− mice for this study. Kras+/− mice are viable and showed no obvious cardiac abnormalities in heart size, structure and function compared to WT controls at baseline (10–12 weeks)(Figure 1A–D). Interestingly, homozygous disruption of Hras is tolerated and mice are born at expected Mendelian ratios26. However, by 10–12 weeks of age, Hras−/− mice developed a mild but significant reduction in cardiac function, as determined by echocardiographic analysis (%LVEF) (Figure 1E–H). Hras−/− mice also had a modest but significant reduction in LV mass compared to WT controls. In contrast, Hras+/− mice showed no difference in cardiac size or function compared to WT mice at baseline (Figure 1E–H).
Figure 1. Basal cardiac characterization of K- and H-Ras mutant mice.
A–H. All mice were between 10–12 weeks old. A. Ventricular homogenates from WT and Kras+/− mice were subjected to western blot. B. Heart sections were stained with Masson’s Trichrome to visualize interstitial fibrosis. C. Post-mortem gravimetric analysis of left ventricular weight/tibia length (LVW/TL) was determined. D. Prior to sacrifice, echocardiographic analysis was performed and left ventricular ejection fraction (%LVEF) determined. N=5 per group. E. Ventricular homogenates from WT, Hras+/− and Hras−/− mice were subjected to western blot. F. Heart sections were stained with Masson’s Trichrome to visualize interstitial fibrosis. G. Post-mortem gravimetric analysis of left ventricular weight/tibia length (LVW/TL) was determined. H. Prior to sacrifice, echocardiographic analysis was performed and left ventricular ejection fraction (%LVEF) determined. N=6–10 per group. Data are mean ± SEM. *,P<0.05. N.S.=not significant.
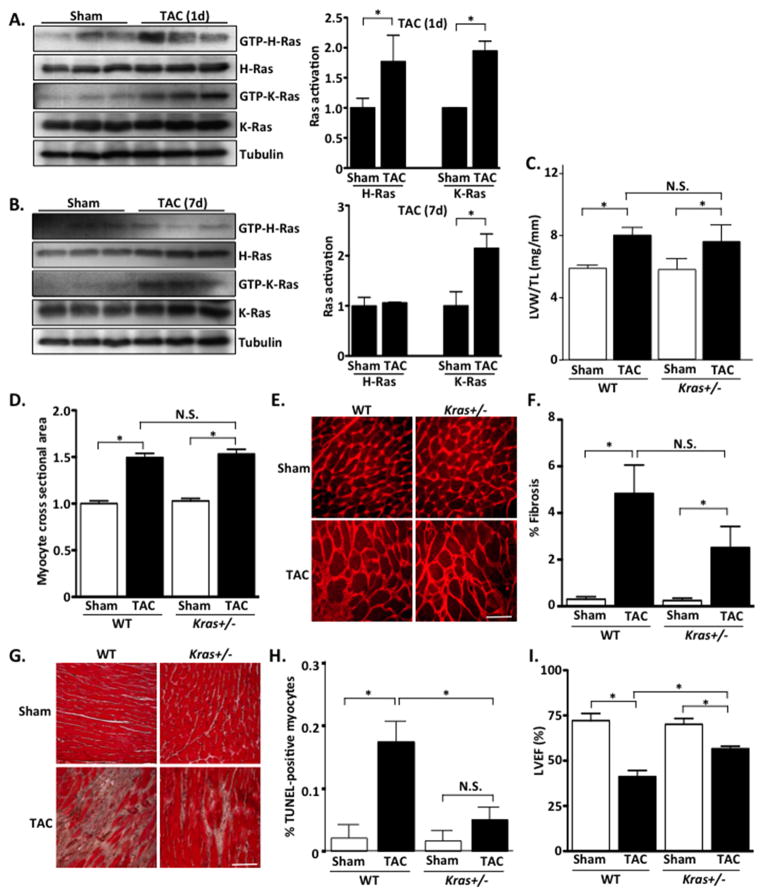
Mechanical stretch is known to activate Ras proteins in cardiomyocytes20. We therefore subjected WT mice to 1 and 7 days of pressure overload by transverse aortic constriction (TAC) and determined H- and K-Ras activation in the myocardium. We found that both isoforms were activated at 1-day post-TAC; however, only K-Ras activation was increased at 7 days post-TAC (Figure 2A and B). To determine the role of these Ras isoforms in mediating pressure overload-induced cardiac hypertrophy and dysfunction, H- and K-Ras mutant mice were subjected to 4-week TAC. There was no significant difference in pressure gradients achieved in all TAC experiments (Supplemental Figure 1). In response to pressure overload, Kras+/− mice showed a similar increase in cardiac hypertrophy, as determined by LVW/TL, cardiomyocyte cross-sectional area (CSA), and fetal gene expression, compared to WT littermates (Figure 2C–E and Supplemental Figure 2). However, we observed a trend toward reduced myocardial fibrosis and a significant reduction in TUNEL-positive cells in Kras+/− hearts following TAC compared to WT (Figure 2F–H). Importantly, %LVEF was significantly greater in Kras+/− mice compared to WT following 4 weeks of TAC (Figure 2I and Table 1). Furthermore, in Kras+/− mice, LVEDD was significantly smaller compared to WT controls after TAC (Table 1) indicating attenuated chamber dilatation and improved systolic function. Taken together these results indicate that reduced K-Ras expression does not alter the hypertrophic capability of the mouse heart but does attenuate cardiac maladaptation to pressure overload stress.
Figure 2. Inhibition of endogenous K-Ras does not alter hypertrophy but protects against pressure-overload-induced cardiac dysfunction.
A and B. WT mice were subjected to TAC or sham operation for 1 or 7 days. Ventricular homogenates were subjected to RBD-pull down assay to determine activation of H- and K-Ras. Quantification of blots is shown in right panels. C. Left ventricular weight/tibia length (LVW/TL) was determined in TAC and sham operated groups. D. Cardiomyocyte cross-sectional area was determined by wheat germ agglutinin (WGA) staining. E. Representative WGA images. F. Fibrosis was determined by Masson’s Trichrome staining. G. Representative images shown. Scale bar, 100 μm. H. Extent of apoptosis was determined by TUNEL staining. I. Echocardiographic analysis was performed to determine left ventricular ejection fraction (%LVEF). N=4–9 per group. Data are mean ± SEM. *,P<0.05. N.S.=not significant.
