Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
Survival was 100% for 18 patients with ADA-SCID treated with genetically modified CD34+ cells (2.3-13.4 years follow up; median, 6.9 years).
Long-term engraftment, immune reconstitution, and fewer severe infections were observed in 15 out of 18 patients without leukemic transformation.
Abstract
Adenosine deaminase (ADA) deficiency is a rare, autosomal-recessive systemic metabolic disease characterized by severe combined immunodeficiency (SCID). The treatment of choice for ADA-deficient SCID (ADA-SCID) is hematopoietic stem cell transplant from an HLA-matched sibling donor, although <25% of patients have such a donor available. Enzyme replacement therapy (ERT) partially and temporarily relieves immunodeficiency. We investigated the medium-term outcome of gene therapy (GT) in 18 patients with ADA-SCID for whom an HLA-identical family donor was not available; most were not responding well to ERT. Patients were treated with an autologous CD34+-enriched cell fraction that contained CD34+ cells transduced with a retroviral vector encoding the human ADA complementary DNA sequence (GSK2696273) as part of single-arm, open-label studies or compassionate use programs. Overall survival was 100% over 2.3 to 13.4 years (median, 6.9 years). Gene-modified cells were stably present in multiple lineages throughout follow up. GT resulted in a sustained reduction in the severe infection rate from 1.17 events per person-year to 0.17 events per person-year (n = 17, patient 1 data not available). Immune reconstitution was demonstrated by normalization of T-cell subsets (CD3+, CD4+, and CD8+), evidence of thymopoiesis, and sustained T-cell proliferative capacity. B-cell function was evidenced by immunoglobulin production, decreased intravenous immunoglobulin use, and antibody response after vaccination. All 18 patients reported infections as adverse events; infections of respiratory and gastrointestinal tracts were reported most frequently. No events indicative of leukemic transformation were reported. Trial details were registered at www.clinicaltrials.gov as #NCT00598481.
Introduction
Adenosine deaminase (ADA) deficiency, an autosomal-recessive monogenic disorder of purine metabolism, leads to accumulation of toxic deoxyadenosine and deoxyadenosine triphosphate (dAXP). In patients with little or no residual enzyme activity, this results in a fatal, if untreated, severe combined immunodeficiency (ADA-SCID), characterized by profound lymphopenia; impaired differentiation and function of T, B, and natural killer (NK) cells1,2; cognitive impairment and auditory defects2,3; and other systemic problems (including hepatic abnormalities4). From birth, patients with ADA-SCID experience life-threatening opportunistic infections, chronic diarrhea, and failure to thrive. The condition is rare, with a reported incidence ranging from 0.22 to 0.68 per 100 000 live births.5-8
There remains a significant unmet need for treatment options that improve long-term survival. Hematopoietic stem cell transplantation (SCT) from an allogeneic human leukocyte antigen (HLA)-compatible sibling donor is the preferred treatment. However, <25% of infants have a suitable HLA-matched related donor available, making HLA-matched unrelated donor (MUD) transplant an acceptable alternative.9 A study investigating outcomes in children with ADA-SCID found 6.5-year survival rates of 86% and 83% from matched sibling and matched family donors, vs 67% from MUD transplants.10 Survival was lowest for haploidentical (43%) and mismatched unrelated donors (29%). Moreover, the use of alternative sources of stem cells typically requires a conditioning regimen and posttreatment immunosuppression to prevent graft-versus-host disease (GVHD).10
ADA-SCID may be treated by enzyme replacement therapy (ERT) with polyethylene-glycol–modified bovine ADA (PEG-ADA).11,12 ERT improves immune function,13 decreases incidence of severe infections, and supports growth.12 Survival may be as high as 78% over 20 years. However, PEG-ADA is expensive and has limited availability in some countries,14 while decreasing lymphocyte counts and functionality over time (possibly because of the development of anti-ADA neutralizing antibodies3) leave patients susceptible to infection, autoimmunity, and malignancy.14,15
Autologous transplant of hematopoietic stem cells corrected by gene transfer has been investigated as an alternative therapeutic approach. Previously, transplant with autologous gene-corrected hematopoietic stem cells in 10 patients with ADA-SCID detoxified purine metabolites, increased T-cell counts, and normalized T-cell function during a clinical follow-up period ranging from 1.8 to 8 years.16-18 Here, we expand on those data with long-term (2.3 to 13.4 years; median, 6.9 years) safety and efficacy results in those and 8 additional patients.
Materials and methods
Study design
We report here on an integrated data set from 18 subjects enrolled via 2 pilot studies,16,17 a pivotal study17 with a long-term follow-up (LTFU) component, and a compassionate use program (CUP) according to Italian Ministerial Decree May 8, 2003 (D.M. 8/5/2003). LTFU permitted enrollment of patients from the pilot studies and CUP to participate in long-term assessments beyond the initial follow-up period. All studies were nonrandomized, single arm, and open label. Patients were screened to determine study eligibility. During the pretreatment phase, a central venous catheter was placed and backup stem cells were harvested and cryopreserved (in case of poor engraftment or technical issues with product manufacture). For patients on PEG-ADA, ERT was discontinued at a median of 18 days (range, 10-22) before gene therapy (GT), depending on ERT schedule. On day 4 before GT, bone marrow was harvested and CD34+ cells purified for transduction. Patients received low-dose busulfan preconditioning followed by infusion of gene-transduced autologous CD34+ cells. Patients were hospitalized for treatment and for up to 3 months post-GT. LTFU is ongoing. The pivotal study and LTFU are registered at www.clinicaltrials.gov as #NCT00598481. LTFU and CUP were administered at San Raffaele Scientific Institute in Milan (Italy) and approved by the institutional ethics committee and the Italian national regulatory authorities. Patient 1 was enrolled through Hadassah Hebrew University Hospital in Jerusalem (Israel).
Patients
Eligibility criteria included patients with ADA-SCID who lacked an HLA-identical sibling donor and (1) had received ≥6 months of PEG-ADA treatment with demonstrated inefficacy or intolerance or (2) for whom PEG-ADA was not a long-term treatment option. Informed consent was signed by all study patients’ parents.
GT
Full details of CD34+ cell transduction have been reported previously.16,17 Mononuclear cells were isolated from bone marrow harvested under general anesthesia. CD34+ cells were purified, stimulated, and transduced with the human ADA complementary DNA sequence carried in a retroviral vector to produce GSK2696273. The same vector was used for all patients. Busulfan preconditioning was administered at 2 mg/kg per day divided into 4 doses of 0.5 mg/kg on days −3 and −2. The total final dose of busulfan was 4 mg/kg, ∼25% of the typical myeloablative regimen.19,20 On day 0, patients were infused with the transduced autologous CD34+-enriched cell fraction. Acyclovir and fluconazole prophylaxis were continued until immune reconstitution.
Outcomes
Survival was the key efficacy end point assessed. Intervention-free survival (defined as survival without receiving a post-GT SCT or continuous PEG-ADA treatment of ≥3 months) and infection rates were also assessed. Vector copy number, lymphocyte ADA activity,21 and red blood cell (RBC) dAXP levels were used to assess engraftment and transgene function. For dAXP analysis, adequate systemic metabolic detoxification was classified as concentrations <100 nmol/mL dAXP based on observations in patients receiving HLA-matched SCTs.21-25 Immune reconstitution measures included lymphocyte subset counts,26,27 T-cell receptor excision circle (TREC) analysis, and T cell proliferative capacity.28 Physical growth was monitored. Post-hoc analyses included transduced cell engraftment in CD15+ and CD34+ cells, antibody response to vaccination, and duration of intravenous immunoglobulin (IVIG) administration. In addition, data for the duration of hospitalization post-GT and intervention-free survival for Patient 1 were collected by San Raffaele Scientific Institute but are not contained within the GSK database.
