Abstract
Hematopoietic cell transplantation (HCT) survivors treated with total body irradiation (TBI) are known to be at increased risk for the development of cardiovascular risk factors (CVRF). We sought to characterize the incidence of CVRF in a TBI-exposed survivor cohort and describe prognostic indicators of their development. Retrospective analysis of CVRF in 1-year survivors of leukemia or lymphoma treated with TBI at Memorial Sloan Kettering from April 1987–May 2011. Eligible participants were≤ 21 years of age at TBI and were not on glucocorticoids at the time of entry to Long-Term Follow-Up. Survivors were assessed for obesity (body mass index [BMI]≥ 95th% for ages≤ 20; ≥30 kg/m2 for ages >20 years), elevated blood pressure, dyslipidemia (elevated triglycerides [TG], low high-density lipoprotein [HDL]), and glucose intolerance (fasting glucose ≥100 mg/dl); those with ≥3 risk factors were deemed to have a CVRF cluster, a surrogate for metabolic syndrome. Cox regression models were used to estimate hazard ratios (HRs) evaluating factors associated with each CVRF. In order to compare prevalence of CVRF in HCT survivors and the general population, survivors were compared to age-, sex-, and race-matched controls from the National Health and Nutrition Examination Survey (NHANES). 123 survivors were evaluated (62.6% male). Median age at TBI was 11.8 years (range 1.6–21.9); median followup was 8.0 years (1.01–24.6); median age at last followup 20.1 years (4.0–41.3). Five-year cumulative incidence of elevated blood pressure, elevated glucose, low HDL, hypertriglyceridemia, and obesity was 14.7%, 10.5%, 26.8%, 39.2%, and 16.0%, respectively, while 10-year cumulative incidence was 28.8%, 33.1%, 52.0%, 65.0%, and 18.6%, respectively. The cumulative incidence of CVRF cluster rose from 10.6% (5.6–17.5) at 5 years to 28.4% (18.8–38.7) at 10 years. In multivariate analysis, growth hormone (GH) deficiency (HR 8.6; 95% CI, 2.1–34.4, p=0.002), history of cranial radiation (HR 4.0; 95% CI, 1.7–9.6, p=0.002), and grade II-IV acute GVHD (HR 4.2; 95% CI, 1.5–12.2, p=0.008) were associated with risk of developing CVRF cluster. HCT survivors had an increased prevalence of hypertriglyceridemia and low HDL, but not glucose intolerance, elevated blood pressure, or CVRF cluster, when compared to a random sample of matched population controls. Given the young age of this HCT survivor cohort, these data highlight the importance of routine screening for CVRF starting in childhood among those exposed to TBI.
Keywords: survivors, transplant, cardiovascular risk factors, metabolic syndrome, total body irradiation
Introduction
Advances in hematopoietic cell transplantation (HCT) and supportive care have resulted in improved survival rates for patients with high-risk hematologic malignancies.1-3 Long-term survivors, however, remain at increased risk for treatment-related morbidity, 4-6 including multiple cardiovascular risk factors (CVRF), such as obesity, elevated blood pressure, glucose intolerance, and dyslipidemia.7-10 Taken together, these factors constitute the so-called metabolic syndrome, a constellation of abnormalities associated with increased all-cause and cardiovascular mortality.11, 12
Prior studies have demonstrated an increased prevalence of metabolic syndrome and its components in HCT survivors.8, 9, 13-22 Exposure to total body irradiation (TBI) has been identified as an independent risk factor for the development of CVRF.8, 14, 23-25 In a large single-institution study of 1885 one-year HCT survivors (median age at HCT: 44.4 years; 30.5% treated prior to age 35; 52.7% treated with TBI), the prevalence of CVRF was significantly higher among HCT survivors when compared to the general population. Additionally when compared to HCT survivors not exposed to TBI-based conditioning regimens, those treated with TBI were at 1.5fold increased risk for the development of diabetes mellitus and 1.4-fold increased risk of dyslipidemia.8 Others have similarly demonstrated an increased risk of metabolic derangements in individuals exposed to TBI.13, 14, 17, 18, 26, 27
For those exposed to TBI during childhood, however, data are lacking on longitudinal changes in CVRF and the contribution of demographic and other therapeutic exposures to risk. The current report seeks to fill this gap by using a single-institutional cohort of long-term HCT survivors treated with TBI during childhood to: (1) determine the longitudinal changes in CVRF as this survivor population ages; (2) identify demographic and treatment factors associated with the development of metabolic derangements in this population; and (3) compare the prevalence of CVRF to age-, race- and sex-matched population controls.