Table 1.
Echocardiographic analysis of K-Ras mutant mice.
| Parameter | WT Sham | WT TAC | Kras+/− Sham | Kras+/− TAC |
|---|---|---|---|---|
| n | 8 | 4 | 9 | 5 |
| DSEP WT (mm) | 0.80±0.05 | 0.85±0.06 | 0.68±0.03 | 0.79±0.06 |
| LVEDD (mm) | 3.69±0.10 | 4.65±0.22* | 3.69±0.10 | 3.82±0.10† |
| DPW WT (mm) | 1.01±0.05 | 0.82±0.04* | 0.93±0.03 | 0.89±0.05 |
| SSEP WT (mm) | 1.32±0.08 | 1.16±0.09 | 1.13±0.05 | 1.24±0.09 |
| LVESD (mm) | 2.37±0.14 | 3.90±0.26* | 2.46±0.16 | 2.89±0.07† |
| SPW WT (mm) | 1.46±0.07 | 1.13±0.06* | 1.34±0.05 | 1.21±0.08 |
| LVEF | 0.72±0.04 | 0.41±0.03* | 0.70±0.03 | 0.57±0.02*† |
| %FS | 35.9±3.0 | 16.2±1.6* | 33.9±2.7 | 24.3±0.9* |
| BW (g) | 27.7±1.3 | 30.6±0.9 | 30.5±1.6 | 32.7±1.5 |
Data are presented as mean ± S.E.M.
p<0.05 versus respective Sham.
p<0.05 versus WT TAC.
DSEP WT (diastolic septum wall thickness), LVEDD (left ventricular end diastolic dimension), DPW WT (diastolic posterior wall thickness), SSEP WT (systolic septum wall thickness), LVESD (left ventricular end systolic dimension), SPW WT (systolic posterior wall thickness, LVEF (left ventricular ejection fraction), %FS (fractional shortening), BW (body weight).
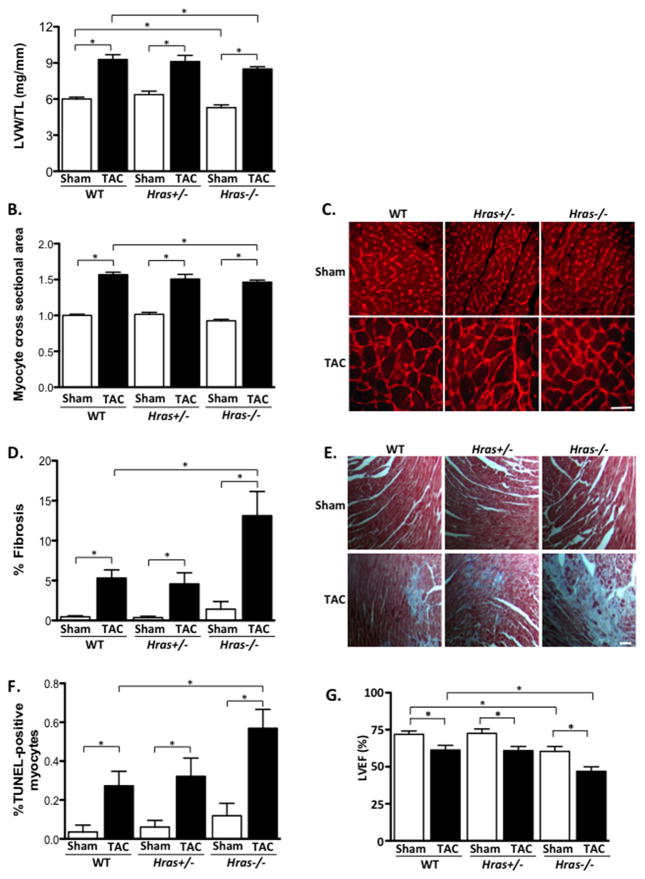
H-Ras mutant mice were also subjected to pressure overload and assessed. Pressure overload-induced increases in LVW/TL and cardiomyocyte CSA were not significantly different between Hras+/− mice and WT controls. However, Hras−/− mice had significantly attenuated LVW/TL and cardiomyocyte CSA following 4 weeks of TAC compared to WT (Figure 3A–C). Hras−/− hearts also had significantly increased TUNEL-positive cells and myocardial fibrosis after 4 weeks of TAC compared to WT controls, whereas Hras+/− mice did not (Figure 3D–F). Cardiac function (%LVEF) in Hras+/− mice declined to a similar extent as WT mice after 4 weeks of TAC. However, %LVEF was significantly further reduced in the Hras−/− mice following pressure overload (Figure 3G and Table 2). Moreover, septal wall thickness was significantly smaller in Hras+/− and Hras−/− mice after TAC compared to WT. These results suggest that H-Ras may contribute to the hypertrophic process in response to pressure overload stress.
Figure 3. Inhibition of endogenous H-Ras attenuates hypertrophy and exacerbates pressure overload-induced cardiac dysfunction.
A. Left ventricular weight/tibia length (LVW/TL) was determined in TAC and sham operated groups. B. Cardiomyocyte cross-sectional area was determined by wheat germ agglutinin (WGA) staining. C. Representative WGA images. D. Fibrosis was determined by Masson’s Trichrome staining. E. Representative images shown. Scale bar, 100 μm. F. Extent of apoptosis was determined by TUNEL staining. G. Echocardiographic analysis was performed to determine left ventricular ejection fraction (%LVEF). N=4–11 per group. Data are mean ± SEM. *,P<0.05.
Table 2.