Adverse events (AEs) were recorded and reported using Good Clinical Practice guidelines. For the purposes of this study, “related to study treatment” was only applied to AEs related to the GT product and not to accompanying procedures (eg, central venous catheter placement). Clinical examinations, hematologic/immunologic/biochemical/molecular testing, and imaging were used to monitor efficacy and safety. Retroviral insertion site (RIS) analysis using linear amplification-mediated polymerase chain reaction (PCR) assessed the overall integration profile.29
Statistical analysis
Not all patients had data available for each time point due to exact timing of follow-up visits. For efficacy end points, all available data through the 8-year time points were used for statistical analysis. Within-patient changes were used for efficacy comparisons. Efficacy data collected after receipt of ≥3 continuous months of PEG-ADA or allogeneic SCT were excluded. For dichotomous and categorical end points, percentages and 95% confidence intervals were calculated. For continuous end points, the changes from pretreatment baseline were analyzed by mixed-model repeated-measures analyses fitting visit and baseline as fixed effects and subject as the random effect. Where normality assumptions were violated, the data were log transformed. No primary end point or adjustments for multiplicity were predefined for the integrated analysis of all 18 patients. The statistical significance of results has therefore not been determined, ie, P values have not been calculated and are not presented.
No deaths occurred during the studies; therefore, no formal survival analysis was performed. However, Kaplan-Meier survival curves were plotted for intervention-free survival. Severe infections were defined as those leading to hospitalization or prolonging hospitalization and were reported as the number of severe infections per person-year of observation. Events during the 0- to 3-month posttreatment monitoring period were not included in AE analysis, as patients were generally confined to the hospital (per protocol) with an expected risk of infections due to incomplete immune reconstitution and the neutropenia following busulfan conditioning. Height and weight for all patients were compared with age-appropriate growth charts.30-32
Data were analyzed by M.A., A.A., J.A., I.B., F. Carlucci, M.P.C., E.D.B., F.D., F.F., S.G., R.R., K.R., M.G.R., and A.T. and were available to all authors.
Results
Study population
All 18 patients with ADA-SCID received a median GT dose of 9.2 × 106 CD34+ cells/kg (range: 0.9 to 18.2 × 106 CD34+ cells/kg) at a median age of 1.7 years (range, 0.5 to 6.1 years) (supplemental Table 1, available on the Blood Web site). Patient 2 received a second GT treatment in the absence of further busulfan conditioning (Table 1). Patients discontinued PEG-ADA a median of 18 days prior to GT. Following GT, patients were hospitalized for a median of 45 days (range, 34 to 110 days).
Table 1.
Patient summaries
| Patient number | Clinical study | Age at GT, y | Sex | ADA mutation | Baseline height, cm (centile) | Baseline weight, kg (centile) | Race | Previous treatment (duration) | Dose, CD34+ cells × 106/kg | VCN, copies/ cell | Duration of follow up, y* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pilot 1 | 0.6 | F | H17P (homozygous) | NR | 6.5 (fifth) | White/Arabic | None | 8.5 | 2.28 | 13.4 |
| 2 | Pilot 2 | 2.4, 5.0† | F | L107P, R211H | 88 (25th) | 11.5 (25th) | White | Haplo-SCT | 0.9 | NR | 13.1‡ |
| 2.1 | 2.15 | ||||||||||
| 3 | Pilot 2 | 1.0 | M | G74V, R282Q | NR | 8.8 (15th) | White | Haplo-SCT | 6.7 | 0.85 | 11.9 |
| 4 | Pivotal | 1.9 | F | R282Q (homozygous) | 72 (less than first)§ | 7.8 (less than first)§ | White/Arabic | Haplo-SCT; PEG-ADA (2 mo) | 3.8 | NR | 11.6 |
| 5 | Pivotal | 1.6 | F | G216R, E319fsX3 | 71 (less than first) | 10.0 (25th) | White | PEG-ADA (15 mo) | 9.6 | 1.89 | 9.9 |
| 6 | Pivotal | 5.6 | M | R211H (homozygous) | 104 (less than third) | 14.5 (less than third) | White | PEG-ADA (65 mo) | 9.5 | 1.05 | 9.0 |
| 7 | Pivotal | 1.5 | M | G216R, S291L | 76 (first) | 9.0 (third) | White | PEG-ADA (13 mo) | 9.0 | 0.83 | 7.0‡ |
| 8 | Pivotal | 2.8 | F | H15D (homozygous) | 79 (less than first) | 7.4 (less than first) | White | PEG-ADA (32 mo) | 10.6 | 0.12 | 2.3ঠ|
| 9 | Pivotal | 1.4 | M | Exon 5 splice-donor site +2 | 76 (5th) | 9.8 (25th) | White | PEG-ADA (10 mo) | 13.6 | 0.57 | 7.0 |
| 10 | Pivotal | 1.8 | F | G216R (homozygous) | 81 (15th) | 10.2 (25th) | White/Arabic | Haplo-SCT; PEG-ADA (11 mo) | 10.7 | 0.35 | 6.9 |
| 11 | Pivotal | 1.6 | M | R211H (homozygous) | 76 (less than first) | 8.1 (less than first) | White | PEG-ADA (8 mo) | 6.34 | 0.17 | 7.0 |
| 12 | Pivotal | 1.3 | M | G216R, E319fsX3 | 82 (85th) | 12.6 (95th) | White | PEG-ADA (12 mo) | 11.5 | 0.14 | 6.2 |
| 13 | Pivotal | 0.5 | M | Exon 6, splice donor site +5 | 60 (less than first) § | 5.9 (less than first) | Asian | PEG-ADA (1 mo) | 18.2 | 0.06 | 6.2 |
| 14 | Pivotal | 6.1 | M | G216R, exon 10, deletion +6 | 114 (15th) | 21.8 (50th) | White | PEG-ADA (71 mo) | 6.0 | 0.54 | 5.1‡ |
| 15 | Pivotal | 2.5 | F | R156C (homozygous) | 82 (less than first) | 11.5 (15th) | White/Arabic | PEG-ADA (12 mo) | 5.9 | 0.38 | 5.0 |
| 16 | CUP | 2.3 | M | c.7C→T (p.Q3X) | 97 (97th)§ | 16.0 (97th)§ | African | PEG-ADA (23 mo) | 6.9 | 0.17 | 3.2 |
| 17 | CUP | 0.7 | M | c.881C→A (p.T294K) | 69 (15th)§ | 7.9 (15th)§ | African | PEG-ADA (7 mo) | 13.0 | 0.24 | 3.7‡# |
| 18 | CUP | 2.1 | M | c.956_960delAAGAG (p.Glu319Glyfsx3) | 88 (50th)§ | 12.0 (25th)§ | White/Arabic | PEG-ADA (24 mo) | 9.9 | 0.11 | 2.6‡ |
Baseline and initial follow-up data for patients 1 to 10 have been previously described.17 Date of data cutoff was 8 May 2014.
F, female; haplo-SCT, haplo-identical stem cell transplant; M, male; NR, not recorded; VCN, vector copy number.
Duration of follow-up was calculated from date of last assessment relative to the date of GT.
Second dose of GT did not include busulfan preconditioning.
Patient remained on IVIG supplementation at data cut off.
Screening/prebaseline values are shown where baseline values are unavailable.
Patient withdrew from study (reason: investigator discretion) when she became a candidate for an allogeneic sibling donor stem cell transplant.
Patient withdrew from study (reason: unsuccessful response to GT) when he became a candidate for an allogeneic sibling donor stem cell transplant.
