Summary
Background
Aim of the present study was to evaluate the role of MRI in staging of malignant lesions of the oral cavity and to correlate MRI findings with clinical/surgical and anatomical-pathological findings, wherever possible.
Material/Methods
The study included 50 patients who presented with malignant lesions of the oral cavity and were referred to radiology departments for MRI. All patients included were subjected to a detailed physical examination following which MRI was carried out on Philips Gyroscan Achieva 1.5 Tesla unit.
Results
In the study, the highest number of patients were found to have tongue malignancy (82%) followed by buccal mucosa and gingivobuccal sulcus malignancy (18%). The highest number of patients was in the age group of 51–60 years (32%). The incidence was higher in males (96%). There was moderate agreement (k=0.537) for T stage between the clinical and MRI staging assessments. The agreement for N stage between clinical and MRI staging assessments was fair (k=0.328). The final diagnosis was made by histopathology in 22 patients. The agreement for T stage was good/substantial (k=0.790) and for N stage was moderate (k=0.458) between MRI and histopathology staging assessments.
Conclusions
MRI provides satisfactory accuracy for preoperative estimation of tumor thickness and predicting occult cervical nodal metastasis. MRI is the preferred modality in evaluation and staging of oral cavity malignancy which helps a clinician for planning of treatment.
MeSH Keywords: Magnetic Resonance Imaging, Mouth, Neoplasm Staging, Tongue Neoplasms
Background
Oral cavity cancers form a significant percentage of cancers in India. Tobacco chewing and alcohol are dominant causes [1]. They are classified into following subsites [2]:
Buccal mucosa;
Alveolus and gingival;
Hard palate;
Tongue and floor of the mouth.
MRI is used to assess the extent of loco-regional tumor spread, depth of invasion and extent of lymphadenopathy. The invasion of the floor of the mouth by the tumor is depicted well in the coronal plane [3–5]. Non-contrast T1W sequences demonstrate cortical erosion and marrow invasion. Contrast-enhanced T1W images help assess marrow invasion [6], perineural spread, soft tissue extent, tumor thickness and best demonstrate necrosis in nodes [7]. The T2W images are sensitive to the presence of tumor tissue, which is usually hyperintense compared with the surrounding muscles [3–5].
The staging of oral cavity squamous cell carcinoma (SCC) is currently based on American Joint Committee on Cancer (AJCC) criteria [3,8] (Table 1).
Table 1.
AMerican Joint Committee on Cancer (AJCC) TNM staging.
| Stage | Description |
|---|---|
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor ≤2 cm in greatest dimension |
| T2 | Tumor >2 cm but ≤4 cm in greatest dimension |
| T3 | Tumor >4 cm in greatest dimension |
| T4a | Moderately advanced local disease, – invades through cortical bone, into deep (extrinsic) muscles of tongue, maxillary sinus, or skin of face |
| T4b | Very advanced local disease, – invades masticator space, pterygoid plates, or skull base and/or encases internal carotid artery |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node, ≤3 cm in greatest dimension |
| N2a | Metastasis in single ipsilateral lymph node, >3 cm but ≤6 cm in greatest dimension |
| N2b | Metastases in multiple ipsilateral lymph nodes, none >6 cm in greatest dimension |
| N2c | Metastases in bilateral or contralateral lymph nodes, none >6 cm in greatest dimension |
| N3 | Metastasis in a lymph node >6 cm in greatest dimension |
| M0 | No distant metastasis |
| M1 | Distant metastasis |
Aims and objectives
Evaluation of the role of MRI in loco-regional staging of malignant lesions of the oral cavity and to correlate the MRI findings with clinical/surgical findings and anatomical-pathological findings, wherever possible.
Material and Methods
This was a prospective study comprising fifty patients of all age groups with malignant lesions involving the oral cavity. An initial Informed consent was taken. The following patients were excluded from the study:
Those with any contraindication to undergo MRI;
Those having any previous history of surgical treatment of the oral cavity.
