Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
PolyP significantly augments the plasminogen activator capacity of FXIIa.
Platelet-bound fibrin acts as a reservoir for plasminogen, FXII(a), and polyP.
Abstract
Activated factor XII (FXIIa) has plasminogen activator capacity but its relative contribution to fibrinolysis is considered marginal compared with urokinase and tissue plasminogen activator. Polyphosphate (polyP) is released from activated platelets and mediates FXII activation. Here, we investigate the contribution of polyP to the plasminogen activator function of αFXIIa. We show that both polyP70, of the chain length found in platelets (60-100 mer), and platelet-derived polyP significantly augment the plasminogen activation capacity of αFXIIa. PolyP70 stimulated the autoactivation of FXII and subsequent plasminogen activation, indicating that once activated, αFXIIa remains bound to polyP70. Indeed, complex formation between polyP70 and αFXIIa provides protection against autodegradation. Plasminogen activation by βFXIIa was minimal and not enhanced by polyP70, highlighting the importance of the anion binding site. PolyP70 did not modulate plasmin activity but stimulated activation of Glu and Lys forms of plasminogen by αFXIIa. Accordingly, polyP70 was found to bind to FXII, αFXIIa, and plasminogen, but not βFXIIa. Fibrin and polyP70 acted synergistically to enhance αFXIIa-mediated plasminogen activation. The plasminogen activator activity of the αFXIIa-polyP70 complex was modulated by C1 inhibitor and histidine-rich glycoprotein, but not plasminogen activator inhibitors 1 and 2. Platelet polyP and FXII were found to colocalize on the activated platelet membrane in a fibrin-dependent manner and decorated fibrin strands extending from platelet aggregates. We show that in the presence of platelet polyP and the downstream substrate fibrin, αFXIIa is a highly efficient and favorable plasminogen activator. Our data are the first to document a profibrinolytic function of platelet polyP.
Introduction
The contact pathway comprises factor XII (FXII), prekallikrein (PK), factor XI (FXI), and a nonenzymatic cofactor, high-molecular-weight kininogen (HK). Reciprocal proteolytic activation of FXII and PK to their active forms, FXIIa and kallikrein, respectively, occurs via interaction with a negatively charged surface and is enhanced by Zn2+.1-5 FXI and PK circulate in complex with HK,6,7 which assembles these proteases on the activating surface. FXIIa can cleave FXI, stimulating the intrinsic pathway and downstream generation of thrombin, leading to its classification as a coagulation factor. However, FXIIa is reported to participate in multiple pathways, including inflammation, complement, and fibrinolysis.8
The profibrinolytic effects of the contact pathway are multifaceted, involving direct and indirect interactions. Kallikrein liberates the vasoactive peptide bradykinin from HK, which induces a host of vascular responses, including release of tissue plasminogen activator (tPA) from endothelial cells.9 Kallikrein also directly cleaves single-chain urokinase plasminogen activator10,11 to active urokinase plasminogen activator (uPA). FXII shows distinct homology to tPA and uPA12-14 and, accordingly, FXIIa exhibits plasminogen activator activity. The kinetics of the reaction are considered unfavorable13,15 but FXII is present in plasma at 4 orders of magnitude higher concentrations compared with tPA and uPA.16,17 During evolution, redundancy has developed in the fibrinolytic system, underscored by the relatively mild abnormalities associated with deficiency in tPA or uPA, whereas the double knockout exhibits a more acute phenotype.18 Under certain circumstances or within specific milieus, it is plausible that FXIIa contributes to plasmin generation to complement or compensate for tPA and uPA activity and may be potentially relevant in vivo.19
Several natural surfaces facilitate FXII activation, including polyphosphate (polyP),20,21 RNA,22 misfolded proteins,23 and collagen.24 PolyP is an ancient biomolecule that is ubiquitous in nature.25 It is highly anionic and, consequently, acts as a propitious surface for activation of the contact pathway.20,21 PolyP is localized in platelet dense granules26 and is secreted upon activation21 alongside adenosine 5′-diphosphate (ADP), serotonin, and metal ions, including Zn2+. Platelet polyP is ∼60 to 100 mers,21 which is significantly shorter than the long-chain polymers (1000-2000 mers) in bacteria.27 Polymer length is crucial to polyP’s biological activity, with shorter chains showing reduced capacity to activate the contact pathway.28 PolyP also interacts with fibrin(ogen) and accumulates in clots, altering their structural properties and susceptibility to tPA-mediated fibrinolysis.29,30 The half-life of polyP in plasma is relatively short (1.5-2 hours)20 but could be preserved within the microenvironment of the thrombus.
The plasminogen activator function of FXIIa is augmented by artificial surfaces and Zn2+.31 Here, for the first time, we show that a natural surface, polyP, amplifies the plasminogen activator function of FXIIa. Indeed polyP, of approximate chain length of that found in platelets, binds both FXII(a) and plasminogen, indicative of a template mechanism of activation. Importantly, we also demonstrate that platelet-associated fibrin acts as a reservoir for FXII(a), plasminogen, and platelet-released polyP.
Methods
Collection of blood and preparation of plasma and platelets
For platelet experiments, peripheral blood was collected into acid citrate dextrose solution A VACUETTE tubes (Greiner Bio-One); the first 3 mL was discarded. Platelets were washed and counted as described previously.32 Pooled normal plasma that is essentially free of platelets was prepared from whole blood of 20 normal donors collected into 3.2% trisodium citrate.33
PolyP preparation
PolyP was extracted from platelets as described previously.28 Experiments were performed with platelet-derived polyP, synthetic polyP65 (Sigma-Aldrich), or polyP70 (a kind gift from BK Giulini, GmbH). Similar results were obtained with both synthetic preparations and, for simplicity, are described as polyP70 throughout and the concentration quoted as phosphate monomer (monomer formula, NaPO3).
Clot lysis
Plasminogen-depleted fibrinogen (3.8 μM), Glu- or Lys-plasminogen (0.24 μM), and αFXIIa (200 nM) were added with or without polyP70 (70 μM) in 10 mM Tris-HCl (pH 7.4), 140 mM NaCl, and 0.01% Tween20 to 96-well plates (Greiner Bio-One). Clotting was initiated with 0.25 U/mL of human thrombin and 10 mM CaCl2. In an analogous set of experiments, 200 nM FXII was preincubated with polyP70 (140 μM) at ambient temperature for 30 minutes. In some cases, tPA (1 pM) was also included. Some experiments incorporated plasminogen activator inhibitor 1 (PAI-1; 0.1-1 nM) and PAI-2 (0.1-1 nM), C1 inhibitor (125-500 nM), or histidine-rich glycoprotein (HRG; 0.25-1 μM). PolyP70 was replaced by HeLa RNA (10 μg/mL) or equine type 1 (Horm) collagen (5 μg/mL) in some assays. Absorbance at 405 nm was recorded every 1 minute using a BioTek PowerWave plate reader and BioTek Gen5 software.
Plasminogen activation assay
Plasminogen activation was analyzed as described previously,30 with the following modifications. αFXIIa (200 nM), Glu- or Lys-plasminogen (0-400 nM), and S2251 (0.5 mM) were added to microtiter plates in 10 mM Tris-HCl (pH 7.4), 140 mM NaCl, and 0.01% Tween,20 with or without polyP70 (0-70 μM). Alternatively, 200 nM FXII was added with or without polyP70 (140 μM). Plasmin activity was detected by measuring the absorbance at 405 nm every 1 minute. In a parallel set of assays, the following inhibitors were incorporated: PAI-1 (0.1-1 nM), PAI-2 (0.1-1 nM), C1 inhibitor (125-500 nM), and HRG (0.25-1 μM). In some experiments, soluble fibrin (3.8 μM), prepared as described previously,34 was coated onto plates overnight at 4°C before performing activity assays. In some cases, RNA (10 μg/mL) or collagen (5 μg/mL) was included in place of polyP70.
FXIIa activity assay
FXIIa activity was analyzed as described previously.35 Briefly, αFXIIa (50 nM) and S2302 (0.25 mM), with or without polyP70 (70 μM), were added to microtiter plates and the absorbance was read at 405 nm for 4 hours, with addition of 5 μL (5 mM) S2302 or 5 μL (50 nM) protein at the 2-hour midpoint.