Four patients previously received an unsuccessful haploidentical SCT, and 15 patients previously received ERT with PEG-ADA (Table 1). At baseline, 8 patients (44%) exhibited failure to thrive (supplemental Table 1). Baseline central nervous system (CNS) history included abnormal findings on brain magnetic resonance imaging in 7 patients (39%), psychomotor retardation or delayed development in 6 patients (33%), and auditory abnormalities in 5 patients (28%).
Survival
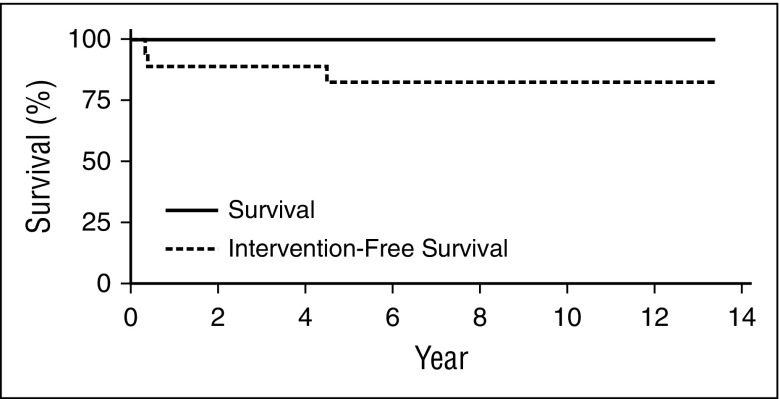
All 18 patients were alive at data cut (May 8, 2014) after a median follow-up of 6.9 years (range, 2.3 to 13.4 years) (Figure 1), and 15 of 18 patients (83%) did not require further intervention.
Figure 1.
GT resulted in 100% survival, with few patients requiring intervention. No deaths have occurred in this patient population. Intervention-free survival was defined as survival without post-GT SCT or PEG-ADA use for a continuous period of ≥3 months. Three patients resumed PEG-ADA therapy following GT; 2 of those patients later received a matched-sibling SCT (previously unavailable before GT). Intervention-free survival represents a sensitivity analysis of the overall survival rate.
Engraftment and purine metabolism
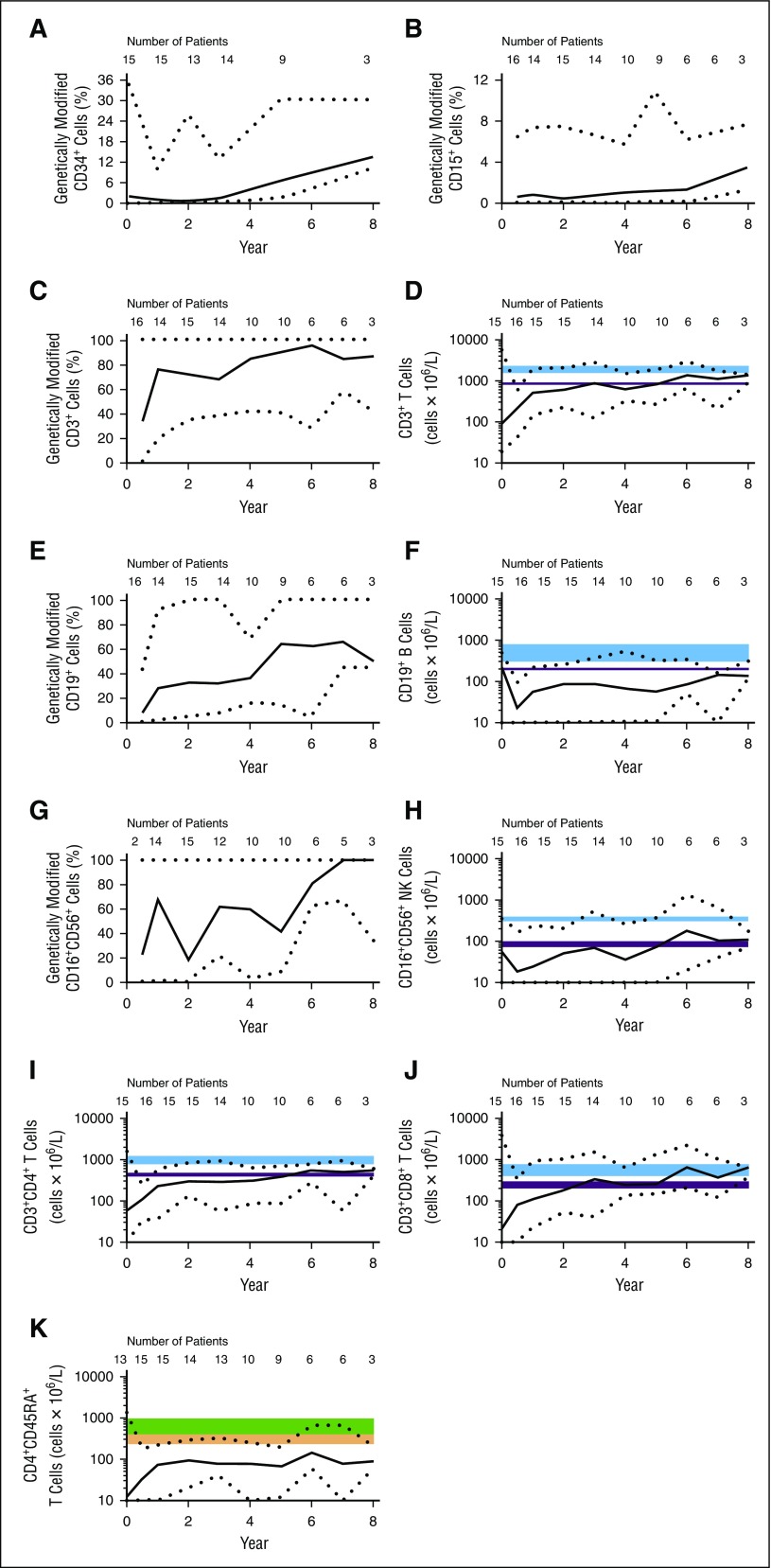
Quantitative real-time PCR (qRT-PCR) was used to evaluate the prevalence of gene-transduced cells following GT. For the first 3 years of follow up, a median of 1% to 2% of the bone marrow CD34+ stem cells were gene modified (Figure 2A). During LTFU, the percentage of gene-modified CD34+ cells appeared to increase, but strong conclusions were hampered by the small patient numbers at later time points. Gene-modified CD15+ granulocytes in peripheral blood (Figure 2B) and multiple bone marrow lineages also persisted long-term (data not shown).
Figure 2.
Transduced cells persist long-term and proliferate. All values shown are medians; black dotted lines represent minimum and maximum values. The number of patients contributing to each data point is indicated on the graph. Day 0 indicates baseline (pre-GT) values. (A) CD34+ cells were purified from bone marrow aspirate. (B-K) CD15+ cells (B), CD3+ cells (C-D), CD19+ cells (E-F), CD16+CD56+ cells (G-H), CD3+CD4+ cells (I), CD3+CD8+ cells (J), and CD4+CD45RA+ cells (K) were purified from peripheral venous blood. (A-C,E,G) Genotype was determined by qRT-PCR. (D,F,H-K) Absolute cell counts are also shown; 10 was set as the minimum value for graphs using a logarithmic scale. The shaded blue and purple regions represent median and fifth percentile values, respectively, in normal children. The top edges correspond to levels in children ages 2 to 5 years; bottom edges correspond to levels in children ages 10 to 16 years. Values for children ages 5 to 10 typically fall within the shaded areas.26 The green and orange shaded areas represent median and 10th percentile values, respectively, in normal children. Top edges correspond to levels in children ages 2 to 6 years; bottom edges correspond to levels in children ages 12 to 18 years. Values for children ages 6 to 12 years fall within the shaded areas.27
As predicted by the known survival advantage for ADA-expressing lymphocytes,33,34 after 1 year, gene-modified cells represented ∼70% of the CD3+ lymphocyte population in peripheral blood; the level of gene-modified CD3+ cells remained high throughout LTFU (Figure 2C). The proportion of gene-modified CD19+ B cells increased more slowly but after year 5 stabilized at ∼60% of the B-cell population (Figure 2E). Gene-modified CD16+CD56+ NK cells also increased (Figure 2G).