The clinical history and detailed local examination of all the patients was recorded. Examination of the mucosa of the cheek, vestibule of the mouth, gums in both upper and lower jaws, hard and soft palate, floor of the mouth, retromolar trigone, tonsils and pillars was done to look for any ulcer, mass and bulge. Examination of the tip, dorsum, lateral borders and undersurface of the tongue was done to look for any ulcers, white patch and proliferative growth. Movements of the tongue were looked for, with the base of the tongue and vallecula being examined by indirect laryngoscopy and finger palpation. Tumor data, including site, subsite and extent (infiltration of the surrounding structures) were collected to carry out clinical TNM staging. (AJCC Staging system – Table 1).
After clinical staging was made, MRI was conducted in each patient included in the study. Afterwards surgery and histopathological staging were performed, wherever possible.
MR technique
MR scan was carried out on Philips Gyroscan Achieva 1.5 Tesla unit.
MR imaging protocol
T1W images were acquired in all planes (axial, sagittal and coronal). The T2W images and diffusion weighted images were acquired in the axial plane. STIR images were acquired in the coronal and sagittal planes. The intravenous contrast used was Gadopentate dimeglumine (0.1 mmol/kg) (Table 2).
Table 2.
Pre- and postcontrast.
| SEQ | FOV | RFV | MATRIX | THK (MM) | GAP (MM) | NSA | TR | TE |
|---|---|---|---|---|---|---|---|---|
| Pre contrast | ||||||||
| STIR (COR) | 250 | 75 | 272/512 | 3.0 | 1.3 | 3 | 4340 | 14 |
| T1W COR | 250 | 75 | 352/512 | 3.0 | 1.3 | 4 | 460 | 12 |
| T2W FS TRA | 250 | 80 | 352/512 | 4.0 | 0.4 | 3 | 2548 | 80 |
| STIR SAG | 250 | 75 | 272/512 | 3.0 | 1.3 | 3 | 4341 | 14 |
| T1W FS TRA | 250 | 80 | 352/512 | 4.0 | 0.4 | 4 | 664 | 12 |
| T1 axial | 250 | 80 | 352/512 | 4.0 | 0.4 | 4 | 460 | 12 |
| Diffusion axial | 230 | 80 | 112/80 | 5.0 | 0.5 | 4 | 460 | 12 |
| Post contrast | ||||||||
| T1W FS | 250 | 80 | 352/512 | 4.0 | 0.4 | 4 | 664 | 12 |
| T1W COR | 250 | 80 | 352/512 | 3.0 | 1.3 | 3 | 460 | 12 |
Results
A total of fifty patients were included in the study. After an initial thorough clinical examination, Magnetic Resonance Imaging was done. Data were analyzed using the Statistical Package for the Social Sciences – version 18. The observations found clinically, on histopathology examination and on MR imaging were correlated using Pearson’s chi-squared test and kappa index. The observations are as follows:
The present study comprised of all oral cavity cancers of squamous cell type. The highest number of patients were found to have tongue malignancy constituting about 82% of the patients followed by gingival and buccal mucosa malignancy which constitutes about 18% of the total number of patients (Table 3). 32% of the patients belonged to age group of 51–60 years, which was the maximum followed by the age group of 41–50 years comprising of 26% of the patients (Table 4). The incidence of the oral cancers is higher in males constituting 96% of total patients (Table 5). There was moderate agreement (k=0.537) for the T stage between the clinical and MRI staging assessments as shown in Table 6. N stage agreement between MRI and clinical staging assessments was fair (k=0.328) as shown in Table 7. In 22 patients, the confirmatory diagnosis was made by surgery/histopathology. Good/substantial (k=0.790) agreement for the T stage was seen between MRI and histopathology staging assessments as depicted in Table 8. The agreement for the N stage was moderate (k=0.458) between MRI and histopathology staging assessments (Table 9). The agreement for the T stage was poor (k=0.085) between the clinical and histopathology staging assessments (Table 10). Table 11 shows that the agreement for the N stage was poor (k=0.185) between the clinical and histopathology staging assessments.
Table 3.
Incidence of individual cancer.
| Site | Number of patients | Percentage |
|---|---|---|
| Tongue | 41 | 82% |
| Buccal mucosa and alveolar | 9 | 18% |
| Hard palate | 0 | 0% |
| Total | 50 | 100% |
Table 3 shows that maximum number of patients were of tongue malignancy followed by buccal mucosa and alveolar malignancy.