Methods
We performed a retrospective analysis of CVRF in one-year HCT survivors treated with TBI at Memorial Sloan Kettering (MSK) between April 1987 and May 2011. All data were obtained from review of the MSK medical record, which includes internal documentation as well as outside correspondence with survivors' local physicians. The protocol was approved by the MSK Institutional Review Board/Privacy Board.
Subjects
Eligible participants had a primary diagnosis of leukemia or lymphoma, were ≤ 21 years of age at the time of TBI, and survived at least one year relapse-free from the date of HCT. All participants had been seen at least once in one of the long-term follow-up (LTFU) clinics at MSK, which provide risk-based comprehensive follow-up care to individuals who have survived at least one year after completion of cancer-directed therapy. All patients underwent serial assessments of height, weight, blood pressure, fasting glucose, and fasting lipid panel, as would be recommended by the Children's Oncology Group LTFU guidelines.28
Patients were censored at the date of relapse. Any survivor who was transplanted more than once was excluded. Additionally, in an effort to exclude those with active graft-versus-host disease (GVHD) and avoid potential confounding associated with glucocorticoid use, individuals taking glucocorticoids at the time of their first LTFU visit for at least three months were excluded as well (n=11). Indications for glucocorticoid use among excluded individuals were: chronic graft versus host disease (n=7); post-transplant autoimmune hemolytic anemia (n=1); nonspecific arthritis (n=1); rejection prophylaxis after renal transplant for post-HCT renal failure (n=1); recurrent bronchiolitis obliterans organizing pneumonia (n=1).
Exposure data
Demographic information was abstracted from the medical record (see Table 1). Race/ethnicity was self-reported by the patient and/or family. Post-treatment complications including thyroid dysfunction, sex hormone deficiency, and documented growth hormone (GH) deficiency, as well as dates of treatment when relevant, were recorded.
Table 1. Characteristics of the study participants.
| Characteristic | All survivors (n=123) |
|---|---|
| Age at TBI, y, median (range) | 11.8 (1.6, 21.9) |
| Age at last followup, y, median (range) | 20.1 (4.0, 41.3) |
| Follow-up since TBI, y, median (range) | 8.0 (1.01, 24.6) |
| Sex, no. (%) | |
| Male | 77 (62.6) |
| Female | 46 (37.3) |
| Race, no. (%) | |
| White, non-Hispanic | 96 (78.0) |
| Other | 23 (18.6) |
| No response | 4 (0.03) |
| TBI dose (cGy), no. (%) | |
| Range | 12–15 Gy |
| ≤ 1410 | 54 (43.9) |
| > 1410 | 69 (56.1) |
| Primary diagnosis, no. (%) | |
| ALL/NHL | 77 (62.6) |
| AML/CML | 46 (37.4) |
| Pre-transplant therapy, no. (%) | |
| Anthracyclines | 115 (93.5) |
| Glucocorticoids* | 100 (81.3) |
| Cranial radiotherapy | 38 (30.9) |
| Pre-transplant BMI, no. (%) | |
| Obese | 20 (16.3) |
| Non-obese | 103 (83.7) |
| HCT type, no. (%) | |
| Autologous | 5 (4.1) |
| Allogeneic | 118 (95.9) |
| Graft source, no. (%) | |
| Bone marrow (BM) | 86 (69.9) |
| Peripheral blood stem cells (PBSC) | 27 (21.9) |
| Cord blood | 8 (6.5) |
| BM + Cord | 1 (0.8) |
| BM + PBSC | 1 (0.8) |
| Donor source, allogeneic transplants only, no. (%) | |
| Related | 59 (50.0) |
| Unrelated | 59 (50.0) |
| GVHD Prophylaxis**, allogeneic transplants only, no. (%) | |
| T-cell depletion | 77 (65.3) |
| Cyclosporine | 35 (29.7) |
| Methotrexate | 28 (23.7) |
| Mycophenolate mofetil | 8 (6.7) |
| Corticosteroids | 6 (5.2) |
| Tacrolimus | 5 (4.3) |
| Sirolimus | 1 (0.9) |
| Acute GVHD, allogeneic transplants only, no. (%) | |
| Grade I or none | 104 (88.1) |
| Grade II-IV | 14 (11.8) |
| Chronic GVHD, allogeneic transplants only, no. (%) | |
| No | 107 (90.7) |
| Yes | 11 (9.3) |
Abbreviations: y indicates years; HCT, hematopoietic stem cell transplant; TBI, total body irradiation; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; and GVHD, graft-versus-host disease.