Gels and binding assays
In autodegradation experiments, αFXIIa (50 nM) was incubated with or without polyP70 (70 μM) for up to 360 minutes before resolving on 4% to 12% Bis-Tris gels (NuPAGE; Thermo Fisher Scientific) under nonreducing conditions and protein staining using InstantBlue.
PolyP70 was bound to Sepabeads as described previously36 and incubated with 5 μg of FXII, αFXIIa, βFXIIa, or plasminogen. Flow-through material and subsequent low-salt (50 mM NaCl) and high-salt (1 M NaCl) washes were collected, and fractions were analyzed by western blotting as described previously32 using horseradish peroxidase–conjugated goat anti-human FXII or horseradish peroxidase–conjugated goat anti-human plasminogen (both Enzyme Research Laboratories).
Visualization of FXII and polyP on single platelets
Slides were coated with collagen (20 μg/mL) and thrombin (100 nM) and subsequently blocked with 5% bovine serum albumin. Platelets (5 × 107/mL) were added to coated slides for 45 minutes with 4′,6-diamidino-2-phenylindole (DAPI; 25 μg/mL; excitation wavelength, 358 nm; emission wavelength, 525 nm), DyLight 488 (Thermo Fisher Scientific)-labeled human FXII (DL488-FXII; 365 nM; excitation wavelength, 493 nm; emission wavelength, 518 nm), and Alexa Fluor 647–conjugated annexin V (AF647-annexin V; 1/20, excitation wavelength, 594 nm; emission wavelength, 633 nm; Thermo Fisher Scientific) in the presence of CaCl2 (2 mM). In control experiments, Benzonase Nuclease (Sigma-Aldrich) was included during stimulation to degrade contaminating DNA and RNA. Platelets were visualized by fluorescent confocal microscopy on a Zeiss LSM710 confocal microscope with ×63/1.40 oil immersion objective. Images were recorded on bright field and on separate channels for each wavelength and analyzed using Zen 2012 software.
FXII and platelet polyP distribution in plasma clots
Washed platelets (6.35 × 108/mL; final concentration in clot, 1.5 × 108/mL) were incubated with DAPI (20 μg/mL) and AF647-annexin V (1/20) before activating with collagen (100 μg/mL), thrombin receptor activator peptide 6 (TRAP-6; 100 µM), and CaCl2 (4 mM). Activated platelets were added to pooled normal plasma (50% final concentration) in the presence of DL488-FXII (365 nM) and DyLight 550 (Thermo Fisher Scientific)-labeled human fibrinogen (120 nM; excitation wavelength, 562 nm; emission wavelength, 576 nm). Thrombin (24 nM) and CaCl2 (10 mM) were added, and clots were allowed to form in μ-Slide VI0.4 ibiTreat chambers (ibidi GmbH) for 2 hours. Images were recorded as detailed in “Visualization of FXII and polyP on single platelets.”
Flow cytometry
DL488-FXII (365 nM) and DAPI (10 μg/mL) were added to washed platelets (2 × 107/mL) in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid resuspension buffer (pH 7.45) containing 2 mM CaCl2 and then were stimulated with either 100 ng/mL of convulxin (CVX) and 20 μM TRAP-6 or with 100 ng/mL of CVX and 100 nM thrombin for 45 minutes at ambient temperature. AF647-annexin V (1/20) was added 5 minutes before the end of stimulation. In some cases, 5 mM Gly-Pro-Arg-Pro (GPRP; Sigma-Aldrich) was included to inhibit fibrin polymerization. Analysis was performed on an LSR II flow cytometer with FACSDiva 6.1.3 software (Beckton Dickinson), with appropriate compensation applied and 10 000 events collected per sample. Data were analyzed using FlowJo V.X.0.6 software. Results are expressed as mean percentage of positive platelets and median fluorescence intensity ± standard error of the mean.
Data analysis
Data analysis was performed in GraphPad Prism 5.04. Clot lysis time (CLT) results are expressed as time to 50% lysis and were derived from the time taken from the maximal amplitude of the clot to reach the midpoint to baseline. Alternatively, graphs were normalized and data plotted as percentage turbidity. Plasmin generation was calculated as described previously.37 Briefly, absorbance at 405 nm was plotted against time squared, and the slope from the initial linear portion was estimated. These values were used to calculate plasminogen activation rates using the specific activity of plasmin against S2251 that was experimentally determined to be 1.055 at A405/min per μM (not shown). Fold-changes in lysis were calculated from mean CLT data. Statistical analysis was performed on CLT, plasmin activity assays, and flow cytometry data using the Student t test or, when multiple parameters were tested, 1-way analysis of variance with the Dunnett multiple comparison post hoc test. Values of P < .05 were considered significant.
Results
αFXIIa plasminogen activator activity is enhanced by polyP70
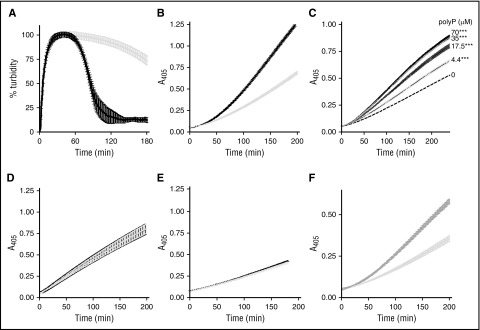
Artificial surfaces can enhance the plasminogen activator capacity of αFXIIa.31 This prompted us to examine the effect of the “natural” activator polyP, of approximately the size (60-100 mers) of that found in platelets, in αFXIIa-mediated plasminogen activation. In line with previous observations, we show that αFXIIa is a relatively weak plasminogen activator.13,15 However, inclusion of polyP70 significantly augments the ability of αFXIIa to drive fibrinolysis, decreasing the CLT by 2.3-fold (105 ± 6 minutes vs 238 ± 14 minutes; P < .0001; Figure 1A). Similarly, polyP70 accelerated αFXIIa-mediated plasmin generation (Figure 1B; P < .0001) in a dose-dependent manner, with concentrations as low as 4.4 μM, demonstrating a significant enhancement over αFXIIa alone (Figure 1C; P < .0001). PolyP had no direct effect on the activity of preformed plasmin (Figure 1D; P = .93). This finding indicates that polyP70 accelerates αFXIIa-mediated conversion of plasminogen to plasmin. The derivative, βFXIIa, lacks the surface binding domain and is less efficient at stimulating plasminogen activation.16 The low level of plasmin generation observed with βFXIIa was not augmented by polyP70, reflecting the requirement of the anion binding domain of αFXIIa for interaction with polyP70 (Figure 1E; P = .71). Inclusion of Zn2+ in the reaction buffer did not further enhance the cofactor function of polyP70 in this reaction (not shown). We found that platelet-derived polyP extracted from human platelets similarly enhanced the plasminogen activator capacity of αFXIIa (Figure 1F).
Figure 1.
αFXIIa plasminogen activator activity is enhanced by polyP70. (A) Clots were formed with 3.8 μM fibrinogen, 0.24 μM Glu-plasminogen, and 200 nM αFXIIa in the absence (gray) and presence (black) of 70 μM polyP70. Clotting was initiated with 0.25 U/mL of thrombin and 10 mM CaCl2, and subsequent lysis was monitored at 405 nm. Mean data ± SEM is expressed as percentage turbidity (n = 5; P < .0001). (B) Plasminogen activation was analyzed by incubating 200 nM αFXIIa and 200 nM plasminogen in the absence (light gray) or presence (black) of 70 μM polyP70. Plasmin activity was detected using the chromogenic substrate S2251 at 405 nm. Data represent mean ± SEM (n = 3; P < .0001). (C) αFXIIa-mediated plasminogen activation was analyzed in the presence of various concentrations of polyP70 by incubating 200 nM αFXIIa and 200 nM plasminogen in the absence (dashed line) or presence of 70, 35, 17.5, or 4.4 μM polyP70, as indicated. Plasmin activity was detected using the chromogenic substrate S2251 at 405 nm. Data represent mean ± SEM (n = 3; P < .0001). (D) Similarly, direct effects of polyP on preformed plasmin (6.25 nM) were analyzed in the absence (gray line) and presence (black line) of 70 μM polyP70 with S2251. Data represent mean ± SEM (n = 3; P = .93). (E) Activation of plasminogen (200 nM) by βFXIIa (200 nM) was monitored in the absence (gray) and presence (black) of 70 μM polyP70 and was detected using S2251. Data represent mean ± SEM (n = 4; P = .71). (F) Plasminogen activation was analyzed by incubating 200 nM αFXIIa and 200 nM plasminogen in the absence (light gray) or presence (dark gray) of 70 μM platelet-derived polyP. Plasmin activity was detected using the chromogenic substrate S2251 at 405 nm. Data represent mean ± SEM (n = 3; P < .0001). A, absorbance; SEM, standard error of the mean.