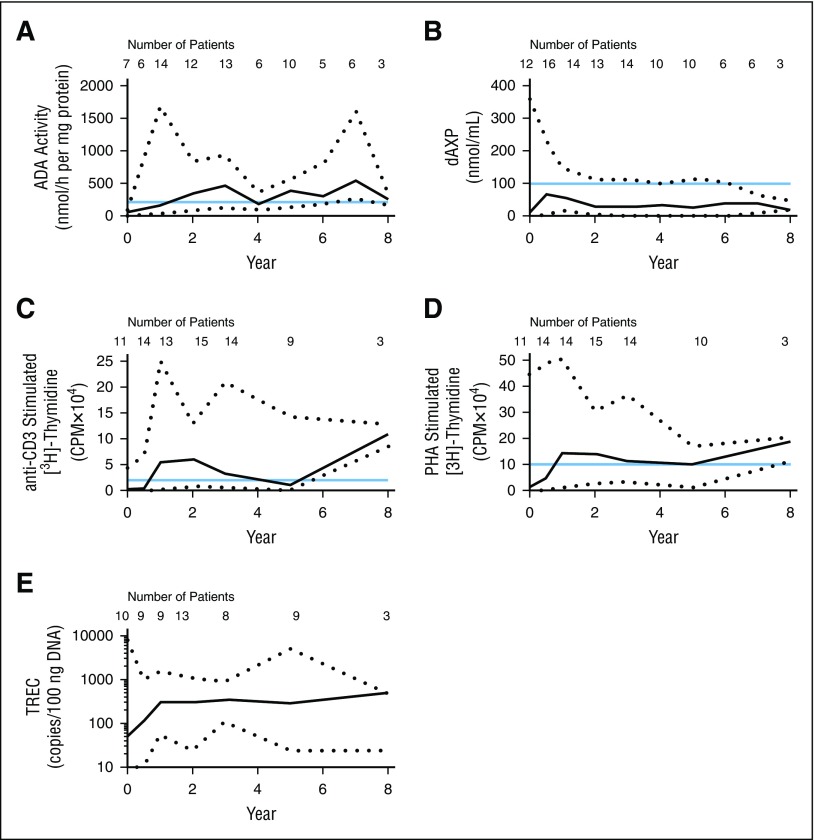
Lymphocyte ADA enzyme activity in peripheral blood increased from a median of 65 to 162 nmol/h/mg at 1 year (Figure 3A). Throughout LTFU, median lymphocyte ADA activity ranged from 184 to 539 nmol/h per mg. The ADA metabolic defect was corrected, producing a durable decrease in dAXP (Figure 3B) to levels in the range of those found in successfully allogeneic transplanted patients (≤100 nmol/mL).10,35 Baseline ADA enzyme activity and RBC dAXP levels were likely affected by ERT carryover effects.
Figure 3.
GT supports ADA activity and lymphocyte function. All values shown are medians; black dotted lines represent minimum and maximum values. The number of patients contributing to each data point is indicated on the graph. (A) Lymphocyte ADA levels were measured in peripheral venous blood. ADA activity of ≥210 nmol/h per mg (blue line) represents 10% of normal and was considered as a threshold for adequate activity in subjects with ADA-SCID following treatment.21 (B) RBC dAXP levels were measured in peripheral venous blood. The blue line denotes dAXP levels found in successfully allogeneic transplanted patients (≤100 nmol/mL).10,17 (C-D) Proliferative capacity was assessed following challenge with (C) anti-CD3 antibody and (D) phytohemagglutinin (PHA). Blue lines indicate normal response in healthy children.28 (E) TREC abundance in whole blood was determined by qRT-PCR.
Immune reconstitution
Immune reconstitution was observed starting 6 months after GT, with increased median numbers of peripheral CD3+, CD4+, and CD8+ T cells (Figure 2D,I,J). CD16+CD56+ NK cells also increased (Figure 2H). Not all circulating lymphocytes were gene marked. Presumably, the general detoxification allowed for survival of nontransduced cells. Functional thymopoiesis was restored, as evidenced by increased numbers of CD4+CD45RA+ naive T cells (Figure 2K). Following GT, the majority of patients had TREC values increasing above 100 copies/100 ng DNA postbaseline (Figure 3E), although median TREC values remained low relative to age-matched healthy controls at all time points. However, proliferation was demonstrated in response to stimulation with anti-CD3 antibody and phytohemagglutinin (Figure 3C-D), suggesting a restoration of T-cell functionality.
Median CD19+ B-cell counts remained low (Figure 2F). However, functional improvements were observed by IVIG withdrawal and response to vaccination. All 18 patients underwent IVIG replacement or supplementation therapy before and after GT. At data cut, 6 patients (patients 2, 7, 8, 14, 17, and 18) remained on IVIG support, including those that subsequently underwent transplantation. A total of 12 patients discontinued IVIG use (7 of them within 3 years of GT), with stable median serum immunoglobulin G levels within normal ranges throughout LTFU and improved levels of serum immunoglobulin A and immunoglobulin M. Protective antibody-forming capacity was demonstrated with detectable antibodies to pertussis, tetanus toxoid, and hepatitis B surface antigen confirmed in 11, 11, and 8 patients with available data, respectively. Antibody production was durable, with antibodies generally detectable at multiple time points during the follow-up period (data not shown). Data available for 2 patients indicate receipt of live attenuated measles/mumps/rubella vaccination followed by development of protective specific antibodies without complications. All 4 patients who were infected with varicella zoster virus (VZV) during the follow-up period developed VZV-specific antibodies during the infection.
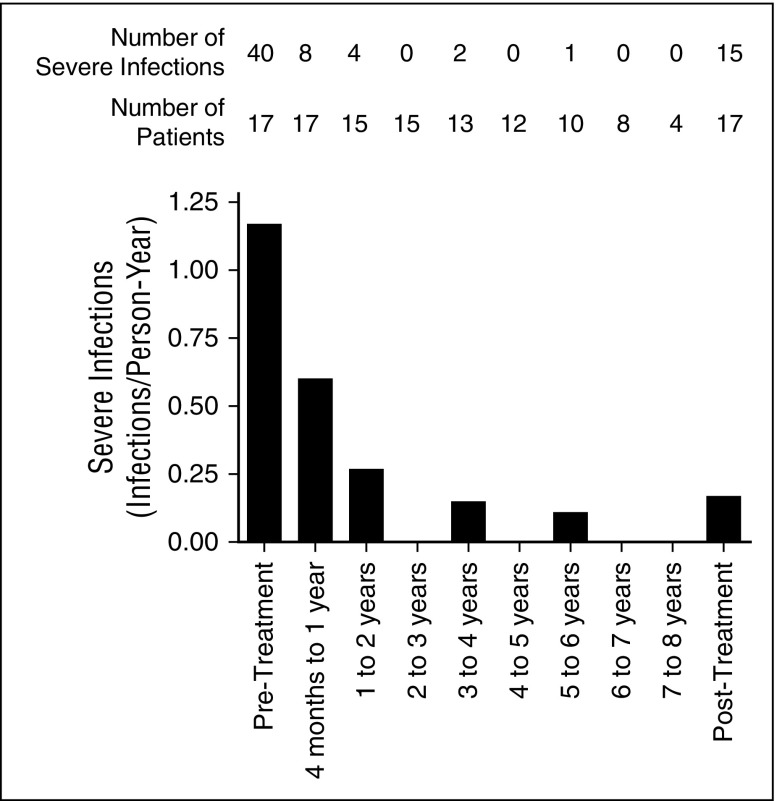
Severe infections
Prior to GT, 40 severe infections occurred in 14 patients (82% of 17 patients with available data) (Figure 4), with an estimated 1.17 severe infections per person-year of observation (data not shown). After GT, 15 severe infections were reported in 10 patients (59% of 17 patients with available data). Of these infections, 12 (80%) occurred between 4 months and 3 years after GT (Figure 4), with a rate of 0.26 severe infections per person-year of observation (data not shown). Between 4 and 8 years of follow up, only 3 severe infections were reported (Figure 4) and the rate dropped to 0.07 severe infections per person-year of observation (data not shown).