Echocardiographic analysis of H-Ras mutant mice.
| Parameter | WT Sham | WT TAC | Hras+/− Sham | Hras+/− TAC | Hras−/− Sham | Hras−/− TAC |
|---|---|---|---|---|---|---|
| n | 6 | 7 | 4 | 6 | 6 | 7 |
| DSEP WT (mm) | 1.01±0.18 | 1.29±0.08 | 0.76±0.03 | 0.76±0.06* | 0.86±0.03 | 0.99±0.06 |
| LVEDD (mm) | 4.24±0.20 | 4.19±0.20 | 4.07±0.15 | 4.45±0.24 | 3.98±0.13 | 4.15±0.12 |
| DPW WT (mm) | 0.65±0.06 | 0.83±0.06 | 1.03±0.10† | 1.01±0.10 | 0.70±0.02 | 0.85±0.06 |
| SSEP WT (mm) | 1.64±0.10 | 1.74±0.07 | 1.40±0.08 | 1.20±0.08* | 1.24±0.05† | 1.31±0.10* |
| LVESD (mm) | 2.76±0.10 | 3.05±0.19 | 2.64±0.17 | 3.25±0.21 | 2.92±0.15 | 3.35±0.11 |
| SPW WT (mm) | 1.03±0.07 | 1.24±0.06 | 1.43±0.12 | 1.44±0.12 | 0.86±0.04‡ | 1.04±0.08§ |
| LVEF | 0.72±0.02 | 0.61±0.03 | 0.72±0.03 | 0.61±0.03 | 0.60±0.03 | 0.47±0.03*§ |
| %FS | 34.6±1.7 | 27.4±2.1 | 35.2±2.4 | 27.1±1.7 | 26.9±2.1 | 19.1±1.5*§ |
| BW (g) | 31.3±0.6 | 29.4±0.4 | 28.4±1.8 | 30.0±1.2 | 26.6±0.7† | 26.8±0.6† |
Data are presented as mean ± S.E.M.
p<0.05 versus WT TAC.
p<0.05 versus WT Sham.
p<0.05 versus Hras+/− Sham.
p<0.05 versus Hras+/− TAC.
DSEP WT (diastolic septum wall thickness), LVEDD (left ventricular end diastolic dimension), DPW WT (diastolic posterior wall thickness), SSEP WT (systolic septum wall thickness), LVESD (left ventricular end systolic dimension), SPW WT (systolic posterior wall thickness, LVEF (left ventricular ejection fraction), %FS (fractional shortening), BW (body weight).
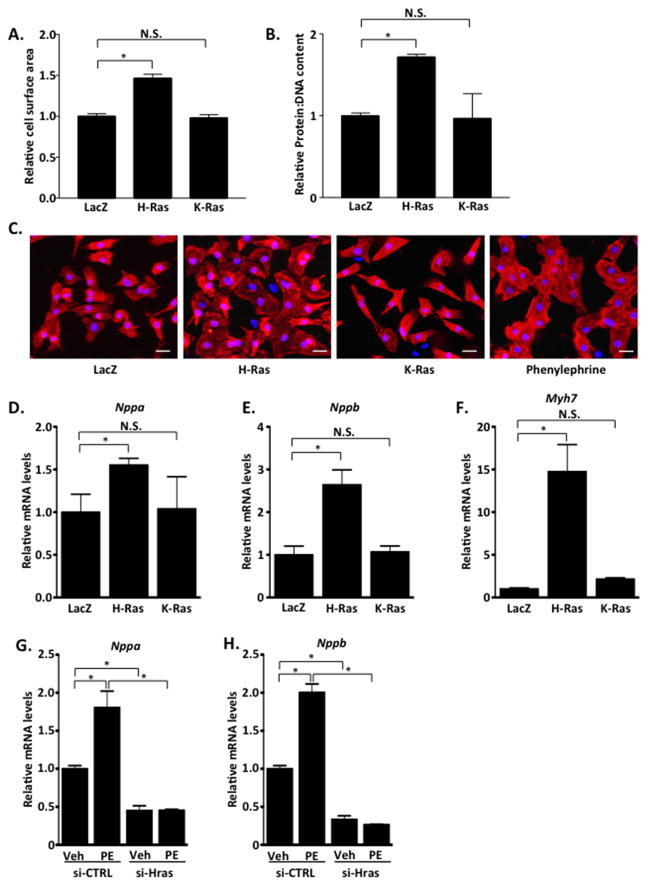
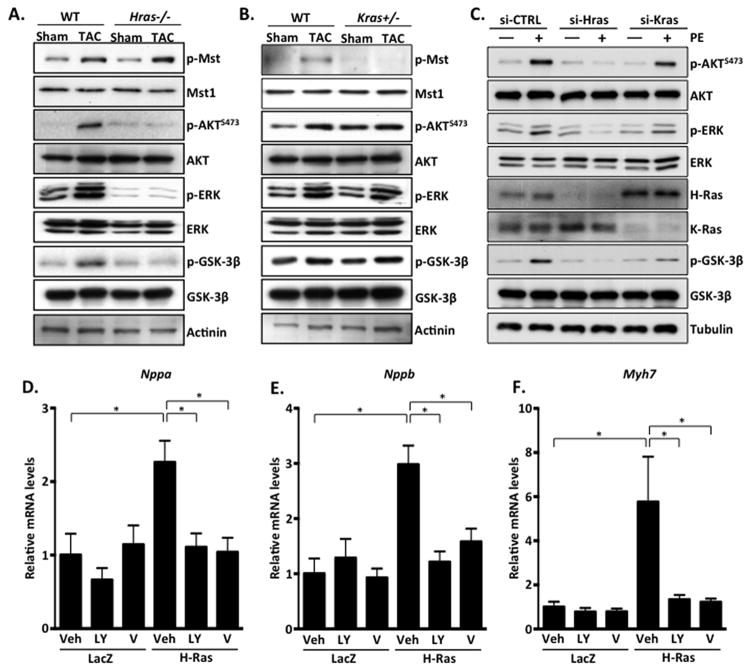
To further investigate the underlying mechanism, we utilized the culture of neonatal rat cardiomyocytes (NRCMs). We ectopically expressed activated H- and K-Ras12V in cultured cardiomyocytes using adenoviral gene transfer. We found that expression of activated H-Ras12V elicited a significant increase in cell surface area as well as protein:DNA content compared to LacZ control. In contrast, K-Ras12V expression did not alter cell surface area or protein:DNA content compared to LacZ suggesting that H-Ras selectively promotes cardiomyocyte hypertrophy in a cell autonomous manner (Figure 4A–C). We further evaluated hypertrophy by measuring fetal gene induction. By qRT-PCR analysis, we determined that H-Ras expression induced significant increases in ANF, BNP and β-MHC mRNA expression, whereas K-Ras expression did not significantly alter ANF, BNP or β-MHC expression, compared to LacZ control-infected NRCMs (Figure 4D–F). These results are in agreement with our previous findings that showed differential signaling elicited by H- and K-Ras in cardiomyocytes27, and demonstrate that H-Ras activation is sufficient to induce a hypertrophic response in NRCMs. We speculate that differences in isoform signaling may be due to differences in subcellular localization, which were observed by fractionation of ventricular tissue (Supplemental Figure 3). Importantly, a complimentary approach using RNAi-mediated depletion of endogenous H-Ras completely abolished the phenylephrine (PE)-induced expression of the hypertrophic markers ANF and BNP indicating that H-Ras is an important mediator of hypertrophy elicited by this α1 adrenergic agonist (Figure 4G and H).