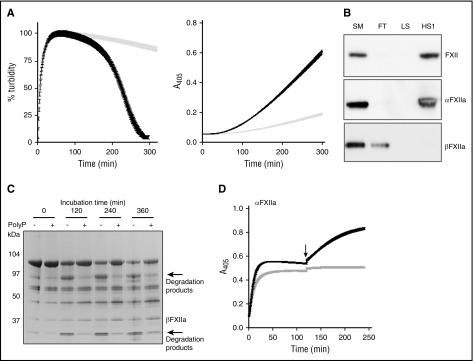
Because polyP is a known activator of FXII,20,21,35 we examined its capacity to both stimulate FXII activation and subsequently stimulate its plasminogen activator function. CLTs were significantly longer with FXII (>300 minutes) compared with αFXIIa (238 ± 58 minutes) and, similarly, rates of plasmin generation were significantly lower with FXII compared with αFXIIa, presumably reflecting the time for transition of zymogen to protease. Nevertheless, polyP70 exhibits significant cofactor activity in clot lysis and activity assays when initiated with FXII-polyP rather than with FXII alone (Figure 2A).
Figure 2.
PolyP70 stimulates FXII activation and modulates its plasminogen activator function. (A) PolyP induces autoactivation of FXII. Left: Clots were formed with 3.8 μM fibrinogen, 0.24 μM Glu-plasminogen, and 200 nM FXII in the absence (gray) or presence (black) of 140 μM polyP70. Clotting was initiated with 0.25 U/mL of thrombin and 10 mM CaCl2, and subsequent lysis was monitored at 405 nm. Mean data ± SEM are expressed as percentage turbidity (n = 3; P < .0001). Right: FXII (200 nM) and Glu-plasminogen (200 nM) were incubated in the absence (gray) or presence (black) of 140 μM polyP70, and plasmin activity was detected using S2251. Data represent mean ± SEM (n = 3; P < .0001). (B) PolyP binds to FXII and αFXIIa. FXII, αFXIIa, or βFXIIa (5 μg) were run through columns containing Sepabeads coated with polyP70 before collecting the flow-through fraction (FT), low-salt wash (LS; 50 mM NaCl), and high-salt wash (HS1; 1 M NaCl) and comparing with starting material (SM). Protein was detected by western blotting with an antibody to FXII. Image is representative of 3 separate experiments. (C) PolyP protects αFXIIa from autodegradation. αFXIIa (5 μg) was incubated with or without polyP70 (100 μg) before resolving on 4% to 12% Bis-Tris gels under nonreducing conditions. Data shown are representative of 3 separate experiments. (D) PolyP preserves αFXIIa activity. αFXIIa (50 nM) activity was analyzed using S2302 in the absence (gray) or presence (black) of 70 μM polyP70. After 2 hours, additional S2302 substrate was added to the reaction (arrow) and readings continued for a further 2 hours. Data represent mean ± SEM (n = 3).
We next investigated the binding of polyP70 to FXII derivatives and found it complexed with FXII and αFXIIa, but not with βFXIIa, confirming the critical role of the anion binding site for this interaction (Figure 2B). When in complex with polyP70, autodegradation of αFXIIa was delayed (Figure 2C). The protective effect of polyP70 on αFXIIa was analyzed using S2302 substrate (Figure 2D). Addition of excess substrate to the αFXIIa reaction at 2 hours generated a further increase in absorbance in the presence of polyP70, whereas no change was observed with αFXIIa alone. No further increase in activity was observed when additional αFXIIa was added to reactions with polyP70 at 2 hours, confirming that protein is not limiting (not shown). We further analyzed changes in αFXIIa activity in terms of cleavage of FXI but were unable to detect any changes in FXI activation after 360 minutes of incubation in the absence or presence of polyP70 (not shown). These results indicate that although polyP70 confers protection against autodegradation, the differences may be too minor to alter functional activity toward physiological targets.
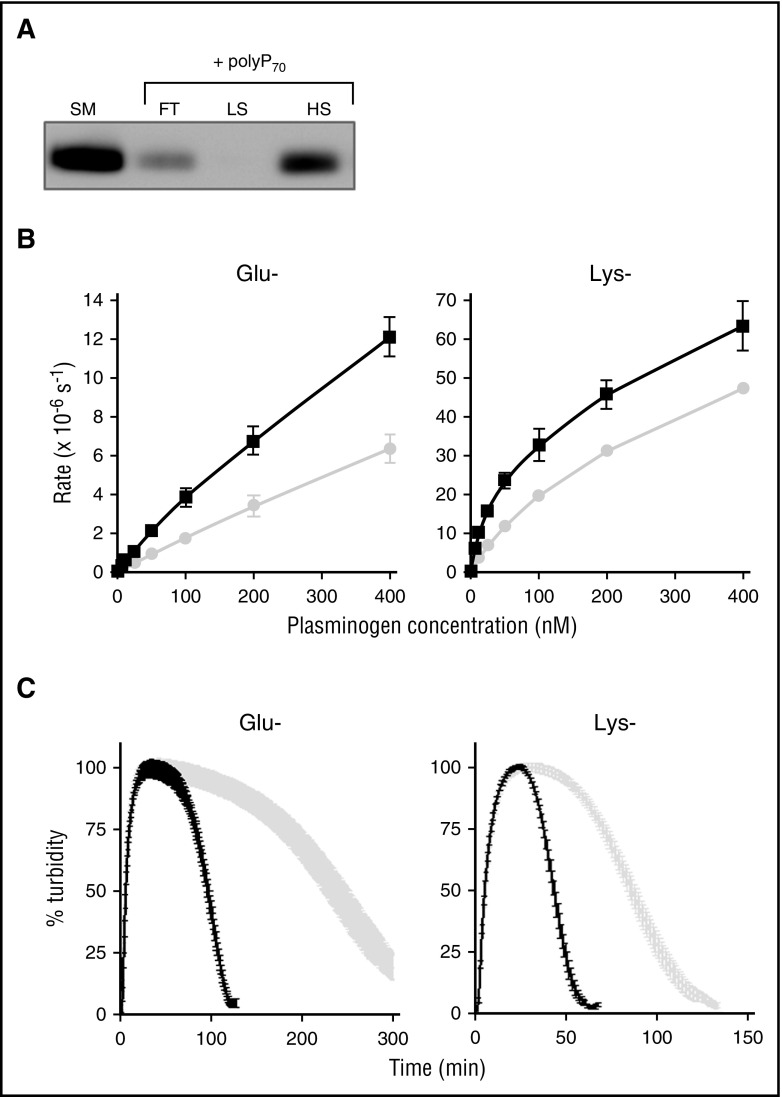
αFXIIa enhances activation of Glu and Lys forms of plasminogen
We found that plasminogen bound immobilized polyP70 and could be released by washing with high-salt buffer (Figure 3A). This is indicative of an electrostatic interaction with the polymer, as previously shown for other proteins.30,34,36 Plasminogen circulates in 2 forms: the predominant Glu-plasminogen is described as the “closed” conformation, whereas the truncated Lys-plasminogen, cleaved at the C-terminus by plasmin, is in an “open” conformation and exhibits a shorter half-life. Enhanced binding of Lys-plasminogen to fibrin38 and the activators tPA and uPA results in more rapid plasmin generation.39,40 We observed faster plasmin generation and clot lysis with Lys-plasminogen (catalytic efficiency [CE] = 257.5 ± 21.0 M−1s−1) than Glu-plasminogen (CE = 18.5 ± 4.2 M−1s−1) when activated with αFXIIa (note the different scales in Figure 3B-C). PolyP70 significantly augmented αFXIIa-mediated activation of Glu-plasminogen (CE = 41.1 ± 6.0 M−1s−1) and Lys-plasminogen (CE = 629.5 ± 75.6 M−1s−1) (Figure 3B) and accelerated clot lysis with both forms by ∼2.6-fold (Figure 3C). These data suggest that polyP does not impact on the transition of the closed (Glu) to open (Lys) conformation of plasminogen but directly facilitates cleavage to plasmin.