Table 4.
Age incidence.
| Age group in years | No. of patients | Percentage |
|---|---|---|
| ≤20 | 0 | 0% |
| 21–30 | 2 | 4% |
| 31–40 | 11 | 22% |
| 41–50 | 13 | 26% |
| 51–60 | 16 | 32% |
| >60 | 8 | 16% |
| Total | 50 | 100% |
Table 4 shows that maximum number of patients were in the age group of 51–60 followed by the age group of 41–50 years.
Table 5.
Sex incidence.
| Sex | Number of patients | Percentage |
|---|---|---|
| Male | 48 | 96% |
| Female | 2 | 4% |
| Total | 50 | 100% |
Table 5 shows that incidence of the oral cancers is higher in males than females. Males were predominant in this study with 96% of the total patients were males and 4% were females. Male to female ratio is 24: 1.
Table 6.
Correlation between clinicaltumour (T) staging and MRI tumour (T) staging.
| Clinical ‘T’ staging | MRI ‘T’ staging | Total | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| T1 | 2 | 0 | 0 | 0 | 2 |
| T2 | 0 | 12 | 1 | 0 | 13 |
| T3 | 0 | 5 | 11 | 3 | 19 |
| T4 | 0 | 1 | 6 | 9 | 16 |
| Total | 2 | 18 | 18 | 12 | 50 |
Table 6 correlates the MRI and clinical tumour (T) staging. By applying the chi square test and kappa statisitics, p value and k value comes out to be 0.01 and 0.537 respectively showing moderate agreement between the clinical and MRI staging assessments.
Table 7.
Correlation between clincal nodal (N) staging and MRI nodal (N) staging.
| Clinical ‘N’ staging | MRI ‘N’ staging | Total | ||
|---|---|---|---|---|
| N0 | N1 | N2 | ||
| N0 | 7 | 4 | 8 | 19 |
| N1 | 1 | 9 | 11 | 21 |
| N2 | 0 | 0 | 10 | 10 |
| Total | 8 | 13 | 29 | 50 |
Table 7 correlates the MRI and clinical nodal (N) staging. By applying the chi square test and kappa statisitics, p and k value comes out to be 0.02 and 0.328 respectively which shows fair agreement between the clinical and MRI staging assessments.
Table 8.
Correlation between MRI tumour (T) staging and histopathological tumour (T) staging.
| MRI ‘T’ staging | Histopathological ‘T’ staging | Total | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| T1 | 1 | 0 | 0 | 0 | 1 |
| T2 | 0 | 9 | 0 | 0 | 9 |
| T3 | 0 | 2 | 6 | 0 | 8 |
| T4 | 0 | 1 | 0 | 3 | 4 |
| Total | 1 | 12 | 6 | 3 | 22 |
Table 8 correlates the MRI and histopathological tumour (T) staging. By applying the chi square test and kappa statisitics, p and k value comes out to be 0.01 and 0.790 respectively which shows good/substantial agreement between the clinical and MRI staging assessments.
Table 9.
Correlation between MRI nodal (N) staging and histopathological nodal (N) staging.
| MRI ‘N’ staging | Histopathological ‘N’ staging | Total | ||
|---|---|---|---|---|
| N0 | N1 | N2 | ||
| N0 | 3 | 0 | 0 | 3 |
| N1 | 3 | 3 | 1 | 7 |
| N2 | 4 | 0 | 8 | 12 |
| Total | 10 | 3 | 9 | 22 |
Table 9 correlates the MRI and histopathological (N) staging. By applying the chi square test and kappa statisitics, p and k value comes out to be 0.01 and 0.458 respectively which shows moderate agreement between the clinical and MRI staging assessments.
Table 10.
Clinical (T) staging vs. histopathological (T) staging.
| Clinical ‘T’ staging | Histopathological ‘T’ staging | Total | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | ||
| T1 | 1 | 0 | 0 | 0 | 1 |
| T2 | 0 | 6 | 1 | 0 | 7 |
| T3 | 0 | 3 | 3 | 1 | 7 |
| T4 | 0 | 3 | 2 | 2 | 7 |
| Total | 1 | 12 | 6 | 3 | 22 |
Table 10 correlates the clinical and histopathological tumour (T) staging. By applying the chi square test and kappa statistics, p and k value comes out to be 0.01 and 0.085 respectively which shows poor agreement between the clinical and MRI staging assessments.