Figure 4. H-Ras, but not K-Ras, promotes cardiomyocyte hypertrophy in vitro.
A–F. neonatal rat cardiomyocytes (NRCMs) were treated with LacZ, H-Ras12V or K-Ras12V adenovirus and assayed 48 hours later. A. NRCMs were stained with troponin T to visualize cardiomyocytes and cell surface area determined. B. Cells were collected and protein and DNA concentrations were determined. C. Representative images shown. Scale bar, 30 μm. mRNA was isolated and qRT-PCR performed to determine Nppa (D), Nppb (E) and Myh7 (F) levels. NRCMs were treated with siRNA to deplete endogenous H-Ras (si-Hras) or control siRNA (si-CTRL). 72 hours later, cells were treated with phenylephrine (100μM; 24 hours) or vehicle and qRT-PCR performed to determine Nppa (G) and Nppb (H) levels. N=3. Data are mean ± SEM. *,P<0.05. N.S.=not significant.
Our recent work demonstrated that activation of K-Ras by oxidative stress in cardiomyocytes promotes the phosphorylation and activation of the pro-apoptotic kinase Mst127. Similarly, during pressure overload, K-Ras appeared to mediate Mst1 activation as phosphorylation of Mst1 was attenuated in Kras+/−, but not Hras−/−, hearts after TAC (Figure 5A and B). Interestingly, phosphorylation of AKT and ERK were not altered in Kras+/− hearts but were strikingly inhibited in Hras−/− heart samples. Examination of downstream signaling in cultured cardiomyocytes revealed that H-Ras12V expression engaged PI3K-AKT, leading to increased phosphorylation of AKT and GSK-3β (an established physiological substrate of active AKT), and increased AKT kinase activity, while the effect of K-Ras12V expression was less pronounced (Supplemental Figure 4A and B). We also found that H-Ras knockdown (si-Hras) abolished PE-induced activation of AKT and ERK, yet si-Kras treatment had a less pronounced effect (Figure 5C). Mechanistically, we observed that H-Ras co-immunoprecipitated with the p110α subunit of PI3K in NRCMs (Supplemental Figures 4C). H-Ras may also negatively regulate expression of PTEN, which could contribute to increased AKT activation (Supplemental Figure 5). Finally, we employed inhibitors of PI3K, AKT and MEK1-ERK to determine their involvement in hypertrophy stimulated by H-Ras. We observed a significant reduction in H-Ras-induced hypertrophic gene expression in NRCMs subjected to PI3K-AKT inhibition, whereas little attenuation was observed following MEK/ERK inhibition (PD98059), suggesting that H-Ras promotes hypertrophy at least in part through activation of AKT signaling (Figure 5D–F and Supplemental Figure 6).
Figure 5. H-Ras activates the PI3K-AKT signaling axis and promotes cardiomyocyte hypertrophy.
A and B. Ventricular homogenates from control and mutant mice were subjected to western blot analysis to examine downstream signaling in response to 4 week TAC. C. NRCMs were treated with control siRNA (si-CTRL), or siRNA targeted to H-Ras (si-Hras) or K-Ras (si-Kras) for 72 hours. Cells were then stimulated with phenylephrine (PE; 100 μM) or vehicle control for 1 hour. NRCMs were transduced with LacZ or H-Ras12V in combination with vehicle or inhibitor for 48 hours and qRT-PCR performed to detect Nppa (D), Nppb (E) and Myh7 (F). N=3–4. Data are mean ± SEM. *,P<0.05.