Figure 3.
αFXIIa enhances activation of Glu and Lys forms of plasminogen. (A) Binding of polyP70 plasminogen was analyzed by running Glu-plasminogen through columns containing Sepabeads coated with polyP70 before collecting the flow-through fraction (FT), low-salt wash (LS; 50 mM NaCl), and high-salt wash (HS; 1 M NaCl) and comparing with starting material (SM). Protein was detected by western blotting with an antibody to plasminogen. Image is representative of 3 separate experiments. (B) The rate of plasmin generation by αFXIIa (200 nM) in the presence (black line) or absence (gray line) of polyP70 (70 μM) was quantified for Glu-plasminogen (left; P < .05) and Lys-plasminogen (right; P < .01). Data are expressed as mean ± standard deviation (n = 3). (C) Fibrin clots were formed with fibrinogen (3.8 μM), αFXIIa (200 nM), and Glu-plasminogen (left) or Lys-plasminogen (right), in the absence (gray) or presence (black) of polyP70 (70 μM). Clotting was initiated with thrombin (0.25 U/mL) and CaCl2 (10 mM), and lysis was monitored at 405 nm. Mean data ± SEM are expressed as percentage turbidity (n = 3; P < .0001). Note the different scales on the Lys-plasminogen plot compared with Glu-plasminogen, due to the different rates of activation of the isoforms of plasminogen.
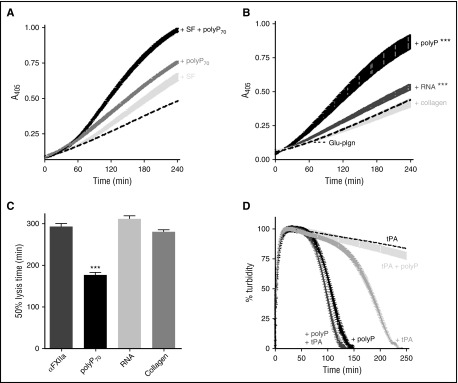
PolyP70 and fibrin augment αFXIIa-mediated plasminogen activation
We have previously shown that polyP binds to fibrin(ogen) and alters the structure of the fibrin network34; therefore, we assessed the impact on αFXIIa-mediated plasminogen activation. Fibrin significantly enhanced the plasminogen activator function of αFXIIa (Figure 4A) and, when combined, fibrin and polyP70 acted in concert to further amplify αFXIIa-mediated plasminogen activation (Figure 4A; P < .001).
Figure 4.
PolyP and fibrin augment αFXIIa-mediated plasminogen activation. (A) Plasminogen activation was analyzed by incubating 200 nM αFXIIa and 200 nM Glu-plasminogen (dotted line) in the presence of either 3.8 μM soluble fibrin (SF; light gray), 70 μM polyP70 (dark gray), or both SF and polyP70 (black). Plasmin activity was detected using the chromogenic substrate S2251 at 405 nm. Data represent mean ± SEM (n = 3; P < .001). (B-C) The plasminogen activator function of αFXIIa (200 nM) was analyzed in the presence of different surfaces, including polyP70 (70 μM), RNA (10 μg/mL), and collagen (5 μg/mL). (B) Plasmin activity, generated from 200 nM plasminogen, was detected using S2251. (C) Fibrinolysis was analyzed by forming clots from fibrinogen (3.8 μM), αFXIIa (200 nM), Glu-plasminogen (200 nM), thrombin (0.25 U/mL), and CaCl2 (10 mM). Lysis was monitored at 405 nm, and mean ± SEM are shown as the time from maximal absorbance of the clot to 50% lysis (n = 3; ***P < .001). (D) Fibrin clots were formed and monitored as described in panel C, with 70 μM polyP70 (black), 1 pM tPA (gray), or both polyP70 and tPA (dark gray). The clots formed with tPA (dashed line) and both tPA and polyP (light gray) were formed in the absence of αFXIIa. Mean data ± SEM are expressed as percentage turbidity (n = 4; P < .0001).
We analyzed whether other natural surfaces that stimulate FXII activation, such as RNA22 and collagen,24 modulated the plasminogen activator function of αFXIIa. RNA slightly enhanced plasmin generation by αFXIIa (Figure 4B; P < .001), but not to the same magnitude as polyP70, whereas collagen was unable to stimulate activation. However, neither collagen nor RNA was as effective in shortening the CLT as polyP70 (Figure 4C; P < .001).
We next addressed whether αFXIIa acted in concert with tPA to mediate fibrinolysis. Inclusion of αFXIIa with a concentration of tPA (15 pM) sufficient to induce clot lysis further shortened the CLT from 69 ± 8.6 minutes to 47 ± 2.7 minutes (P < .01; CLT of αFXIIa alone was >300 minutes). We then performed assays at a suboptimal dose of tPA (1 pM), which, alone, was not sufficient to induce lysis (CLT >300 minutes). Addition of αFXIIa to clots containing 1 pM tPA significantly shortened the CLT (180 ± 9.4 minutes), but not to the extent of polyP70 (106 ± 6.5 minutes; P < .0001; Figure 4D). A marginal decrease in CLT was observed with both αFXIIa and tPA in the presence of polyP70 (97 ± 1.4 minutes; P < .01). Similarly, visualization of lysis in real time (supplemental Video 1, available on the Blood Web site) demonstrated significantly faster lysis in the presence of both αFXIIa and tPA compared with tPA alone. Interestingly, the pattern of lysis in the presence of αFXIIa was different, with clots lysing from the edge inward, rather than proceeding with a defined lysis front as observed with tPA alone. These data illustrate that fibrin degradation is enhanced in the presence of both tPA and αFXIIa, compared with either activator alone.
C1 inhibitor and HRG regulate the plasminogen activator activity of αFXIIa
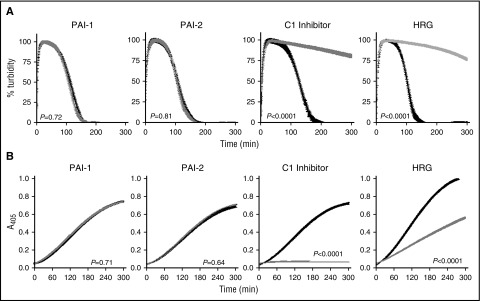
Inclusion of PAI-1 and PAI-2 at plasma concentrations did not impact αFXIIa-mediated lysis (Figure 5A) or plasmin generation (Figure 5B) in the absence (not shown) or presence of polyP70 (Figure 5A). In contrast, C1 inhibitor significantly attenuated αFXIIa-mediated clot lysis (Figure 5A; P < .0001) and plasmin generation (Figure 5B; P < .001), with or without polyP70. HRG was also effective in downregulating CLT (Figure 5A; P < .0001) and plasminogen activation (Figure 5B; P < .001) by αFXIIa, with or without polyP70. The inhibition of αFXIIa-mediated plasminogen activation by α2-antiplasmin could not be examined due to its dominant inhibition of plasmin, but we found no discernible effects on direct inhibition of αFXIIa using S2302, with or without polyP70 (not shown).
Figure 5.
C1 inhibitor and HRG regulate the plasminogen activator function of αFXIIa. The impact of inhibitors on αFXIIa-polyP70 plasminogen activator function was monitored in the absence (black) or the presence (gray) of PAI-1 (1 nM), PAI-2 (1 nM), C1 inhibitor (500 nM), or HRG (1 μM) by absorbance-based clot lysis (A) or by plasminogen activation assay in which plasmin was detected by cleavage of S2251 (B). For simplicity, the controls in the absence of polyP70 are not shown, because no lysis was observed in either the absence or presence of the inhibitor. Data represent mean ± SEM (n = 3).