Refers to glucocorticoids used for upfront chemotherapy;
Numbers do not sum to 100 percent since most allogeneic HCT recipients received more than one agent for GVHD prophylaxis
Treatment exposures included pre-HCT chemotherapy, high-dose chemotherapy related to the conditioning regimen, and sites and doses of radiation therapy were abstracted from the medical record. Details related to transplant-related exposures, including donor type, stem cell source, and TBI-based conditioning regimen, were obtained from the MSK medical record and transplant database. Presence and severity of acute (aGVHD) and/or chronic GVHD (cGVHD) were recorded as well.29, 30
Outcome measurements
For this analysis, CVRFs of interest included: elevated blood pressure, elevated triglycerides (TG), low high-density lipoprotein (HDL) cholesterol, elevated fasting glucose, and obesity. Waist circumference was not routinely recorded in the medical record.
Individual CVRF were defined according to current adult International Diabetes Foundation Consensus criteria, as well as pediatric-adapted values when indicated,31 which have been previously used in other analyses of CVRF in childhood cancer survivors.32 Table 2 summarizes pediatric- and adult-specific criteria used to define each CVRF in the present analysis.
Table 2. Definitions of cardiovascular risk factors (CVRF) in adult and pediatric individuals.
| Consensus criteria | ||
|---|---|---|
| Adult31 | Pediatric23 | |
| Obesity | BMI ≥ 30 kg/m2 | BMI ≥ 95th percentile for age and sex |
| Elevated blood pressure | ≥ 130/85 mmHg | ≥ 90th percentile for age, sex, and height |
| Elevated glucose | Fasting glucose≥ 100 mg/dl | Fasting glucose≥ 100 mg/dl |
| Low HDL-cholesterol | Males < 40 mg/dl Females < 50 mg/dl |
≤ 40 mg/dl |
| Hypertriglyceridemia | ≥ 150 mg/dl | ≥ 110 mg/dl |
HDL indicates high density lipoprotein; any individual taking drugs for hypertension and/or diabetes was classified as fulfilling the associated criterion
Consensus criteria for obesity suggest that if BMI is greater than 30 kg/m2, then waist circumference does not need to be measured, as over 95% of these individuals will have a waist circumference above gender- and ethnic-specific threshold values for obesity
For each participant, serial measurements of height, weight, and blood pressure were abstracted from the medical record. Normative pediatric data were used to calculate age- and gender-specific body mass index (BMI) 33 for those between the ages of 2-20 years, and age-, gender, and height-specific blood pressure percentiles for those between the ages of 2-17 years.34 Fasting glucose, triglyceride, and HDL cholesterol levels at each time point were recorded as well. The onset of elevated blood pressure or hypertension, glucose intolerance, or dyslipidemia was defined as an abnormal value in the medical record according to the predefined criteria (Table 2) or the start of drug therapy for the associated outcome of interest. However, any individual receiving antihypertensive medication for non-hypertensive conditions (renal dysfunction, cardiac dysfunction) was considered inevaluable for the elevated blood pressure outcome; similarly those taking statins or other lipid-lowering medications (except omega-3-acid ethyl esters) were considered inevaluable for the high triglyceride/low HDL outcome. The term CVRF cluster, a surrogate for metabolic syndrome, was used to characterize the occurrence of three or more of the five pre-defined CVRFs.32
Statistical considerations
We estimated the cumulative incidence function for each CVRF with a nonparametric estimate35 treating death as a competing risk and using the time since TBI as the time scale. Patients were considered at risk for this analysis beginning at one year after TBI.
Cause-specific Cox proportional hazards regression models were used to evaluate the association between risk factors and each CVRF, stratifying by treatment year (≤ 2000, >2000), and using time since treatment as the scale. Patients were considered at risk of a CVRF beginning at one year after TBI until development of a CVRF, death, relapse, or the date of their last clinic visit without a CVRF. GH deficiency was included as a time-dependent covariate in all models. Multivariate models were constructed using a forward selection procedure which considered all univariate risk factors having p-values < 0.1 as candidates and retaining those with an adjusted p-value < 0.05 in the final multivariate models. All multivariate models were adjusted for age at TBI.