Table 11.
Clinical (N) staging vs. histopathological (N) staging.
| Clinical ‘N’ staging | Histopathological ‘N’ staging | Total | ||
|---|---|---|---|---|
| N0 | N1 | N2 | ||
| N0 | 4 | 0 | 3 | 7 |
| N1 | 5 | 3 | 4 | 12 |
| N2 | 1 | 0 | 2 | 3 |
| Total | 10 | 3 | 9 | 22 |
Table 11 correlates the clinical and histopathological (N) staging. By applying the chi square test and kappa statistics, p and k value comes out to be 0.01 and 0.185 respectively which shows poor agreement between the clinical and MRI staging assessments.
Discussion
Oral cavity cancers include buccal mucosa, alveolus and gingival, hard palate and tongue and floor of the mouth cancers. Tumor Node Metastasis (TNM) classification is currently the most commonly used system for describing malignant tumors and their extent of spread (both regional and distant). This staging system is the guide for every radiologist for assessment of oral carcinomas as well as for reporting relevant studies.
MRI is a very useful tool for providing the details of structures within the oral cavity and also of the adjacent structures. Excellent soft-tissue discrimination of MRI readily reveals tumor invasion and spread to surrounding structures (Figure 1). MRI is used to assess the extent of local and regional tumor spread, the depth of invasion, and the extent of lymphadenopathy (Figure 2). The major advantage of MRI over computed tomography (CT) is that it provides excellent soft tissue details as compared to CT and does not expose the patients to any harmful radiations. MRI can detect marrow invasion by tumor earlier than CT. MRI provides information about the involvement of the base of the tongue, floor of the mouth, and is very useful to see the tumor extension into the oro-pharynx that is extremely difficult to be seen on CT (Figure 3). The gadolinium chelates used as MRI contrast agents are associated with a much lower incidence of allergic/anaphylactic reactions and are less nephrotoxic in the doses used than the iodinated contrast agents used in CT [9].
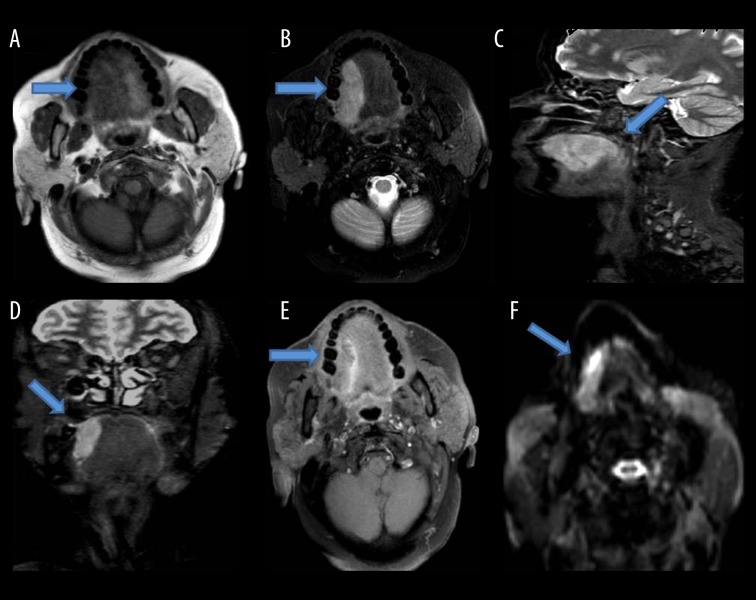
Figure 1.
Shows a mass lesion with soft tissue signal intensity involving the right lateral border of the tongue, appearing isointense on T1W images (A), hyperintense on T2W (B), STIR sagittal (C), STIR coronal images (D), extending posteriorly to involve the posterior margin of the tongue, showing mild contrast enhancement on post-contrast T1W FS images (E) and restriction on diffusion weighted images (F).