FXII and polyP bind to the surface of stimulated platelets
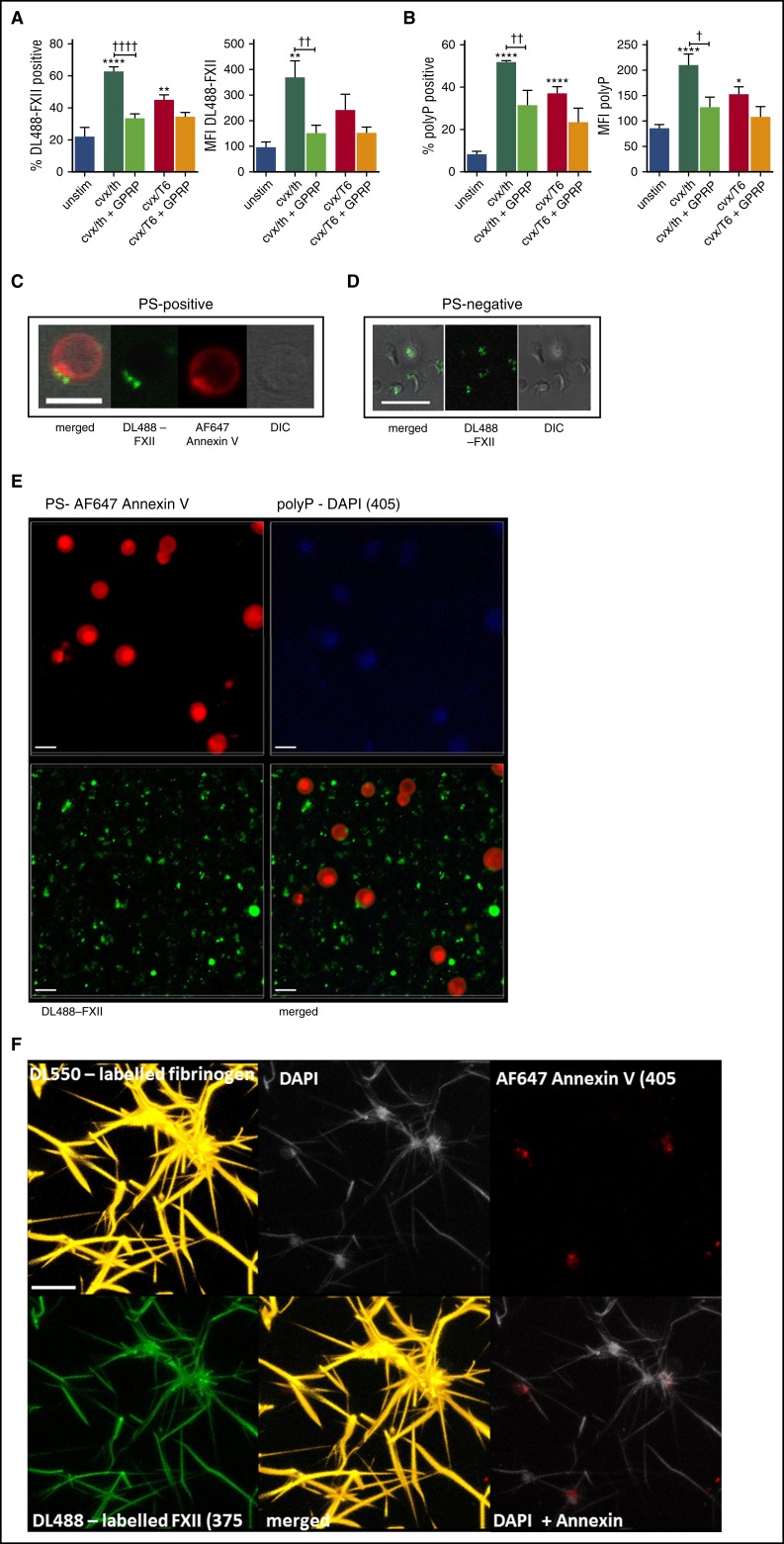
We examined the interaction of DL488-FXII with platelets by flow cytometry and found that binding to CVX/thrombin-stimulated platelets was significantly augmented compared with unstimulated platelets (62.6% ± 6.9% vs 22.4% ± 13.5%; P < .0001; Figure 6A). Platelets stimulated with CVX/TRAP-6 displayed a reduced capacity to bind DL488-FXII (44.7% ± 8.9%; P < .01). Inclusion of GPRP, to impede fibrin polymerization, markedly decreased the percentage of CVX/thrombin-stimulated platelets that bound DL488-FXII (33.4% ± 6.9%; P < .0001). Together, these data suggest that platelet-bound fibrin plays a crucial role in the association of FXII with the activated platelet surface.
Figure 6.
FXII and polyP bind to the surface of stimulated platelets. (A-B) Washed human platelets were incubated with DL488-labeled FXII and DAPI to detect platelet-derived polyP and were left unstimulated (unstim) or stimulated with 100 ng/mL of CVX and 100 nM thrombin (cvx/th) or with 100 ng/mL of CVX and 20 μM TRAP-6 (cvx/T6), with or without 5 mM GPRP, for 45 minutes at ambient temperature before analyzing DL488-FXII-positive cells (A) and DAPI-positive cells (B) by flow cytometry. **P < .01, ****P < .0001 vs unstim platelets; †P < .05, ††P < .01, ††††P < .0001 cvx/th-stimulated platelets vs cvx/th + GPRP. (C-E) Washed platelets (5 × 107/mL) were activated with 20 μg/mL of collagen and 100 nM thrombin in the presence of DL488-FXII (green) and DAPI (blue; seen in panel E) and stained using AF647-annexin V to detect PS (red). Images represent 3-dimensional render of z-stacks. Scale bars represent 5 μm. Representative images of 3 separate experiments. (F) Platelets (1.5 × 108/mL final concentration) were activated with 100 μg/mL of collagen and 100 μM TRAP-6 in the presence of CaCl2 before adding to plasma clots (50%) in the presence of DyLight 550 (DL550)-labeled fibrinogen (120 nM; orange), DL488-FXII (365 nM; green), DAPI (20 μg/mL; light gray), and AF647-annexin V (1/20; red). Thrombin (24 nM) and CaCl2 (10 mM) were added, and clots were allowed to form for 2 hours. The image is representative of 3 separate experiments and displays a 3-dimensional render of a z-stack. Scale bar represents 10 μm. Images in C-F were obtained using a Zeiss LSM710 confocal microscope with a ×63/1.40 oil immersion objective and were analyzed using Zen 2012 software. DIC, differential interference contrast; MFI, median fluorescence intensity.
We found a significantly higher degree of positivity for platelet-derived polyP on the surface of CVX/thrombin-stimulated platelets compared with unstimulated platelets (52% ± 2% vs 8.1% ± 4.0%; P < .0001). Surface-bound polyP was slightly decreased when platelets were stimulated with CVX/TRAP-6 (37.1% ± 7.2%; P < .01) and upon inclusion of GPRP (31.5% ± 12.1%; P < .01), suggesting a role for platelet-bound fibrin in its retention on the activated platelet membrane (Figure 6B).
Using fluorescence confocal microscopy, we examined the localization of FXII on the activated platelet surface stimulated with CVX/thrombin. Phosphatidylserine (PS)-positive platelets bound DL488-FXII in a single protruding “cap” on the platelet surface that is also rich in PS (Figure 6C; supplemental Video 2). DL488-FXII also bound to PS–negative spread platelets in a central diffuse pattern over the area of the granulomere (Figure 6D). Staining for platelet-derived polyP was dispersed over the activated membrane of PS-positive platelets (Figure 6E). Control experiments performed with nuclease, to degrade contaminating DNA and RNA, did not alter DAPI staining on activated platelets (supplemental Figure 1).
Platelet-derived polyP associates with the platelet surface in clots and colocalizes with FXII on adjacent fibrin fibers
The location of platelet-derived polyP and FXII was studied in plasma clots formed in the presence of activated platelets. Platelet-derived polyP associates with the surface of activated platelets, particularly procoagulant PS-positive platelets and on platelet-bound fibrin (Figure 6F.) No DAPI staining was observed in clots formed in the absence of platelets (data not shown). DL488-FXII also decorated fibrin fibers extending from platelet aggregates, in clear colocalization with platelet-derived polyP.