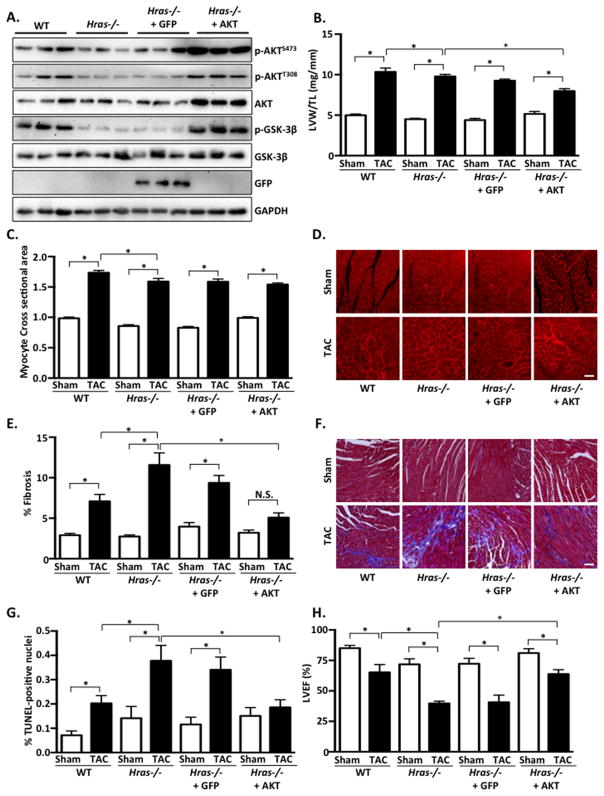
AKT has been implicated in cardiomyocyte hypertrophy and survival28–31. Based on our mouse studies and cardiomyocyte results, we hypothesized that restoration of AKT activity might ameliorate the deleterious phenotype observed in Hras−/− mice following pressure overload. To test this hypothesis we generated adeno-associated virus (AAV) to express exogenous AKT, or GFP as a control, and treated mice 2 weeks prior to TAC operation. Under basal conditions, LV mass, cardiomyocyte CSA and %LVEF were modestly reduced in Hras−/− compared to WT mice (Figure 6A–D, H). Similar to results shown in Figure 3, we also observed attenuated hypertrophy, augmented fibrosis and apoptosis, and exacerbated cardiac dysfunction in Hras−/− mice following TAC compared to WT (Figure 6A–H and Table 3). TAC-induced fetal gene expression was attenuated in Hras−/− mice compared to WT and was reversed to varying degrees by AKT-AAV, but not GFP-AAV (Supplemental Figure 7). Administration of AKT-AAV, but not GFP-AAV, significantly ameliorated the enhanced fibrosis, apoptosis and cardiac dysfunction observed in Hras−/− mice (Figure 6A–H and Table 3). Somewhat unexpectedly, we observed a modest attenuation of the hypertrophic response as determined by LV mass and cardiomyocyte CSA in Hras−/− mice supplemented with AKT-AAV after TAC (Figure 6B–D and Table 3). However, AKT normalization also significantly attenuated both TAC-induced lung congestion and increased wall stress in Hras−/− mice (Supplemental Figure 8). We hypothesize that the reduction in stress achieved by AKT-AAV, and the diminished need for compensatory hypertrophy, may explain why LV mass and cardiomyocyte CSA were not normalized in AKT-AAV treated Hras−/− mice.
Figure 6. Adeno-associated virus-mediated expression of AKT attenuates the deleterious effects of H-Ras deletion in response to pressure overload stress.
A–G. Four groups of mice (WT, Hras−/−, Hras−/− + GFP-AAV and Hras−/− + AKT-AAV) were subjected to sham operation or TAC for 4 weeks. AAV constructs were administered 2 weeks prior to TAC or sham surgery. A. Western blot analysis of GFP and AKT expression from ventricular homogenates. B. Left ventricular weight/tibia length (LVW/TL) was determined in TAC and sham operated groups. C. Cardiomyocyte cross-sectional area was determined by wheat germ agglutinin (WGA) staining. D. Representative WGA images. E. Fibrosis was determined by Masson’s Trichrome staining. F. Representative images shown. Scale bar, 100 μm. G. Extent of apoptosis was determined by TUNEL staining. H. Echocardiographic analysis was performed to determine left ventricular ejection fraction (%LVEF). N=3–8 per group. Data are mean ± SEM. *,P<0.05. N.S.=not significant.
Table 3.
Echocardiographic analysis of AAV treated H-Ras−/− mice.
| Parameter | WT Sham | WT TAC | KO Sham | KO TAC | KO + GFP Sham | KO + GFP TAC | KO + Akt Sham | KO + Akt TAC |
|---|---|---|---|---|---|---|---|---|
| n | 6 | 6 | 5 | 9 | 4 | 3 | 6 | 6 |
| DSEP WT (mm) | 1.01±0.06 | 1.25±0.05 | 0.92±0.04 | 0.95±0.09* | 0.79±0.02 | 0.93±0.04* | 0.94±0.08 | 1.11±0.03 |
| LVEDD (mm) | 3.27±0.08 | 4.26±0.19† | 3.35±0.08 | 4.66±0.18† | 3.59±0.25 | 4.78±0.11† | 3.33±0.12 | 4.08±0.14† |
| DPW WT (mm) | 0.91±0.02 | 1.01±0.08 | 1.06±0.09 | 1.09±0.11 | 0.96±0.12 | 0.92±0.13 | 0.99±0.10 | 0.99±0.06 |
| SSEP WT (mm) | 1.66±0.08 | 1.64±0.11 | 1.30±0.12 | 1.29±0.05 | 1.35±0.11 | 1.10±0.05* | 1.52±0.10 | 1.42±0.05 |
| LVESD (mm) | 1.72±0.11 | 2.96±0.29† | 2.18±0.15 | 3.86±0.21† | 2.33±0.20 | 4.01±0.16*† | 1.90±0.17 | 2.90±0.15†‡ |
| SPW WT (mm) | 1.29±0.09 | 1.40±0.10 | 1.29±0.10 | 1.02±0.01 | 1.32±0.19 | 1.06±0.04 | 1.37±0.05 | 1.39±0.07 |
| LVEF | 0.85±0.02 | 0.65±0.07† | 0.72±0.05 | 0.40±0.02*† | 0.72±0.04 | 0.41±0.06*† | 0.81±0.04 | 0.64±0.04‡§ |
| %FS | 47.5±2.6 | 31.3±4.7† | 35.1±3.4 | 17.2±1.4† | 35.2±3.3 | 16.1±2.7† | 43.5±3.1 | 29.1±2.3† |
| BW (g) | 24.9±0.4 | 29.2±0.6† | 25.6±0.7 | 32.5±1.6† | 27.0±0.7 | 28.9±0.6 | 28.5±1.3 | 30.4±1.4 |
Data are presented as mean ± S.E.M.
p<0.05 versus WT TAC.
p<0.05 versus respective Sham.
p<0.05 versus KO + GFP TAC.
p<0.05 versus KO TAC.