In order to compare prevalence of CVRF in HCT survivors and the general population we used a random sample of controls from the National Health and Nutrition Examination Survey (NHANES). For each visit an HCT survivor contributed to the data, three controls from NHANES were selected and matched on sex, age at assessment (10-year group), and race/ethnicity. Differences in prevalence of CVRFs between the HCT and NHANES cohort were evaluated using generalized estimating equations with an independent working correlation matrix and adjusted for era of assessment (1991-2000, 2001-2006, and 2007-2013).
Results
Description of the cohort
Baseline demographic characteristics of the cohort are summarized in Table 1. Among 123 childhood HCT survivors treated with TBI, 62.6% were male (n=77). TBI exposure occurred at a median age of 11.8 years (range, 1.6–21.9). Median age at last follow-up was 20.1 years (range, 4.0–41.3 years) and the duration of follow-up ranged from 1.01–24.6 years (median: 8.0). The number of visits recorded per patient ranged from one to 18.
During the post-transplant period, 27 survivors (22.0%) developed documented GH deficiency; 18 of the 27 (66.7%) elected to receive treatment with GH. Gonadal function could be assessed in 42 females and 72 males, all of whom were 10 years of age or older. One hundred percent of females (n=42) and 25.0% of males (n=18) had evidence of sex hormone deficiency, as defined by elevated FSH values (> 15 mU/ml) in females and elevated LH levels (> 15 mU/ml) with low testosterone levels (<250 ng/dl) in sexually mature males, or use of hormone replacement therapy in either gender. Thirty-eight individuals had evidence of primary hypothyroidism and 36 (94.7%) were on levothyroxine replacement therapy.
Cumulative incidence of CVRF and CVRF cluster
Five- and ten-year cumulative incidence estimates of CVRF and CVRF cluster are listed in Table 3.
Table 3. Cumulative incidence estimates of cardiovascular risk factors (CVRF) in childhood HCT survivors treated with TBI (n=123).
| Outcome | N | Events | 5-year cumulative incidence* (95% CI) | 10-year cumulative incidence* (95% CI) |
|---|---|---|---|---|
| Elevated blood pressure | 118 | 34 | 14.7 (8.6, 22.4) | 28.8 (19.2, 39.2) |
| Elevated glucose | 121 | 47 | 10.5 (5.5, 17.3) | 33.1 (22.4, 44.1) |
| Low HDL-cholesterol | 116 | 58 | 26.8 (18.8, 35.5) | 52.0 (40.7, 62.2) |
| Hypertriglyceridemia | 117 | 77 | 39.2 (29.8, 48.4) | 65.0 (53.8, 74.2) |
| Obesity | 123 | 21 | 16.0 (9.9, 23.4) | 18.6 (11.7, 26.7) |
| CVRF cluster1 | 123 | 35 | 10.6 (5.6, 17.5) | 28.4 (18.8, 38.7) |
HCT indicates hematopoietic cell transplantation; TBI, total body irradiation; CI, confidence interval; and HDL, high-density lipoprotein.
Cumulative incidence estimates reflect the time since TBI with time zero defined as one year after the date of TBI;
Defined as having 3 or more of the following CVRF: obesity, elevated blood pressure, elevated fasting glucose, low HDL, and elevated triglycerides.