Discussion
In the present study, we reveal a cofactor function for platelet-derived polyP in modulating the plasminogen activator activity of αFXIIa. Platelet-derived polyP colocalized with αFXIIa on the fibrin matrix extending from platelet aggregates. Blocking fibrin polymerization with GPRP reduced binding of FXII, highlighting its importance in the accrual of FXII in platelet-rich areas. Our findings demonstrate that in the presence of platelet polyP, αFXIIa is an efficient plasminogen activator. In addition, we show that fibrin augments the plasminogen activator capacity of αFXIIa in a synergistic manner to polyP. There have been several reports on the modulation of fibrinolysis by polyP,20,29,30 but to our knowledge, this is the first study to document a profibrinolytic function of platelet-derived polyP.
Although the plasminogen activator function of αFXIIa has been described previously,15,16,31 it has been largely ignored due to unfavorable kinetics. Despite displaying a 20-fold lower catalytic efficacy for plasminogen than for uPA, the relative abundance of FXII (375 nM) in plasma implies that it may be relevant as a plasminogen activator.17 Nevertheless, studies on the role of αFXIIa as a direct plasminogen activator are limited.19,41,42 Here, we demonstrate that polyP (of approximately the size found in platelets) and platelet-derived polyP significantly augment αFXIIa-mediated plasmin generation. PolyP accelerates αFXIIa-mediated activation of Glu- and Lys-plasminogen to a similar degree, indicating that it does not facilitate transition of the closed to the open conformation, nor does it directly impact on plasmin activity. We observed binding of polyP to FXII(a) and plasminogen, indicating that the cofactor function of this polymer is potentially mediated via a template mechanism on direct conversion of plasminogen to plasmin (Figure 7). PolyP also stimulates autoactivation of FXII to αFXIIa35 and, indeed, affords some protection against autodegradation. This could be relevant in terms of time frame, because FXII may become activated during clot formation; however, by interacting with platelet-derived polyP, its activity may be protected, allowing it to subsequently participate in clot degradation. Indeed, a recent publication highlighted the dual role of FXII in supporting fibrin formation and degradation of the clot.43 It is interesting to speculate on the complex role of this enzyme in modulating both fibrin formation (via generation of thrombin) and fibrin degradation (via plasmin). Further work is necessary to define the procoagulant and profibrinolytic properties of αFXIIa and the role of effector molecules such as polyP in these processes. Indeed, opposing functions of a hemostatic enzyme is not an uncommon phenomenon. The central enzyme thrombin is a prime example, because its activity can be directed from procoagulant to anticoagulant processes, depending on the effector molecule bound to exosite I or exosite II of the protease.44
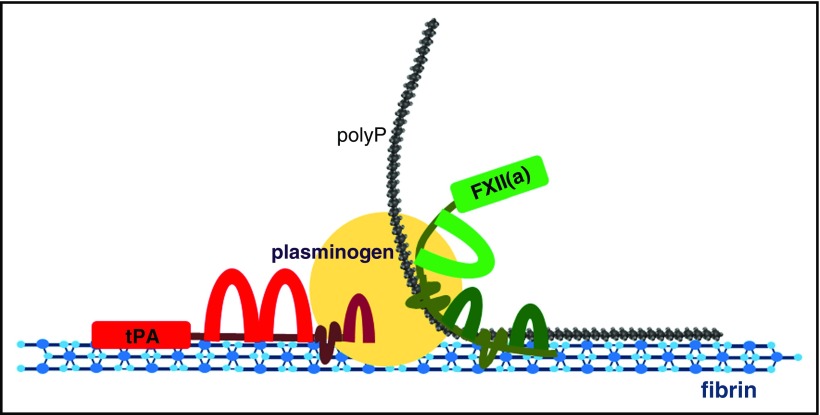
Figure 7.
Interaction of αFXII(a), plasminogen, polyp, and fibrin. Depiction of the potential template interactions among αFXII(a), plasminogen, and polyP on polymerized fibrin. Fibrin forms the initial network and acts as a template for both tPA- and FXIIa-mediated fibrinolysis due to its capacity to bind FXII(a), polyP, plasminogen, and tPA. PolyP binds to fibrin, αFXII(a), and plasminogen, potentially acting as an anchor to reinforce the association among these proteins. When αFXII(a) is bound to fibrin and polyP, its activation and plasminogen-activator activity is enhanced, facilitating plasmin generation on fibrin and subsequent degradation of the network. The cofactor capacity of fibrin in the stimulation of tPA-mediated plasminogen activation is well documented. Binding of the aFXIIa-polyP70 complex may to further facilitate plasminogen activation on the fibrin surface to accelerate fibrinolysis.
Fibrin also amplifies αFXIIa-mediated plasminogen activation, with maximal stimulation observed when both fibrin and polyP are present, suggesting that these molecules act synergistically to drive this process. PolyP has the capacity to bind to fibrin29,30 and FXII(a), but these observations indicate that their respective binding sites on FXII(a) must be distinct. Fibrin is a well-established cofactor for tPA-mediated plasminogen activation. αFXIIa was able to act in conjunction with tPA to significantly accelerate fibrinolysis, suggesting that incorporation of these activators into the forming fibrin network may facilitate clot degradation, as depicted in the model shown in Figure 7. Interestingly, other surfaces that promote FXII activation and procoagulant function, specifically collagen24 and RNA,22 were not effective in promoting αFXIIa-mediated fibrinolysis. This finding suggests that cofactor molecules may drive αFXIIa activity toward distinct downstream target substrates.
Antifibrinolytic functions of polyP have been described in the past.20,29,30 The first of these relates to enhanced activation of the thrombin activatable fibrinolysis inhibitor, due to acceleration of thrombin generation in the presence of polyP.20 Activated thrombin activatable fibrinolysis inhibitor downregulates fibrinolysis by removing C-terminal lysine residues on fibrin that are important for binding plasminogen and tPA.45 PolyP also exerts antifibrinolytic function by altering the structure of the forming fibrin network.29,30 Our recent work has shown that this arises from impaired fibrin polymerization.46 The modifications to the fibrin architecture by polyP alter its capacity to bind tPA and plasminogen, particularly that of partially degraded fibrin, thereby reducing tPA-mediated plasmin generation.30 The profibrinolytic capacity of polyP described here, in terms of augmenting αFXIIa-mediated plasminogen activation, is intriguing, particularly in light of the fact that fibrin also plays a role in this process. It seems that the functions of polyP are more diverse than first thought and that this polymer may exert different levels of control over the hemostatic system, depending on timing and perhaps local concentrations of available reactants.
Of interest, PAI-1 and PAI-2 were ineffective in modulating polyP-αFXIIa-mediated plasminogen activation and fibrinolysis, whereas C1-inhibitor and HRG both effectively neutralized activity. C1-inhibitor is the predominant inhibitor of the contact pathway and regulates tPA-mediated plasminogen activation in situations when tPA is in excess over PAI-1.47-49 C1-inhibitor has also been shown to bind to polyP.50 Recent data highlighted the important role of HRG in modulating FXIIa activity and function.51-53 HRG binds to FXIIa with incredibly high affinity in the presence of Zn2+ ions,51 and both are released from platelet α-granules upon activation.54,55 HRG also associates with DNA and RNA and attenuates nucleic acid–driven activation of FXII.52 Here, we show for the first time that HRG dampens αFXIIa activity directed toward the fibrinolytic pathway, adding to the complexity of this unusual adapter protein in regulation of hemostatic pathways.