DSEP WT (diastolic septum wall thickness), LVEDD (left ventricular end diastolic dimension), DPW WT (diastolic posterior wall thickness), SSEP WT (systolic septum wall thickness), LVESD (left ventricular end systolic dimension), SPW WT (systolic posterior wall thickness, LVEF (left ventricular ejection fraction), %FS (fractional shortening), BW (body weight).
Discussion
Prior to this report, studies employing a loss-of-function approach to investigate the role of Ras isoforms in mediating cardiac hypertrophy and dysfunction in response to hemodynamic stress were lacking. Using H- and K-Ras mutant mice, we observed a beneficial function of endogenous H-Ras that appears to promote hypertrophy and cardioprotection, whereas Kras+/− mice had an improved cardiac phenotype after TAC suggesting that K-Ras contributes to dysfunction in response to chronic pressure overload. Our previous work demonstrated divergent outcomes downstream of H- and K-Ras during myocardial ischemia/reperfusion (I/R). In response to I/R, Hras−/− mice had greater injury likely due to attenuated PI3K-AKT activation while Kras+/− mice were protected due to inhibition of Mst1 and reduced mitochondria-mediated cardiomyocyte apoptosis27. This study is consistent with those findings and implicates PI3K-AKT signaling as an important mechanism downstream of H-Ras that is needed for compensation in response to TAC-induced cardiac stress.
Mechanisms that mediate cardiac hypertrophy are complex and many32. For Ras proteins alone, there are several well-established downstream signaling pathways that have the potential to alter heart growth and function, including Raf-MEK-ERK, PI3K-AKT and MEKK1-JNK. Investigation into each of these cascades has provided a wealth of information, yet the contribution of each to pathophysiology and disease remains somewhat unclear. Despite studies implicating a role for MEKK1-JNK in cardiomyocyte hypertrophy in vitro21, genetic deletion of MEKK1 in mice, the upstream kinase responsible for activating JNK, did not affect cardiac hypertrophy following pressure overload suggesting that this pathway may not be critical for pathological heart growth33. Inhibition of Raf through cardiac expression of a dominant-negative mutant, was shown to attenuate hypertrophy in response to pressure overload in mice, implicating this signaling pathway as a mediator of heart growth34. However, ERK1−/−ERK2+/− double mutant mice showed no change in hypertrophy after pressure overload35. Taken together, these results suggest that Raf signaling may diverge upstream of ERK to modulate the hypertrophic response. Forced expression of active PI3K (p110α) caused increased heart growth, while dominant-negative PI3K transgenic mice had smaller hearts at baseline and showed blunted hypertrophy following exercise but not pressure overload36, 37. Similarly, AKT transgenic mice had concentric hypertrophy and maintained systolic function, while Akt1 null mice were resistant to swimming-induced hypertrophy but showed exacerbated responses to pressure overload28–31. These studies suggest that basal and physiological heart growth rely on PI3K-AKT signaling, whereas pathological hypertrophy elicited by hemodynamic stress may be mediated through alternate mechanisms.
Based on this background, we sought to determine the mechanism underlying H-Ras mediated heart growth. Our results revealed a clear down regulation of AKT activation after TAC in hearts lacking H-Ras. Moreover, we employed AAV-mediated gene expression in vivo to show that restoration of AKT function was sufficient to ameliorate the detrimental effects of H-Ras deletion following pressure overload – specifically augmented fibrosis, apoptosis and impaired cardiac function. While we cannot rule out the contribution of additional signaling pathways downstream of H-Ras, these data indicate that AKT is an important target mediating a cardioprotective effect.
In addition to modulating cardiomyocyte growth and survival, AKT can also regulate angiogenesis and cardiomyocyte contractility, which may indirectly influence myocardial hypertrophy. Akt1−/− mice showed reduced angiogenesis, as well as reduced eNOS activation and NO production, following ischemia38. Akt1 null mice also had impaired vascular maturation leading to leaky vessels compared to WT counterparts39. On the other hand, mice engineered to express AKT in cardiomyocytes40, or selectively in endothelial cells in an inducible manner41, had increases in angiogenesis, capillary density, NO production and heart function after pressure overload, while endothelial cell apoptosis was attenuated. These findings demonstrate that enhanced vascular AKT activity can promote vessel formation, maturation and protection against insult, indicating its importance for adaptation to pathological stress.
AKT also modulates calcium handling and contractility of cardiomyocytes. Gain of function AKT transgenic mice had significantly increased contractility concomitant with increases in SERCA expression, SR Ca2+ load, and phosphorylation of PLN28, 42, 43. Adenoviral AKT transduction of rat hearts showed similar results44. However, prolonged expression of AKT elicited cardiac dysfunction, which was attributed to insufficient coupling of angiogenesis, indicating that the duration of AKT activation as well as myocyte/vessel growth are critical determinants of heart function45. Although the heart typically responds to increased demand via enlargement, it is plausible that AAV-AKT administration may increase cardiac contractility and/or angiogenesis independent of growth, and could explain why we observed attenuated hypertrophy in Hras−/− + AAV-AKT mice but improved cardiac function.
We have shown previously that H- and K-Ras isoforms can have divergent signaling and outcomes in cardiomyocytes27. Our prior findings, and current data, point to differences in subcellular localization of Ras isoforms that may be responsible for different effects of each in cardiomyocytes. In other cell types, Ras isoforms have been shown to localize to different cellular compartments, which can modulate association with downstream effectors and altered signaling46–49. Interestingly, differences in post-translational modification and intracellular processing of H- and K-Ras are established, and can affect protein trafficking, localization and signaling1. While less is known in cardiomyocytes, this is one possible explanation for the disparities observed between these two isoforms and warrants further exploration.