Figure 4.
The rate of severe infections declined after GT. Severe infections were defined as infections leading to, or prolonging, hospitalization. The number of patients contributing to each data point and the number of severe infections reported are indicated on the graph. Severe infections in the 3 months immediately following GT were not included in the analysis. Severe infections after the reintroduction of long-term PEG-ADA treatment (patients 2, 8, and 17) were excluded from the analysis.
Growth and activity
Although patients generally remained below the 50th percentile for a normal, age-matched population, most continued to track along their original percentiles for growth (supplemental Figure 1). At data cut, 12 out of 14 patients (86%) surveyed were attending preschool or school, as appropriate for their age. Lansky performance status index was queried in 14 patients; all patients were reported as “fully active, normal” during LTFU, except for patient 10, who had minor restrictions in strenuous physical activity recorded at year 7.
Baseline predictors of efficacy
A variety of baseline covariates were assessed for their ability to predict long-term patient outcomes following GT (supplemental Figure 2 and data not shown). The covariates assessed included age at GT, CD34+ cells/kg dose, cells/kg × vector copy number dose, baseline values for peripheral CD3+ T-cell counts, TREC counts, peripheral RBC levels of dAXP, and body mass index. The outcomes assessed included intervention-free survival and T-cell counts and RBC dAXP levels at year 3. Efficacy was achieved across a range of GT doses and a variety of patient characteristics without any specific predictor for unsuccessful response to GT. Efficacy was observed in patients both with and without previous exposure to PEG-ADA, regardless of the length of the washout period (10-22 days).
Unsuccessful responses to therapy
Three patients (2, 8, and 17) resumed long-term PEG-ADA treatment due to poor immune reconstitution after GT. Patient 2 received the lowest dose of GT, and a second dose was infused without conditioning (Table 1).17 There was no increase in engraftment following the second dose of GT; patient 2 remained on ERT at data cut. For patient 8, engraftment may have been limited by baseline chronic autoimmunity, which required long-term immunosuppressive treatment.17 Patients 8 and 17 subsequently received sibling-matched SCTs, not available before GT, with successful engraftment of donor stem cells.
AEs
AEs concomitant with, or following, GT were reported for all 18 patients (supplemental Table 2). AEs were predominantly grade 1 and grade 2. Patient 1 (treated at Hadassah) had 2 AEs that were considered by the investigator to be related to study treatment: grade 1 hepatic steatosis (fatty liver) after 8.3 years and grade 2 T-cell repertoire alteration (2 Vβ families had decreased polyclonal repertoires) after 13 years. In this cohort of patients, cytopenia, elevated transaminases, and hypertension generally occurred in the 3 months immediately after GT and usually resolved over time. The timing of these events suggests that they may have been related to busulfan conditioning.
A total of 15 patients (83%) reported 39 serious adverse events (SAEs) after treatment, all of which resolved and none of which were fatal or considered related to study treatment (supplemental Table 3). Patient 8 and patient 13 each reported 7 events.
Infection AEs
As expected, AEs of infective etiology were reported for all 18 patients. The 3 most frequently reported of these were usual childhood infections: upper respiratory tract infection, gastroenteritis, and rhinitis (supplemental Table 2).36 Urinary tract infections were also some of the most commonly reported infections. Other infections that are important in immune-compromised populations were not common and occurred predominantly early after GT, including Clostridium difficile infection (patients 5, 13, and 16), Aspergillus infection (patients 3 and 13), cryptosporidial gastroenteritis (patient 7), pulmonary mycosis and cytomegalovirus infection (patient 17), and Epstein-Barr virus infection (patient 6). All these infections resolved and, of these patients, patient 17 was the only one considered to have an unsuccessful response to therapy.
Twenty-four of the 39 SAEs reported after treatment were infections (62%), with the most common including device-related infections (n = 5), gastroenteritis (n = 3), and pneumonia (n = 3). There were 2 infectious SAEs of VZV (2 additional patients had nonserious events of VZV); all were treated with acyclovir and resolved.
Neurologic AEs
A total of 14 patients had neurologic, CNS, or hearing impairments ongoing at baseline; 10 of these 14 patients also had similar events on or after GT. Most were grade 1 or grade 2 (none were grade 4), none were serious, and none were considered by the investigator to be related to GT. These events are consistent with the underlying ADA-SCID condition.23,25
Autoimmunity AEs
Hypothyroidism, antinuclear antibody–positive test results, and autoimmune hemolytic anemia were reported prior to GT in 5 patients. Following GT, 12 patients reported 26 autoimmunity-related events, which included 6 SAEs. Grade 1 antinuclear antibody positivity was the most frequently reported autoimmune event across patients, with 5 events in 5 patients. However, the clinical relevance of these isolated laboratory events is not clear. A total of 4 patients had 6 autoimmune SAEs, which all resolved, including antibody-mediated neutropenia, autoimmune thrombocytopenia (2 events), autoimmune aplastic anemia, autoimmune hepatitis, and Guillain-Barré syndrome (supplemental Table 3).
Vitals and laboratory measures
Over the course of these studies, there were no clinically significant changes from baseline in mean vital sign values or ECGs; no worrying pattern in clinical chemistry was observed. Hemoglobin, hematocrit, platelet count, and white blood cell count all initially decreased between baseline and day 14, as expected, and then increased over the following year. Total white blood cell counts increased above pretreatment values but remained within normal ranges.
Oncogenic potential
No events indicative of myelodysplasia or leukemic transformation were reported in any patient at any time. B-cell immunoglobulin production, T-cell receptor V-β repertoire, peripheral blood smears, cytogenetic karyotype analysis, bone marrow morphology, and immunophenotype showed no changes of clinical concern (data not shown). An abdominal adipose tumor was found in 1 patient but was likely not related to the GT.37
RIS analysis evaluated the genomic locations of viral integrations. Data were available from 14 patients; a total of 2333 unique insertion sites were evaluated, and insertion sites present at >10% abundance were mapped against the nearest genes. As expected for a semirandomly inserting retrovirus, all but 1 patient had insertion sites close to genes linked with cancer. RISs were detected relatively frequently adjacent to or within CCND2 (5 patients) and LMO2 (6 patients), but clones with these inserts have been present for several years and are stable.38 Additional details from RIS analyses will be reported in a separate article.
Discussion
All patients treated in this GT program have survived after median follow up of 6.9 years. These studies did not incorporate a control group; however, published historical reference data for 15 patients who underwent SCT from MUDs suggest survival with that approach is ∼67% after a median follow-up period of 6.5 years.10 For patients treated with PEG-ADA, the estimated 20-year survival rate is 78%.3 Meaningful comparisons between these various patient populations are limited by differences in the baseline characteristics and by differences in data collection (survived patients vs all patients). Last-observation carried forward data are currently unavailable for the standard-of-care therapies.