To date, the binding sites for αFXIIa on platelets have not been elucidated, but unlike other coagulation factors, it does not bind directly to PS-positive platelets via Gla domains.56 We have previously shown that FXII(a) binds to fibrin and is actively incorporated into clots,57 and elegant flow studies confirmed that FXII(a) interacts with platelet-associated fibrin.56 We observed significantly more FXII on the surface of platelets activated with CVX/thrombin than with CVX/TRAP-6. Blocking fibrin polymerization with GPRP during CVX/thrombin stimulation, but not CVX/TRAP-6 stimulation, significantly reduced the amount of FXII associated with platelets. Together, these data indicate that fibrin anchors FXII to the activated platelet membrane. PS-positive platelets display a cap of FXII on the activated surface that we have recently shown to be rich in fibrin(ogen) and plasminogen.58 We also show that platelet-derived polyP is retained on the membrane of PS-positive platelets, but unlike FXII, fibrinogen, and plasminogen, it is homogenously distributed. Inhibition of fibrin polymerization attenuates the association of polyP with the platelet surface, consistent with its known affinity for fibrin,30 but does not completely abrogate binding. This suggests the presence of an as-yet-unidentified second mechanism that mediates retention of polyP on activated platelets. Nevertheless, we also show that polyP can translocate from the “hot-spots” of PS-positive platelets into the surrounding fibrin network. FXII also decorates fibrin strands, accentuating the importance of platelet-bound fibrin in localizing FXII and plasminogen58 in the vicinity of activated platelets. The release of polyP from stimulated platelets could stimulate activation of FXII on fibrin and enhance the plasminogen activator capacity of αFXIIa; in this sense, fibrin will be acting as a surface for its own destruction, as it does in tPA-mediated plasminogen activation.
Thrombi are composed of different regions, the inner “core” and the outer “shell,” which differ in their levels of platelet activation, aggregation, and packing.59 The core comprises tightly packed, degranulated platelets encased in fibrin.59 ADP released from dense granules regulates α-granule secretion,60-62 forming dense regions that stabilize the platelet aggregate.63 Concomitant release of polyP with ADP from dense granules suggests that it may be retained in these low-solute transport areas. α-Granule release occurs at lower agonist concentrations than dense granule release,64,65 suggesting that platelets at the edge of the core and within the shell may release fibrinogen and bind fibrin before polyP secretion, thereby providing an anchor to retain polyP in the locale of the activated platelet aggregates.
Multiple questions remain over how the pleotropic effects of FXII(a) are mediated in biological systems. Further investigations on the role of αFXIIa as a plasminogen activator in vivo are warranted, particularly in light of the current interest in FXII as an antithrombotic target. It is plausible that different surfaces act as cofactors that direct the function of FXIIa to procoagulant and profibrinolytic pathways, analogous to mechanisms seen with other proteins in the hemostatic cascade. Platelet-bound fibrin acts as reservoir for polyP and αFXII(a) and, within the thrombus microenvironment, may preserve their functional activity. Clearly, there is still much to be learned about the mysterious FXII(a) and its contribution to various physiological processes.
Acknowledgments
The authors thank students Natasha Walker and Thomas Nolan for contributions to the project; the Microscopy and Histology Core Facility and the Iain Fraser Cytometry Centre at the University of Aberdeen for excellent advice and use of facilities; and Jeffrey Weitz from McMaster University, Canada, for the kind gift of HRG.
This work was supported by the British Heart Foundation grant FS/11/2/28579 (N.J.M. and A.S.L.) and the University of Aberdeen Development Trust (J.L.M. and N.J.M.). P.Y.K. is supported by an Early Career Award and by the New Investigator Fund from Hamilton Health Sciences.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.L.M. performed the research, analyzed the data, and wrote the manuscript; A.S.L., A.K., G.G., and C.B. performed the research and analyzed the data; P.Y.K. analyzed the data; and N.J.M. provided the initial concept of the study, supervised the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicola J. Mutch, Institute of Medical Sciences, School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Foresterhill, Aberdeen AB25 2ZD, United Kingdom; e-mail: n.j.mutch@abdn.ac.uk.
References
- 1.Shimada T, Kato H, Iwanaga S. Accelerating effect of zinc ions on the surface-mediated activation of factor XII and prekallikrein. J Biochem. 1987;102(4):913-921. [DOI] [PubMed] [Google Scholar]
- 2.Shore JD, Day DE, Bock PE, Olson ST. Acceleration of surface-dependent autocatalytic activation of blood coagulation factor XII by divalent metal ions. Biochemistry. 1987;26(8):2250-2258. [DOI] [PubMed] [Google Scholar]
- 3.Bernardo MM, Day DE, Halvorson HR, Olson ST, Shore JD. Surface-independent acceleration of factor XII activation by zinc ions. II. Direct binding and fluorescence studies. J Biol Chem. 1993;268(17):12477-12483. [PubMed] [Google Scholar]
- 4.Bernardo MM, Day DE, Olson ST, Shore JD. Surface-independent acceleration of factor XII activation by zinc ions. I. Kinetic characterization of the metal ion rate enhancement. J Biol Chem. 1993;268(17):12468-12476. [PubMed] [Google Scholar]
- 5.Schousboe I. The inositol-phospholipid-accelerated activation of prekallikrein by activated factor XII at physiological ionic strength requires zinc ions and high-Mr kininogen. Eur J Biochem. 1990;193(2):495-499. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RE, Mandle R Jr, Kaplan AP. Association of factor XI and high molecular weight kininogen in human plasma. J Clin Invest. 1977;60(6):1376-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott CF, Colman RW. Function and immunochemistry of prekallikrein-high molecular weight kininogen complex in plasma. J Clin Invest. 1980;65(2):413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90(10):3819-3843. [PubMed] [Google Scholar]
- 9.Fuhrer G, Gallimore MJ, Heller W, Hoffmeister HE. F XII [in German]. Blut. 1990;61(5):258-266. [DOI] [PubMed] [Google Scholar]
- 10.Ichinose A, Fujikawa K, Suyama T. The activation of pro-urokinase by plasma kallikrein and its inactivation by thrombin. J Biol Chem. 1986;261(8):3486-3489. [PubMed] [Google Scholar]
- 11.Binnema DJ, Dooijewaard G, Turion PN. An analysis of the activators of single-chain urokinase-type plasminogen activator (scu-PA) in the dextran sulphate euglobulin fraction of normal plasma and of plasmas deficient in factor XII and prekallikrein. Thromb Haemost. 1991;65(2):144-148. [PubMed] [Google Scholar]
- 12.McMullen BA, Fujikawa K. Amino acid sequence of the heavy chain of human alpha-factor XIIa (activated Hageman factor). J Biol Chem. 1985;260(9):5328-5341. [PubMed] [Google Scholar]
- 13.Tans G, Rosing J. Structural and functional characterization of factor XII. Semin Thromb Hemost. 1987;13(1):1-14. [DOI] [PubMed] [Google Scholar]
- 14.Ponczek MB, Gailani D, Doolittle RF. Evolution of the contact phase of vertebrate blood coagulation. J Thromb Haemost. 2008;6(11):1876-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsmith GH Jr, Saito H, Ratnoff OS. The activation of plasminogen by Hageman factor (factor XII) and Hageman factor fragments. J Clin Invest. 1978;62(1):54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles LA, Greengard JS, Griffin JH. A comparison of the abilities of plasma kallikrein, beta-factor XIIa, Factor XIa and urokinase to activate plasminogen. Thromb Res. 1983;29(4):407-417. [DOI] [PubMed] [Google Scholar]
- 17.Schousboe I, Feddersen K, Røjkjaer R. Factor XIIa is a kinetically favorable plasminogen activator. Thromb Haemost. 1999;82(3):1041-1046. [PubMed] [Google Scholar]
- 18.Carmeliet P, Schoonjans L, Kieckens L, et al. . Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368(6470):419-424. [DOI] [PubMed] [Google Scholar]