Interestingly, previous work demonstrated that levels of H-Ras expression correlated with cardiomyocyte size in patients with hypertrophic cardiomyopathy50. It has also been shown that the developmental disorders termed RASopathies, which are caused by dysfunctional Ras signaling, share phenotypic overlap including cardiovascular defects such as hypertrophic cardiomyopathy among others. These findings suggest that H-Ras may be an important modulator of cardiac growth in human disease. Furthermore, our data demonstrate that H-Ras activation is increased by acute pressure overload (1 day) but not by 7 days post-TAC in the murine heart, whereas K-Ras activation appears to be maintained. Since endogenous H-Ras seems to counteract the progression to heart failure, it may be of interest to determine whether maintenance of physiological H-Ras activity could prove beneficial in the face of hemodynamic stress. On the other hand, since K-Ras disruption afforded cardioprotection, perhaps selective inhibition of this isoform could improve outcomes during hypertension.
Supplementary Material
CLINICAL PERSPECTIVE.
Ras proteins are small GTPases that regulate numerous functions within the cell. Mutations in Ras proteins and Ras-related signaling genes are linked to several disorders collectively known as “RASopathies” that can present with cardiac abnormalities, yet the underlying molecular mechanisms remain largely unclear. In cardiomyocytes, Ras proteins are thought to mediate hypertrophy in response to agonist stimulation through activation of downstream signaling pathways and altered gene expression. However, in vivo, the role of Ras proteins in response to hypertrophic stress, and whether selective Ras isoforms have redundant functions, was not known. In this study we utilized H- and K-Ras knockout mice to determine the role of endogenous Ras isoforms in mediating cardiac hypertrophy and progressive dysfunction in response to chronic pressure overload stress. Our findings indicate that mice lacking H-Ras have attenuated hypertrophy and worsened heart function following stress, while K-Ras deficiency did not alter cardiac hypertrophy but improved function after pressure overload. H-Ras null hearts had downregulation of AKT, a kinase known to mediate cardiomyocyte survival and hypertrophy. Treatment of H-Ras knockout mice with adeno-associated virus to restore AKT expression was able to ameliorate the stress-induced cardiac dysfunction indicating involvement of AKT signaling. These results suggest that H- and K-Ras isoforms have distinct functions in the heart and selective targeting should be considered for potential therapeutic interventions.
Acknowledgments
The authors would like to thank T. Jacks (MIT) and E. Santos (U. of Salamanca) for generously providing mice, J. Sadoshima for helpful discussion, C. Brady for critical reading of the manuscript, and Y. Tian and L. Reed for assistance with virus production and injection. The recombinant adeno-associated virus (rAAV) was produced at the AAV Core, Department of Cell Biology and Molecular Medicine, Rutgers, New Jersey Medical School.
Sources of Funding: This work was supported by NIH grants HL127339 and HL122669 and an American Heart Association Scientist Development Grant (11SDG7240067).
Footnotes
Disclosures: None.
References
- 1.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moodie SA, Willumsen BM, Weber MJ, Wolfman A. Complexes of Ras. GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–61. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 3.Warne PH, Viciana PR, Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993;364:352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XF, Settleman J, Kyriakis JM, Takeuchi-Suzuki E, Elledge SJ, Marshall MS, Bruder JT, Rapp UR, Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993;364:308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]
- 5.Vojtek AB, Hollenberg SM, Cooper JA. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 7.Hofer F, Fields S, Schneider C, Martin GS. Activated Ras interacts with the Ral guanine nucleotide dissociation stimulator. Proc Natl Acad Sci U S A. 1994;91:11089–11093. doi: 10.1073/pnas.91.23.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi A, Demo SD, Ye ZH, Chen YW, Williams LT. ralGDS family members interact with the effector loop of ras p21. Mol Cell Biol. 1994;14:7483–7491. doi: 10.1128/mcb.14.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spaargaren M, Bischoff JR. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc Natl Acad Sci U S A. 1994;91:12609–12613. doi: 10.1073/pnas.91.26.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C(epsilon): a novel Ras effector. Embo J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert JM, Lambert QT, Reuther GW, Malliri A, Siderovski DP, Sondek J, Collard JG, Der CJ. Tiam1 mediates Ras activation of Rac by a PI(3)K-independent mechanism. Nat Cell Biol. 2002;4:621–625. doi: 10.1038/ncb833. [DOI] [PubMed] [Google Scholar]
- 12.Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, Menzies A, Teague JW, Futreal PA, Stratton MR. The Catalogue of Somatic Mutations in Cancer (COSMIC) Curr Protoc Hum Genet. 2008;Chapter 10(Unit 10):11. doi: 10.1002/0471142905.hg1011s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, Cruz MS, McCormick F, Rauen KA. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311:1287–1290. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- 14.Niihori T, Aoki Y, Narumi Y, Neri G, Cave H, Verloes A, Okamoto N, Hennekam RC, Gillessen-Kaesbach G, Wieczorek D, Kavamura MI, Kurosawa K, Ohashi H, Wilson L, Heron D, Bonneau D, Corona G, Kaname T, Naritomi K, Baumann C, Matsumoto N, Kato K, Kure S, Matsubara Y. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38:294–296. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- 15.Schubbert S, Zenker M, Rowe SL, Boll S, Klein C, Bollag G, van der Burgt I, Musante L, Kalscheuer V, Wehner LE, Nguyen H, West B, Zhang KY, Sistermans E, Rauch A, Niemeyer CM, Shannon K, Kratz CP. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–336. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 16.Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, Li L, Yassin Y, Tamburino AM, Neel BG, Kucherlapati RS. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39:70–74. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- 17.Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, Sarkozy A, Pandit B, Oishi K, Martinelli S, Schackwitz W, Ustaszewska A, Martin J, Bristow J, Carta C, Lepri F, Neri C, Vasta I, Gibson K, Curry CJ, Siguero JP, Digilio MC, Zampino G, Dallapiccola B, Bar-Sagi D, Gelb BD. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007;39:75–79. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- 18.Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, Tanaka Y, Filocamo M, Kato K, Suzuki Y, Kure S, Matsubara Y. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 19.Thorburn A, Thorburn J, Chen S, Powers S, Shubeita HE, Feramisco JR, Chien KR. HRas-dependent pathways can activate morphological and genetic markers of cardiac muscle cell hypertrophy. J Biol Chem. 1993;268:2244–2249. [PubMed] [Google Scholar]