The 83% intervention-free survival rate of GT-treated patients is similar to the matched sibling/family donor overall survival rate (86% and 83%, respectively) described elsewhere, which included patients who received >1 transplant.10 Although there are limitations when comparing outcomes between different patient cohorts, it is relevant that intervention-free survival following GT was higher than overall survival in children who received SCTs from MUD (67%) or haploidentical donors (43%)10 (ie, the target population for these studies).
Increases in peripheral CD3+ T cells and T-cell subsets, evidence of increased thymopoiesis (TRECs), and T-cell functionality were observed, suggesting development of functional T-cell populations with diverse repertoires. Gene-modified cells in multiple lineages (granulocytes, erythroid cells, T cells, B cells, and NK cells) were stable and maintained from year 1 throughout LTFU. These changes parallel the decrease in severe infection rates, which dropped post-GT from year 1 onwards.
Consistent with previous reports regarding B-cell reconstitution after SCT or GT,10,39 peripheral B-cell counts remained low and did not increase after GT. However, improvements in B-cell function were demonstrated, as late as 4 years after GT, by increased immunoglobulin production, detectable antibodies following common childhood vaccinations, and decreased dependence on IVIG use.
Peripheral blood lymphocyte ADA activity levels increased after treatment and remained stable during follow up, resulting in normalization of dAXP levels in RBCs. This correction of the metabolic defect matches the timing of immune recovery. Improved metabolic function and immune recovery are stable, maintained throughout the duration of follow up, and provide strong evidence supporting the persistence of efficacy. Although comprehensive long-term outcomes were not formally collected as part of the study, regular school attendance and appropriate height and weight-gain data also suggest that GT is a treatment that produces improved quality of life over the medium-term.
There was no evidence of GT mitigation of preexisting CNS abnormalities, and some patients were diagnosed with CNS abnormalities following GT. This is consistent with CNS outcomes for ADA-SCID patients treated with SCT40 and supports the hypothesis that CNS manifestations in ADA-SCID patients do not improve with transplantation of either wild-type cells or gene-modified autologous cells.
Clinical benefit was achieved across a range of GT doses as well as diverse baseline disease characteristics and demographics. Previously, older age at the time of treatment has been associated with multiple disease-relevant comorbidities, which complicate treatment and lead to a lower survival rate for conventional SCT.41 In the studies reported here, patient ages ranged from 0.5 years to 6.1 years, with no correlation between age at the time of GT and treatment outcomes. Efficacy was observed in both very young and older children.
Overall, the safety findings are consistent with those for an ADA-SCID population that has undergone low-dose myeloablative busulfan conditioning. Following GT, all patients reported infection AEs, including severe, opportunistic, and urinary tract infections. Infections of the respiratory and gastrointestinal tracts were the most frequently reported AEs; these organ systems are targets for infections in both ADA-SCID42 and normal pediatric populations.36 Following GT, the majority of infectious AEs were not serious, and all infectious AEs resolved. Overall, the rate of severe infections declined from 1.17 infections per person-year at baseline to 0.07 infections per person-year from 4 to 8 years after treatment.
γ retroviral vectors using a similar Moloney murine leukemia virus backbone, but with slightly different envelopes and gene expression systems, have demonstrated clinical and biological efficacy in previous trials.43 Unfortunately, in some cases, hematologic malignancies occurred, including 5 of 20 patients affected by X-linked SCID,44 3 of 13 patients treated for chronic granulomatous disease,45-47 and 7 of 10 patients with Wiskott-Aldrich syndrome.48 In some patients, leukemia and myelodysplasia occurred within 3 years of GT.44 In contrast, none of the 18 patients with ADA-SCID who have received GSK2696273 or other comparable γ retroviral vectors (n = 22) have developed leukemia in an extended follow-up period.49,50
Retroviral-mediated cell transduction introduces a risk of oncogenesis through either activation of proto-oncogenes, such as LMO2, EVI1, and CCND2, or the formation of de novo gene expression products.51 Among other ADA-SCID gene therapies, vector integrations have demonstrated stable engraftment in multipotent hematopoietic stem cells.38 Integrations were also found in patients with ADA-SCID within and/or near potentially oncogenic loci (LMO2 and CCND2), but to date, they have not resulted in selection or expansion of malignant cell clones in vivo.38,52 The absence of leukemia in patients described here, despite the detection of insertions close to LMO2 and CCND2, is further evidence of the favorable medium-term safety of this treatment. Given the similarities between the viral backbones, differences in safety profiles between patients with ADA-SCID and patients with Wiskott-Aldrich syndrome and X-linked SCID may involve the nature of the gene of interest or the disease background. In contrast to both WASp and IL2RG proteins, which are involved in cell response to proliferative stimuli, ADA is a metabolic “housekeeping” protein that is constitutively expressed in all cell types.
Treatment with ADA GT has several advantages that complement existing treatment options. Patients who receive SCTs from unrelated HLA-matched donors are at risk of poor engraftment and GVHD; chemotherapy and immunosuppressive drugs are generally required, which increase the risk of toxicity events and severe infections.10 In contrast, ADA GT eliminates the risk of GVHD and reduces the need for ongoing immunosuppressive treatment following the initial busulfan exposure. Furthermore, GT can be administered promptly following diagnosis, reducing the risk of continued clinical impairment during a protracted donor search.
Gene-modified hematopoietic stem cells are stable for up to 13 years post-GT. The immune system supported by these gene-modified cells is also stable and provides functional benefit over the long-term. In contrast, the immune reconstitution provided by ERT may decrease over time,9,14 perhaps because exogenous PEG-ADA corrects T-cell function less effectively than the intracellular enzyme or because dATP is not completely cleared in the thymus.3 Furthermore, the development of anti–PEG-ADA antibodies may require an increase in dosage, administration of corticosteroids, or cessation of therapy.53-56 PEG-ADA requires lifelong weekly or biweekly intramuscular injections. In contrast, most of our patients display improvement that is sustained over many years following a single GT treatment. No difference in outcome was observed between patients with prior PEG-ADA exposure and those who were PEG-ADA naive (data not shown).
The leukemia-free, 100% survival rate for this program is consistent with those reported from other γ retroviral GT trials in ADA-SCID, with over 50 patients treated, using a variety of vectors, and reported to be alive.57 However, the exact mechanisms that support the improved safety of γ retroviral vectors with intact long terminal repeats in ADA-SCID relative to other primary immune deficiencies are not well understood. It is therefore important to continue long-term outcome monitoring in all ADA-SCID patients to allow accurate comparisons of this potential new treatment with alternative options and the emerging class of lentiviral-based GT vectors, which do not include viral enhancers but support expression of the same ADA gene product.58 Most patients described here, for whom other treatment options were limited, achieved stable ADA enzyme activity in lymphoid cells and correction of the metabolic defect in RBCs and have been clinically well, without needing reintroduction of ERT or subsequent SCT. Follow up of these patients is ongoing, with additional future focus on quality-of-life measures in this population.
Acknowledgments
The authors thank all the medical and nurse personnel of the Pediatric Immunohematology and Hematology and Bone Marrow Transplant Unit of San Raffaele Hospital (Milan), the personnel of the San Raffaele Telethon Institute for Gene Therapy Clinical Trial Office, and all patients who participated in this study and their families. The authors would like to thank, in particular, Clara Soliman for supervision of nursing activities, Luciano Callegaro for regulatory and management support to the clinical trial, and Professor Roberto Miniero (University of Catanzaro) for his contribution to patients’ treatment in the initial phase of the study. The authors thank Michela Gabaldo for her continuous support in the alliance between Telethon/San Raffaele and GSK. Writing assistance was provided by Molly Nixon and Angela Winnier of Synchrogenix, a Certara Company (funded by GSK). Project management support was provided by Christopher Cornell and Barbara Kravitz of GSK. In addition, the authors acknowledge Giuliana Tomaselli and Samih El Hossary for their support of patients.