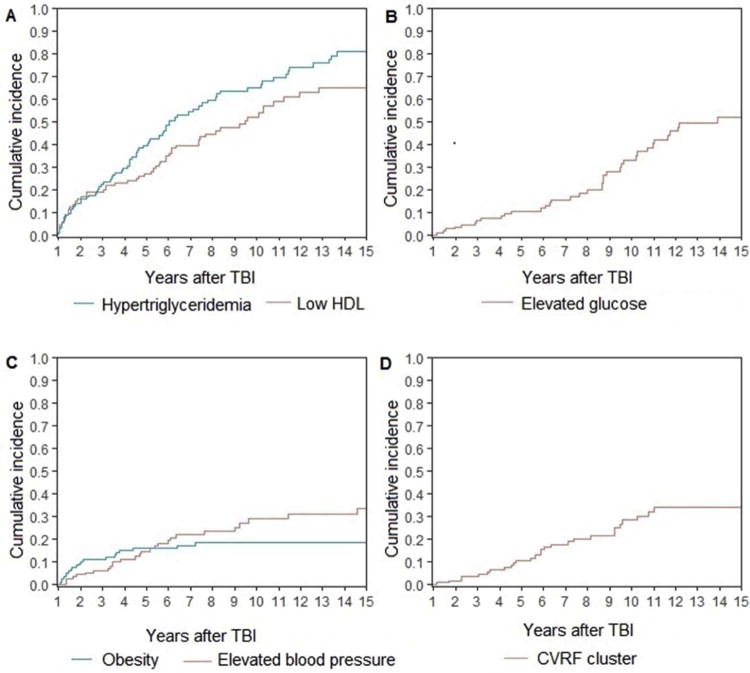
Among 123 HCT survivors treated with TBI, the estimated cumulative incidence of low HDL rose 1.9-fold from 26.8% (95% CI, 18.8–35.5) at 5-years to 52.0% (95% CI, 40.7–62.2) at 10-years [Figure 1a]. Similarly, the cumulative incidence of hypertriglyceridemia increased 1.7-fold from 39.2% (95% CI, 29.8–48.4) at 5-years to 65.0% (95% CI, 53.8–74.2) at 10-years [Figure 1a]. The cumulative incidence of elevated fasting glucose levels increased 3.2-fold from 10.5% at 5-years (95% CI, 5.5–17.3) to 33.1% (95% CI, 22.4–44.1) at 10-years [Figure 1b]. The estimated cumulative incidence of elevated blood pressure doubled from 14.7% (95% CI, 8.6–22.4) at 5-years to 28.8% (95% CI, 19.2–39.2) at 10-years [Figure 1c]. Only obesity remained relatively stable over time, with 5-year cumulative incidence estimated at 16.0% (95% CI, 9.9–23.4) and 10-year cumulative incidence estimated at 18.6% (95% CI, 11.7–26.7) [Figure 1c].
Figure 1.
Cumulative incidence of (a) hypertriglyceridemia [blue] and low HDL [red]; (b) glucose intolerance [red]; (c) obesity [blue] and elevated blood pressure [red]; and (d) cardiovascular risk factor cluster [red] among 123 childhood transplant survivors treated with total body irradiation
Overall, the cumulative incidence of CVRF cluster increased 2.7-fold from 10.6% (95% CI, 5.6– 17.5) at 5 years to 28.4% (95% CI, 18.8–38.7) at 10 years [Figure 1d].
Factors associated with CVRF and CVRF cluster after TBI-based HCT
Results of the multivariate analysis are presented in Table 4. None of the covariates of interest were significantly associated with hypertriglyceridemia, obesity, or glucose intolerance after adjusting for age at TBI (HR 0.8; 95% CI, 0.8–0.9, p<0.001). Higher grade of aGVHD (HR 4.3; 95% CI, 1.5–12.3, p=0.007) and documented GH deficiency (HR 3.9; 95% CI, 1.3–12.3, p=0.02) were associated with elevated blood pressure Higher dose of doxorubicin was associated with low HDL (HR 2.0; 95% CI, 1.04–3.9, p=0.04). Exposure to CRT (HR 4.0; 95% CI, 1.7–9.6, p=0.002), GH deficiency (HR 8.6; 95% CI, 2.1–34.4, p=0.002), and history of grade II-IV aGVHD (HR 4.3; 95% CI, 1.5–12.2, p=0.008) were associated with risk of developing CVRF cluster.
Table 4. Predictors of individual cardiovascular risk factors (CVRF) and CVRF cluster in multivariate analysis.