- 20.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autorine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez MT, Sah VP, Zhao X, Hunter JJ, Chien KR, Brown JH. The MEKK-JNK pathway Is stimulated by a1-andrenergic receptor and Ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 22.LaMorte VJ, Thorburn J, Absher D, AS, Brown JH, Chien KR, Feramisco JR, Knowlton KU. Gq and ras-dependent pathways mediate hypertrophy of neonatal rat ventricular myocytes following a1-adrenergic stimulation. J Biol Chem. 1994;269:13490–13496. [PubMed] [Google Scholar]
- 23.Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270:23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 24.Zheng M, Dilly K, Dos Santos Cruz J, Li M, Gu Y, Ursitti JA, Chen J, Ross J, Jr, Chien KR, Lederer JW, Wang Y. Sarcoplasmic reticulum calcium defect in Ras-induced hypertrophic cardiomyopathy heart. Am J Physiol Heart Circ Physiol. 2004;286:H424–433. doi: 10.1152/ajpheart.00110.2003. [DOI] [PubMed] [Google Scholar]
- 25.Johnson L, Greenbaum D, Cichowski K, Mercer K, Murphy E, Schmitt E, Bronson RT, Umanoff H, Edelmann W, Kucherlapati R, Jacks T. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11:2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esteban LM, Vicario-Abejon C, Fernandez-Salguero P, Fernandez-Medarde A, Swaminathan N, Yienger K, Lopez E, Malumbres M, McKay R, Ward JM, Pellicer A, Santos E. Targeted genomic disruption of H-ras and N-ras, individually or in combination, reveals the dispensability of both loci for mouse growth and development. Mol Cell Biol. 2001;21:1444–1452. doi: 10.1128/MCB.21.5.1444-1452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Del Re DP, Matsuda T, Zhai P, Maejima Y, Jain MR, Liu T, Li H, Hsu CP, Sadoshima J. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol Cell. 2014;54:639–650. doi: 10.1016/j.molcel.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- 31.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 32.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadoshima J, Montagne O, Wang QM, Yang GP, Warden J, Liu J, Takagi G, Karoor V, Hong C, Johnson GL, Vatner DE, Vatner SF. The MEKK1-JNK pathway plays a protective role in pressure overload, but does not mediate cardiac hypertrophy. J Clin Invest. 2002;110:271–279. doi: 10.1172/JCI14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris IS, Zhang S, Treskov I, Kovacs A, Weinheimer C, Muslin AJ. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 35.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–10479. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. Embo J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115:2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceci M, Gallo P, Santonastasi M, Grimaldi S, Latronico MV, Pitisci A, Missol-Kolka E, Scimia MC, Catalucci D, Hilfiker-Kleiner D, Condorelli G. Cardiac-specific overexpression of E40K active Akt prevents pressure overload-induced heart failure in mice by increasing angiogenesis and reducing apoptosis. Cell death and differentiation. 2007;14:1060–1062. doi: 10.1038/sj.cdd.4402095. [DOI] [PubMed] [Google Scholar]
- 41.Mukai Y, Rikitake Y, Shiojima I, Wolfrum S, Satoh M, Takeshita K, Hiroi Y, Salomone S, Kim HH, Benjamin LE, Walsh K, Liao JK. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest. 2006;116:334–343. doi: 10.1172/JCI26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim YK, Kim SJ, Yatani A, Huang Y, Castelli G, Vatner DE, Liu J, Zhang Q, Diaz G, Zieba R, Thaisz J, Drusco A, Croce C, Sadoshima J, Condorelli G, Vatner SF. Mechanism of enhanced cardiac function in mice with hypertrophy induced by overexpressed Akt. J Biol Chem. 2003;278:47622–47628. doi: 10.1074/jbc.M305909200. [DOI] [PubMed] [Google Scholar]
- 43.Catalucci D, Latronico MV, Ceci M, Rusconi F, Young HS, Gallo P, Santonastasi M, Bellacosa A, Brown JH, Condorelli G. Akt increases sarcoplasmic reticulum Ca2+ cycling by direct phosphorylation of phospholamban at Thr17. J Biol Chem. 2009;284:28180–28187. doi: 10.1074/jbc.M109.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cittadini A, Monti MG, Iaccarino G, Di Rella F, Tsichlis PN, Di Gianni A, Stromer H, Sorriento D, Peschle C, Trimarco B, Sacca L, Condorelli G. Adenoviral gene transfer of Akt enhances myocardial contractility and intracellular calcium handling. Gene Ther. 2006;13:8–19. doi: 10.1038/sj.gt.3302589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choy E, Chiu VK, Silletti J, Feoktistov M, Morimoto T, Michaelson D, Ivanov IE, Philips MR. Endomembrane trafficking of ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 47.Prior IA, Harding A, Yan J, Sluimer J, Parton RG, Hancock JF. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol. 2001;3:368–375. doi: 10.1038/35070050. [DOI] [PubMed] [Google Scholar]
- 48.Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL, 2nd, Cox AD, Philips MR. Ras signalling on the endoplasmic reticulum and the Golgi. Nat Cell Biol. 2002;4:343–350. doi: 10.1038/ncb783. [DOI] [PubMed] [Google Scholar]
- 49.Bivona TG, Quatela SE, Bodemann BO, Ahearn IM, Soskis MJ, Mor A, Miura J, Wiener HH, Wright L, Saba SG, Yim D, Fein A, Perez de Castro I, Li C, Thompson CB, Cox AD, Philips MR. PKC regulates a farnesyl-electrostatic switch on K-Ras that promotes its association with Bcl-XL on mitochondria and induces apoptosis. Mol Cell. 2006;21:481–493. doi: 10.1016/j.molcel.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Kai H, Muraishi A, Sugiu Y, Nishi H, Seki Y, Kuwahara F, Kimura A, Kato H, Imaizumi T. Expression of proto-oncogenes and gene mutation of sarcomeric proteins in patients with hypertrophic cardiomyopathy. Circ Res. 1998;83:594–601. doi: 10.1161/01.res.83.6.594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.