Financial support for these studies was provided to A.A. and M.G.R. by Fondazione Telethon and European Union Project FP7 CELL PID and to A.A. by GlaxoSmithKline.
Footnotes
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.A. oversaw the study, enrolled patients, and analyzed data. A.A. designed and coordinated the studies as principal investigator, had full access to all the data, and takes responsibility for the integrity of the data. J.A. oversaw the study and data analysis. F.B. and R.P. clinically managed patients and collected clinical data for case report forms. M.B. served as regulatory officer and carried out essential documentation management. R.G.B. referred and treated patients and provided advice on the busulfan target dose. I.B., F.D., and S.G. performed experimental molecular and biological analyses on patient samples. F. Carlucci and A.T. designed the biochemical approach to ADA-SCID diagnosis and therapy monitoring, analyzed samples, and take responsibility for the integrity of biochemical data. M.C. coordinated patient evaluations and managed biological samples. L.C. collected data and performed quality control. M.P.C. was a study investigator, enrolled patients in the study, treated and followed up the patients, oversaw the study, and reviewed and analyzed the data. F. Ciceri was a study co-investigator, enrolled patients, and interpreted data. E.D.B. reviewed, verified, and interpreted data. F.F. was a study investigator, provided clinical care of patients, and collected, reviewed, and analyzed data. E.G., J.M.P., P.S., and I.T. enrolled patients. R.R.R. participated in the review, analysis, and interpretation of data. K.R. planned and performed data analysis. M.G.R. conceived of and oversaw the study and reviewed the data; and all authors reviewed drafts of the paper and approved the final version of the manuscript.
Conflict-of-interest disclosure: J.A., E.D.B., R.R.R., and K.R. are employees of, and own shares in, GlaxoSmithKline. The remaining authors declare no competing financial interests.
Correspondence: Alessandro Aiuti, San Raffaele Telethon Institute for Gene Therapy, Via Olgettina 58, Dibit 2A2, 20132 Milan, Italy; e-mail: alessandro.aiuti@hsr.it.
References
- 1.Hirschhorn R. Immunodeficiency disease due to deficiency of adenosine deaminase. In: Ochs HD, Smith CIE, Puck JM, editors. Primary Immunodeficiency Diseases. New York, NY: Oxford University Press; 1999. [Google Scholar]
- 2.Picard C, Al-Herz W, Bousfiha A, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency 2015. J Clin Immunol. 2015;35(8):696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaspar HB, Aiuti A, Porta F, Candotti F, Hershfield MS, Notarangelo LD. How I treat ADA deficiency. Blood. 2009;114(17):3524–3532. doi: 10.1182/blood-2009-06-189209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bollinger ME, Arredondo-Vega FX, Santisteban I, Schwarz K, Hershfield MS, Lederman HM. Brief report: hepatic dysfunction as a complication of adenosine deaminase deficiency. N Engl J Med. 1996;334(21):1367–1371. doi: 10.1056/NEJM199605233342104. [DOI] [PubMed] [Google Scholar]
- 5.Ryser O, Morell A, Hitzig WH. Primary immunodeficiencies in Switzerland: first report of the national registry in adults and children. J Clin Immunol. 1988;8(6):479–485. doi: 10.1007/BF00916954. [DOI] [PubMed] [Google Scholar]
- 6.Yee A, De Ravin SS, Elliott E, Ziegler JB Contributors to the Australian Paediatric Surveillance Unit. Severe combined immunodeficiency: a national surveillance study. Pediatr Allergy Immunol. 2008;19(4):298–302. doi: 10.1111/j.1399-3038.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- 7.Verbsky JW, Baker MW, Grossman WJ, et al. Newborn screening for severe combined immunodeficiency; the Wisconsin experience (2008-2011). J Clin Immunol. 2012;32(1):82–88. doi: 10.1007/s10875-011-9609-4. [DOI] [PubMed] [Google Scholar]
- 8.Vogel BH, Bonagura V, Weinberg GA, et al. Newborn screening for SCID in New York State: experience from the first two years. J Clin Immunol. 2014;34(3):289–303. doi: 10.1007/s10875-014-0006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirschhorn R, Grunebaum E, Roifman C, Candotti F. Immunodeficiency due to defects of purine metabolism. In: Ochs HD, Smith CIE, Puck JM, editors. Primary immunodeficiency diseases: a molecular and genetic approach. New York, New York: Oxford University Press; 2014 [3rd edition] [Google Scholar]
- 10.Hassan A, Booth C, Brightwell A, et al. Inborn Errors Working Party of the European Group for Blood and Marrow Transplantation and European Society for Immunodeficiency. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood. 2012;120(17):3615–3624, quiz 3626. doi: 10.1182/blood-2011-12-396879. [DOI] [PubMed] [Google Scholar]
- 11.Hershfield MS, Buckley RH, Greenberg ML, et al. Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med. 1987;316(10):589–596. doi: 10.1056/NEJM198703053161005. [DOI] [PubMed] [Google Scholar]
- 12.Hershfield MS. PEG-ADA replacement therapy for adenosine deaminase deficiency: an update after 8.5 years. Clin Immunol Immunopathol. 1995;76(3 Pt 2):S228–S232. doi: 10.1016/s0090-1229(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg K, Hershfield MS, Bastian J, et al. T lymphocyte ontogeny in adenosine deaminase-deficient severe combined immune deficiency after treatment with polyethylene glycol-modified adenosine deaminase. J Clin Invest. 1993;92(2):596–602. doi: 10.1172/JCI116626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan B, Wara D, Bastian J, et al. Long-term efficacy of enzyme replacement therapy for adenosine deaminase (ADA)-deficient severe combined immunodeficiency (SCID). Clin Immunol. 2005;117(2):133–143. doi: 10.1016/j.clim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Baffelli R, Notarangelo LD, Imberti L, et al. Diagnosis, treatment, and long-term follow-up of patients with ADA deficiency: a single-center experience. J Clin Immunol. 2015;35(7):624–637. doi: 10.1007/s10875-015-0191-z. [DOI] [PubMed] [Google Scholar]
- 16.Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296(5577):2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 17.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 18.Selleri S, Brigida I, Casiraghi M, et al. In vivo T-cell dynamics during immune reconstitution after hematopoietic stem cell gene therapy in adenosine deaminase severe combined immune deficiency. J Allergy Clin Immunol. 2011;127(6):1368–75.e8. doi: 10.1016/j.jaci.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Bartelink IH, Bredius RGM, Belitser SV, et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(2):231–241. doi: 10.1016/j.bbmt.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 20. Pierre Fabre Médicament. Busilvex [summary of product characteristics]. 2014. Available at: www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000472/WC500052066.pdf. Accessed September 1, 2015. [Google Scholar]
- 21.Carlucci F, Tabucchi A, Aiuti A, et al. Capillary electrophoresis in diagnosis and monitoring of adenosine deaminase deficiency. Clin Chem. 2003;49(11):1830–1838. doi: 10.1373/clinchem.2003.021576. [DOI] [PubMed] [Google Scholar]
- 22.Hirschhorn R, Roegner-Maniscalco V, Kuritsky L, Rosen FS. Bone marrow transplantation only partially restores purine metabolites to normal in adenosine deaminase-deficient patients. J Clin Invest. 1981;68(6):1387–1393. doi: 10.1172/JCI110389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers MH, Lwin R, Fairbanks L, Gerritsen B, Gaspar HB. Cognitive and behavioral abnormalities in adenosine deaminase deficient severe combined immunodeficiency. J Pediatr. 2001;139(1):44–50. doi: 10.1067/mpd.2001.115023. [DOI] [PubMed] [Google Scholar]