- 19.Levi M, Hack CE, de Boer JP, Brandjes DP, Büller HR, ten Cate JW. Reduction of contact activation related fibrinolytic activity in factor XII deficient patients. Further evidence for the role of the contact system in fibrinolysis in vivo. J Clin Invest. 1991;88(4):1155-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci USA. 2006;103(4):903-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller F, Mutch NJ, Schenk WA, et al. . Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139(6):1143-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannemeier C, Shibamiya A, Nakazawa F, et al. . Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci USA. 2007;104(15):6388-6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maas C, Govers-Riemslag JW, Bouma B, et al. . Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118(9):3208-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Meijden PE, Munnix IC, Auger JM, et al. . Dual role of collagen in factor XII-dependent thrombus formation. Blood. 2009;114(4):881-890. [DOI] [PubMed] [Google Scholar]
- 25.Brown MR, Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci USA. 2004;101(46):16085-16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279(43):44250-44257. [DOI] [PubMed] [Google Scholar]
- 27.Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89-125. [DOI] [PubMed] [Google Scholar]
- 28.Smith SA, Choi SH, Davis-Harrison R, et al. . Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116(20):4353-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112(7):2810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutch NJ, Engel R, Uitte de Willige S, Philippou H, Ariëns RA. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 2010;115(19):3980-3988. [DOI] [PubMed] [Google Scholar]
- 31.Schousboe I. Factor XIIa activation of plasminogen is enhanced by contact activating surfaces and Zn2+. Blood Coagul Fibrinolysis. 1997;8(2):97-104. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell JL, Lionikiene AS, Fraser SR, Whyte CS, Booth NA, Mutch NJ. Functional factor XIII-A is exposed on the stimulated platelet surface. Blood. 2014;124(26):3982-3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Booth NA, Simpson AJ, Croll A, Bennett B, MacGregor IR. Plasminogen activator inhibitor (PAI-1) in plasma and platelets. Br J Haematol. 1988;70(3):327-333. [DOI] [PubMed] [Google Scholar]
- 34.Mutch NJ, Myles T, Leung LL, Morrissey JH. Polyphosphate binds with high affinity to exosite II of thrombin. J Thromb Haemost. 2010;8(3):548-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engel R, Brain CM, Paget J, Lionikiene AS, Mutch NJ. Single-chain factor XII exhibits activity when complexed to polyphosphate. J Thromb Haemost. 2014;12(9):1513-1522. [DOI] [PubMed] [Google Scholar]
- 36.Choi SH, Collins JN, Smith SA, Davis-Harrison RL, Rienstra CM, Morrissey JH. Phosphoramidate end labeling of inorganic polyphosphates: facile manipulation of polyphosphate for investigating and modulating its biological activities. Biochemistry. 2010;49(45):9935-9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim PY, Tieu LD, Stafford AR, Fredenburgh JC, Weitz JI. A high affinity interaction of plasminogen with fibrin is not essential for efficient activation by tissue-type plasminogen activator. J Biol Chem. 2012;287(7):4652-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucas MA, Straight DL, Fretto LJ, McKee PA. The effects of fibrinogen and its cleavage products on the kinetics of plasminogen activation by urokinase and subsequent plasmin activity. J Biol Chem. 1983;258(20):12171-12177. [PubMed] [Google Scholar]
- 39.Markus G, Evers JL, Hobika GH. Comparison of some properties of native (Glu) and modified (Lys) human plasminogen. J Biol Chem. 1978;253(3):733-739. [PubMed] [Google Scholar]
- 40.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257(6):2912-2919. [PubMed] [Google Scholar]
- 41.Jansen PM, Pixley RA, Brouwer M, et al. . Inhibition of factor XII in septic baboons attenuates the activation of complement and fibrinolytic systems and reduces the release of interleukin-6 and neutrophil elastase. Blood. 1996;87(6):2337-2344. [PubMed] [Google Scholar]
- 42.Braat EA, Dooijewaard G, Rijken DC. Fibrinolytic properties of activated FXII. Eur J Biochem. 1999;263(3):904-911. [DOI] [PubMed] [Google Scholar]
- 43.Konings J, Hoving LR, Ariëns RS, et al. . The role of activated coagulation factor XII in overall clot stability and fibrinolysis. Thromb Res. 2015;136(2):474-480. [DOI] [PubMed] [Google Scholar]
- 44.Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005;106(8):2605-2612. [DOI] [PubMed] [Google Scholar]
- 45.Nesheim M. Fibrinolysis and the plasma carboxypeptidase. Curr Opin Hematol. 1998;5(5):309-313. [DOI] [PubMed] [Google Scholar]
- 46.Whyte CS, Chernysh IN, Domingues MM, et al. . Polyphosphate delays fibrin polymerisation and alters the mechanical properties of the fibrin network [published online ahead of print 25 August 2016]. Thromb Haemost [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Booth NA, Anderson JA, Bennett B. Plasminogen activators in alcoholic cirrhosis: demonstration of increased tissue type and urokinase type activator. J Clin Pathol. 1984;37(7):772-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Booth NA, Walker E, Maughan R, Bennett B. Plasminogen activator in normal subjects after exercise and venous occlusion: t-PA circulates as complexes with C1-inhibitor and PAI-1. Blood. 1987;69(6):1600-1604. [PubMed] [Google Scholar]
- 49.Huisman LG, van Griensven JM, Kluft C. On the role of C1-inhibitor as inhibitor of tissue-type plasminogen activator in human plasma. Thromb Haemost. 1995;73(3):466-471. [PubMed] [Google Scholar]
- 50.Wat JM, Foley JH, Krisinger MJ, et al. . Polyphosphate suppresses complement via the terminal pathway. Blood. 2014;123(5):768-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacQuarrie JL, Stafford AR, Yau JW, et al. . Histidine-rich glycoprotein binds factor XIIa with high affinity and inhibits contact-initiated coagulation. Blood. 2011;117(15):4134-4141. [DOI] [PubMed] [Google Scholar]
- 52.Vu TT, Leslie BA, Stafford AR, Zhou J, Fredenburgh JC, Weitz JI. Histidine-rich glycoprotein binds DNA and RNA and attenuates their capacity to activate the intrinsic coagulation pathway. Thromb Haemost. 2016;115(1):89-98. [DOI] [PubMed] [Google Scholar]
- 53.Vu TT, Zhou J, Leslie BA, et al. . Arterial thrombosis is accelerated in mice deficient in histidine-rich glycoprotein. Blood. 2015;125(17):2712-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marx G, Korner G, Mou X, Gorodetsky R. Packaging zinc, fibrinogen, and factor XIII in platelet alpha-granules. J Cell Physiol. 1993;156(3):437-442. [DOI] [PubMed] [Google Scholar]
- 55.Leung LL, Harpel PC, Nachman RL, Rabellino EM. Histidine-rich glycoprotein is present in human platelets and is released following thrombin stimulation. Blood. 1983;62(5):1016-1021. [PubMed] [Google Scholar]
- 56.Kuijpers MJ, van der Meijden PE, Feijge MA, et al. . Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler Thromb Vasc Biol. 2014;34(8):1674-1680. [DOI] [PubMed] [Google Scholar]
- 57.Konings J, Govers-Riemslag JW, Philippou H, et al. . Factor XIIa regulates the structure of the fibrin clot independently of thrombin generation through direct interaction with fibrin. Blood. 2011;118(14):3942-3951. [DOI] [PubMed] [Google Scholar]
- 58.Whyte CS, Swieringa F, Mastenbroek TG, et al. . Plasminogen associates with phosphatidylserine-exposing platelets and contributes to thrombus lysis under flow. Blood. 2015;125(16):2568-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stalker TJ, Traxler EA, Wu J, et al. . Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng R, Wu J, Harper DC, et al. . Defective release of α granule and lysosome contents from platelets in mouse Hermansky-Pudlak syndrome models. Blood. 2015;125(10):1623-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharda A, Kim SH, Jasuja R, et al. . Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome. Blood. 2015;125(10):1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harper MT, van den Bosch MT, Hers I, Poole AW. Platelet dense granule secretion defects may obscure α-granule secretion mechanisms: evidence from Munc13-4-deficient platelets. Blood. 2015;125(19):3034-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109(2):566-576. [DOI] [PubMed] [Google Scholar]
- 64.Witte LD, Kaplan KL, Nossel HL, Lages BA, Weiss HJ, Goodman DS. Studies of the release from human platelets of the growth factor for cultured human arterial smooth muscle cells. Circ Res. 1978;42(3):402-409. [DOI] [PubMed] [Google Scholar]
- 65.Berman CL, Yeo EL, Wencel-Drake JD, Furie BC, Ginsberg MH, Furie B. A platelet alpha granule membrane protein that is associated with the plasma membrane after activation. Characterization and subcellular localization of platelet activation-dependent granule-external membrane protein. J Clin Invest. 1986;78(1):130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]