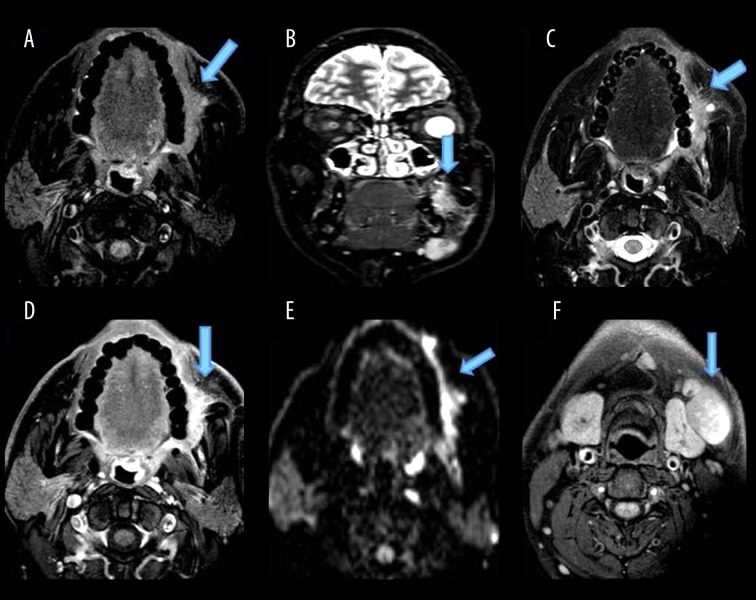
Figure 2.
Shows a mass lesion with ill-defined soft tissue signal intensity, involving the buccal mucosa on the left side, opposite the second, third molars, appearing isointense on T1W (A), hyperintense on STIR coronal (B), T2W (C) images, extending to the left retromolar trigone with loss of fat planes with the buccinator muscle, showing minimal enhancement (D) and restriction on DWI (E). (F) shows enlarged lymph nodes at levels Ib, II on both sides.
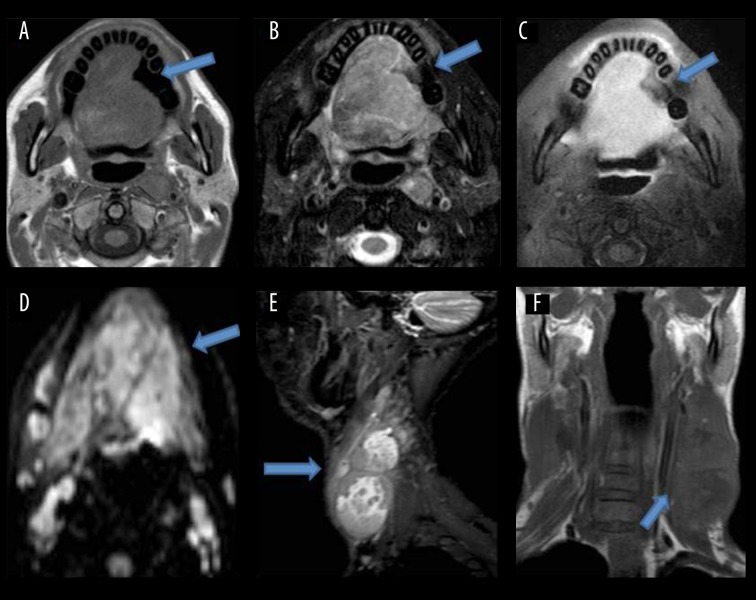
Figure 3.
Shows a lesion with ill-defined soft tissue signal intensity, involving almost the whole tongue, appearing isointense on T1W (A), heterogeneously hyperintense on T2W images (B), showing moderate contrast enhancement (C). On DWI, it shows restriction (D). Posteriorly, it involves the base of the tongue and obliterates the oropharyngeal lumen. Multiple heterogeneously-enhancing lymph node masses seen along both jugular chains (E, F).