- 24.Ochs HD, Buckley RH, Kobayashi RH, et al. Antibody responses to bacteriophage phi X174 in patients with adenosine deaminase deficiency. Blood. 1992;80(5):1163–1171. [PubMed] [Google Scholar]
- 25.Booth C, Hershfield M, Notarangelo L, et al. Management options for adenosine deaminase deficiency; proceedings of the EBMT satellite workshop (Hamburg, March 2006). Clin Immunol. 2007;123(2):139–147. doi: 10.1016/j.clim.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J Pediatr. 1997;130(3):388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 27.Shearer WT, Rosenblatt HM, Gelman RS, et al. Pediatric AIDS Clinical Trials Group. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112(5):973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Kruisbeek AM, Shevach E, Thornton AM. Proliferative assays for T cell function. Curr Protoc Immunol. 2004;Chapter 3:Unit 3.12. [DOI] [PubMed] [Google Scholar]
- 29.Aiuti A, Biasco L, Scaramuzza S, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341(6148):1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva: World Health Organization; 2006. [Google Scholar]
- 31.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Head Circumference-for-Age, Arm Circumference-for-Age, Triceps Skinfold-for-Age, and Subscapular Skinfold-for-Age: Methods And Development. Geneva: World Health Organization; 2007. [Google Scholar]
- 32.Cole TJ, Freeman JV, Preece MA. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med. 1998;17(4):407–429. [PubMed] [Google Scholar]
- 33.Brigida I, Sauer AV, Ferrua F, et al. B-cell development and functions and therapeutic options in adenosine deaminase-deficient patients. J Allergy Clin Immunol. 2014;133(3):799–806.e10. doi: 10.1016/j.jaci.2013.12.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohn DB, Hershfield MS, Carbonaro D, et al. T lymphocytes with a normal ADA gene accumulate after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates. Nat Med. 1998;4(7):775–780. doi: 10.1038/nm0798-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaspar HB. Bone marrow transplantation and alternatives for adenosine deaminase deficiency. Immunol Allergy Clin North Am. 2010;30(2):221–236. doi: 10.1016/j.iac.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Hay AD, Heron J, Ness A ALSPAC study team. The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract. 2005;22(4):367–374. doi: 10.1093/fampra/cmi035. [DOI] [PubMed] [Google Scholar]
- 37.Grunebaum E, Chung CT, Dadi H, et al. Purine metabolism, immune reconstitution, and abdominal adipose tumor after gene therapy for adenosine deaminase deficiency. J Allergy Clin Immunol. 2011;127(6):1417–9.e3. doi: 10.1016/j.jaci.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Biasco L, Ambrosi A, Pellin D, et al. Integration profile of retroviral vector in gene therapy treated patients is cell-specific according to gene expression and chromatin conformation of target cell. EMBO Mol Med. 2011;3(2):89–101. doi: 10.1002/emmm.201000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaspar HB, Cooray S, Gilmour KC, et al. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci Transl Med. 2011;3(97):97ra80. doi: 10.1126/scitranslmed.3002716. [DOI] [PubMed] [Google Scholar]
- 40.Hönig M, Albert MH, Schulz A, et al. Patients with adenosine deaminase deficiency surviving after hematopoietic stem cell transplantation are at high risk of CNS complications. Blood. 2007;109(8):3595–3602. doi: 10.1182/blood-2006-07-034678. [DOI] [PubMed] [Google Scholar]
- 41.Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000-2009. N Engl J Med. 2014;371(5):434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckley RH, Win CM, Moser BK, Parrott RE, Sajaroff E, Sarzotti-Kelsoe M. Post-transplantation B cell function in different molecular types of SCID. J Clin Immunol. 2013;33(1):96–110. doi: 10.1007/s10875-012-9797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cicalese MP, Aiuti A. Clinical applications of gene therapy for primary immunodeficiencies. Hum Gene Ther. 2015;26(4):210–219. doi: 10.1089/hum.2015.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavazzana-Calvo M, Fischer A, Hacein-Bey-Abina S, Aiuti A. Gene therapy for primary immunodeficiencies: part 1. Curr Opin Immunol. 2012;24(5):580–584. doi: 10.1016/j.coi.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Aiuti A, Bacchetta R, Seger R, Villa A, Cavazzana-Calvo M. Gene therapy for primary immunodeficiencies: Part 2. Curr Opin Immunol. 2012;24(5):585–591. doi: 10.1016/j.coi.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 46.Siler U, Paruzynski A, Holtgreve-Grez H, et al. Successful combination of sequential gene therapy and rescue allo-HSCT in two children with X-CGD—importance of timing. Curr Gene Ther. 2015;15(4):416–427. doi: 10.2174/1566523215666150515145255. [DOI] [PubMed] [Google Scholar]
- 47.Grez M, Reichenbach J, Schwäble J, Seger R, Dinauer MC, Thrasher AJ. Gene therapy of chronic granulomatous disease: the engraftment dilemma. Mol Ther. 2011;19(1):28–35. doi: 10.1038/mt.2010.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Braun CJ, Boztug K, Paruzynski A, et al. Gene therapy for Wiskott-Aldrich syndrome: long-term efficacy and genotoxicity. Sci Transl Med. 2014;6(227):227ra33. doi: 10.1126/scitranslmed.3007280. [DOI] [PubMed] [Google Scholar]
- 49.Candotti F, Shaw KL, Muul L, et al. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood. 2012;120(18):3635–3646. doi: 10.1182/blood-2012-02-400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukherjee S, Thrasher AJ. Gene therapy for PIDs: progress, pitfalls and prospects. Gene. 2013;525(2):174–181. doi: 10.1016/j.gene.2013.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knight S, Collins M, Takeuchi Y. Insertional mutagenesis by retroviral vectors: current concepts and methods of analysis. Curr Gene Ther. 2013;13(3):211–227. doi: 10.2174/1566523211313030006. [DOI] [PubMed] [Google Scholar]
- 52.Aiuti A, Cassani B, Andolfi G, et al. Multilineage hematopoietic reconstitution without clonal selection in ADA-SCID patients treated with stem cell gene therapy. J Clin Invest. 2007;117(8):2233–2240. doi: 10.1172/JCI31666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaffee S, Mary A, Stiehm ER, Girault D, Fischer A, Hershfield MS. IgG antibody response to polyethylene glycol-modified adenosine deaminase in patients with adenosine deaminase deficiency. J Clin Invest. 1992;89(5):1643–1651. doi: 10.1172/JCI115761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chun JD, Lee N, Kobayashi RH, Chaffee S, Hershfield MS, Stiehm ER. Suppression of an antibody to adenosine-deaminase (ADA) in an ADA-deficient patient receiving polyethylene glycol modified adenosine deaminase. Ann Allergy. 1993;70(6):462–466. [PubMed] [Google Scholar]
- 55.Lainka E, Hershfield MS, Santisteban I, et al. polyethylene glycol-conjugated adenosine deaminase (ADA) therapy provides temporary immune reconstitution to a child with delayed-onset ADA deficiency. Clin Diagn Lab Immunol. 2005;12(7):861–866. doi: 10.1128/CDLI.12.7.861-866.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sauer AV, Brigida I, Carriglio N, Aiuti A. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol. 2012;3:265. doi: 10.3389/fimmu.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivers L, Gaspar HB. Severe combined immunodeficiency: recent developments and guidance on clinical management. Arch Dis Child. 2015;100(7):667–672. doi: 10.1136/archdischild-2014-306425. [DOI] [PubMed] [Google Scholar]
- 58.Gaspar B, Buckland K, Rivat C, et al. Immunological and metabolic correction after lentiviral vector mediated haematopoietic stem cell gene therapy for ADA deficiency [abstract]. J Clin Immunol. 2014;34 suppl 2:S167. Abstract ESID-0018.