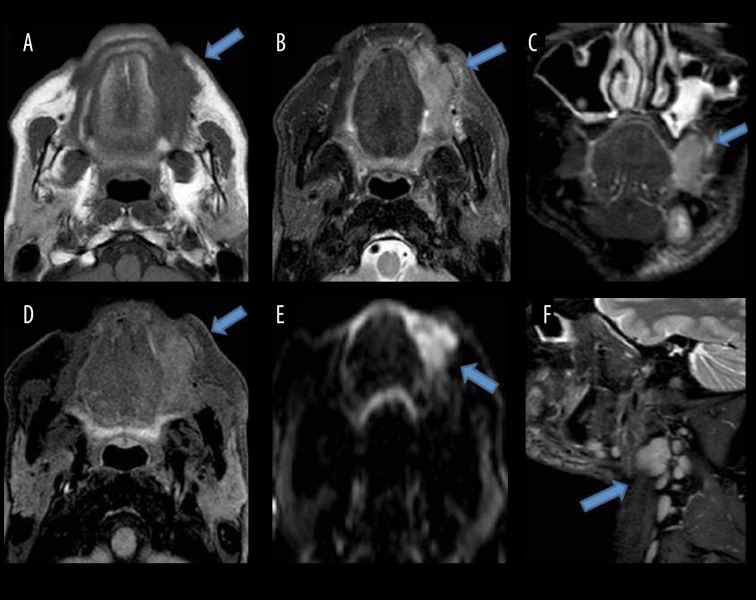
Results of the study showed that the highest number of patients had tongue malignancy (about 82% of the patients). Early tumors of the tongue tend to be confined within the tongue (Figure 1). The advanced tumors can invade the surrounding structures like the floor of the mouth, tongue base, mandible and the anterior tonsillar pillar (Figure 4). Contrast-enhanced T1W images help assess marrow invasion, soft tissue extent, tumor thickness and best demonstrate necrosis in nodes (Figure 3). MRI is also used to assess the extent of lymphadenopathy. It is used to assess the presence of occult metastatic lymph nodes. So the present study emphasized the importance of MR imaging as a valuable tool for carrying out the loco-regional staging of oral cavity cancers with an extreme degree of precision. The extent of primary tumor (T) and metastasis to regional lymph nodes (N) was initially evaluated by clinical examinations followed by MR imaging in this study. The final diagnosis was made by histopathological examination. Kappa Index was used for data analysis. Our study shows that there is moderate agreement (kappa value 0.537) between the clinical and MRI T staging. Clinical T staging changes in 32% of patients after performing MRI, which is finally proven by histopathological examination. This is consistent with the studies performed by Paiva RR et al. [10] and Paiboon JJ et al. [11] which also showed that mis-staging by clinical examination in the overall stage grouping was high.
Figure 4.
Shows a mass lesion with ill-defined soft tissue signal intensity, involving the buccal space on the left side, appearing isointense on T1W (A), hyperintense on T2W (B) and STIR coronal images (C), showing mild enhancement (D). Restriction on DWI (E). Laterally, reaching up to subcutaneous tissues. Medially, indenting the left lateral margin of the tongue with well-defined fat planes in between. (F) shows enlarged lymph nodes at levels 1b, II, III on the left side.
Our study shows good/substantial agreement (kappa value 0.790) for the T staging (tumor depth and width) between MRI and pathological assessments. The final staging as assessed by MR imaging in our study remains the same in 19 out of 22 patients who underwent surgery and final staging by histopathological means. These results are consistent with the study conducted by Tetsumura A et al. [12] in which the tumor depth and width were measured on both MR images and histo-pathological sections and the authors observed a high correlation between the values measured by MRI and histopathology.
Zeng et al. [13] also conducted similar studies and founded that MRI showed good performance in displaying tumor invasion, invasion depth and extension. This is consistent with the present study which also shows good/substantial agreement (kappa value 0.790) for the T staging but shows only fair agreement (kappa value 0.458) for the N staging between MRI and pathological staging assessments.
Our study shows that MRI is an adequate technique for the assessment of oral cavity malignancies, in the evaluation of depth invasion, presence and extension of mandibular involvement (T stage), and shows excellent agreement with the final T staging by histopathology. This is consistent with the study conducted by Vidiri et al. [14] which also shows similar results.
Paul Lam et al. [15] also conducted a study in which the radiological tumor thickness on contrast-enhanced T1-weighted and T2-weighted images was compared with the histological tumor thickness. They concluded that MR images provide satisfactory accuracy for the measurement of tumor thickness and staging of oral tongue cancer. This is consistent with the results of our present study which also shows good/substantial agreement (kappa value 0.790) for the T staging between MRI and pathological staging assessments.
Conclusions
A high correlation was found between the values measured by MRI and histopathology for thickness of the mucosal epithelium and both depth and width of tumors. MRI provides satisfactory accuracy for preoperative estimation of tumor thickness and paralingual distance, valuable for predicting occult cervical nodal metastasis. The mucosal epithelium, lamina propria and muscles of the tongue were clearly identifiable on MRI. MRI is the imaging modality of choice for staging of malignancy of the oral cavity and tongue using TNM classification which helps a clinician in planning treatment like glossectomy/marginal mandibulectomy or radiotherapy or both.
Footnotes
Conflicts of interest
None.
References
- 1.Arya S, Chaukar D, Pai P. Imaging in oral cancers. Indian J Radiol Imaging. 2012;22:195–20. doi: 10.4103/0971-3026.107182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobin LH, Wittekind CH. UICC TNM classification of malignant tumors. 6th Ed. New York: Wiley-Liss; 2002. pp. 52–56. [Google Scholar]
- 3.Castelijns JA. Diagnostic radiology of head and neck oncology. Curr Opin Oncol. 1991;3(3):512–18. doi: 10.1097/00001622-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Van den Brekel MW, Castelijns JA, Snow GB. The role of modern imaging studies in staging and therapy of head and neck neoplasms. Semin Oncol. 1994;21(3):340–48. [PubMed] [Google Scholar]
- 5.Madison MT, Remley KB, Latchaw RE, Mitchell SL. Radiologic diagnosis and staging of head and neck squamous cell carcinoma. Radiol Clin North Am. 1994;32(1):163–81. [PubMed] [Google Scholar]
- 6.Imaizumi A, Yoshino N, Yamada I, et al. A potential pitfall of MR imaging for assessing mandibular invasion of squamous cell carcinoma in the oral cavity. Am J Neuroradiol. 2006;27(1):114–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Yasumoto M, Shibuya H, Takeda M, Korenaga T. Squamous cell carcinoma of the oral cavity: MR findings and value of T1- versus T2-weighted fast spin-echo images. Am J Roentgenol. 1995;164(4):981–87. doi: 10.2214/ajr.164.4.7726062. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 241–49. [Google Scholar]
- 9.Singh D, Sahoo S, Gupta V, Srivastava D. Latest advancements in imaging of oral and maxillofacial neoplasm: A comprehensive review. J Oral Maxillofac Radiol. 2013;1:37–42. [Google Scholar]
- 10.Rogerio RP, de Souza PT, Leite AF, et al. Oral cancer staging established by magnetic resonance imaging. Braz Oral Res. 2011;25(6):512–18. doi: 10.1590/s1806-83242011000600007. [DOI] [PubMed] [Google Scholar]
- 11.Hirunpat S, Paiboon JJ, Angunsri N, Chowchuvech V. When should MRI be recommended for the accurate clinical staging of base of tongue carcinoma. Asian Pacific J Cancer Prev. 2007;8(2):310–14. [PubMed] [Google Scholar]
- 12.Tetsumura A, Yoshino N, Amagasa T, et al. High-resolution magnetic resonance imaging of squamous cell carcinoma of the tongue: An in vitro study. Dentomaxillofac Radiol. 2001;30(1):14–21. doi: 10.1038/sj/dmfr/4600565. [DOI] [PubMed] [Google Scholar]
- 13.Zeng H, Liang CH, Zhou ZG, et al. Study of preoperative MRI staging of tongue carcinoma in relation to pathological findings. Di Yi Jun Yi Da Xue Xue Bao. 2003;23(8):841–43. [PubMed] [Google Scholar]
- 14.Vidiri A, Ruscito P, Pichi B, et al. Oral cavity and base of the tongue tumors. Correlation between clinical, MRI and pathological staging of primary tumor. J Exp Clin Cancer Res. 2007;26(4):575–82. [PubMed] [Google Scholar]
- 15.Lam P, Au-Yeung KM, Cheng PW, et al. Correlating MRI and histologic tumor thickness in the assessment of oral tongue cancer. Am J Roentgenol. 2004;182(3):803–8. doi: 10.2214/ajr.182.3.1820803. [DOI] [PubMed] [Google Scholar]