| Elevated blood pressure | Elevated glucose | Low HDL | CVRF cluster* | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age at TBI | 1.0 (0.9, 1.2) | 0.64 | 0.8 (0.8, 0.9) | <0.001 | 1.1 (0.98,1.3) | 0.09 | 0.9 (0.8, 1.0) | 0.16 |
| Sex | ||||||||
| Male | ||||||||
| Female | ||||||||
| Race | ||||||||
| White, non-Hispanic | ||||||||
| Other | ||||||||
| Cranial RT | 0.002 | |||||||
| No | Reference | |||||||
| Yes | 4.0 (1.7, 9.6) | |||||||
| Anthracycline dose (mg/m2) | 0.04 | |||||||
| ≤ 300 mg/m2 | Reference | |||||||
| >300 mg/m2 | 2.0 (1.04, 3.9) | |||||||
| Glucocorticoids | ||||||||
| No | ||||||||
| Yes | ||||||||
| HCT type | ||||||||
| T-cell depleted | ||||||||
| Unmodified | ||||||||
| aGVHD grade | 0.007 | 0.008 | ||||||
| Grade 0-1 | Reference | Reference | ||||||
| Grade 2-4 | 4.3 (1.5,12.3) | 4.2 (1.5, 12.2) | ||||||
| GH deficiency** | 0.02 | 0.002 | ||||||
| No | Reference | Reference | ||||||
| Yes | 3.9 (1.3,12.3) | 8.6 (2.1, 34.4) | ||||||
Note: All analyses are stratified by treatment year ≤ 2000 vs > 2000 with time zero defined as age at TBI + 1 year; multivariate models constructed via forward selection with age at TBI in the initial model and considering all univariate covariates with p<0.1 as candidate predictors; full results for each model are shown. No significant predictors at 0.05 level for obesity or hypertriglyceridemia, thus omitted from the table above
CVRF cluster defined as having 3 or more of the following CVRF: obesity, elevated glucose, elevated blood pressure, low HDL, high triglycerides
Time dependent covariate;
Abbreviations: TBI, indicates total body irradiation; HCT, hematopoietic cell transplantation; GVHD, graft-versus-host disease; GH, growth hormone; HDL, high density lipoprotein
Prevalence of CVRF in HCT survivors and the general US population
Overall, the prevalence of low HDL and hypertriglyceridemia was significantly higher among HCT survivors exposed to TBI during childhood when compared to sex-, age-, and race/ethnicity-matched controls from the general population (Table 5). Matched controls were more likely to be obese than TBI-exposed HCT survivors. There was no difference in the prevalence of glucose intolerance, elevated blood pressure, or CVRF cluster between the TBI-exposed survivor cohort and controls.
Table 5. Prevalence of cardiovascular risk factors among TBI-exposed HCT survivors and the general population by era.
| Prevalence | NHANES (%) | HCT survivors (%) | p-value |
|---|---|---|---|
| Elevated blood pressure | 0.63 | ||
| 1991-2000 | 11.9 | 11.1 | |
| 2001-2006 | 13.4 | 11.9 | |
| 2007-2013 | 14.0 | 12.9 | |
| Elevated glucose | 0.07 | ||
| 1991-2000 | 12.0 | 7.7 | |
| 2001-2006 | 14.4 | 17.8 | |
| 2007-2013 | 22.4 | 31.0 | |
| Low HDL cholesterol | 0.02 | ||
| 1991-2000 | 27.5 | 52.4 | |
| 2001-2006 | 19.1 | 29.4 | |
| 2007-2013 | 25.1 | 32.9 | |
| Hypertriglyceridemia | < 0.001 | ||
| 1991-2000 | 22.6 | 52.6 | |
| 2001-2006 | 29.3 | 55.8 | |
| 2007-2013 | 28.5 | 46.9 | |
| Obesity | 0.009 | ||
| 1991-2000 | 13.9 | 20.9 | |
| 2001-2006 | 18.4 | 9.5 | |
| 2007-2013 | 25.7 | 12.5 | |
| CVRF cluster1 | 0.70 | ||
| 1991-2000 | 5.5 | 5.9 | |
| 2001-2006 | 8.0 | 6.3 | |
| 2007-2013 | 12.1 | 14.4 |
TBI indicates total body irradiation; HCT, hematopoietic cell transplantation; NHANES, National Health and Nutrition Examination Survey; HDL, high density lipoprotein; CVRF, cardiovascular risk factor
Defined as having 3 or more of the following CVRF: obesity, elevated blood pressure, elevated fasting glucose, low HDL cholesterol, and elevated triglycerides.
Discussion
We found that the risk of elevated blood pressure, low HDL, hypertriglyceridemia, glucose intolerance, and CVRF cluster increases over time in childhood HCT survivors treated with TBI. For all CVRF, except obesity, the cumulative incidence increased 1.7 to 3.2-fold from five to ten years post-TBI. However, when compared to a random sample of matched population controls, survivors were at increased risk for hypertriglyceridemia and low HDL, but not for the other outcomes of interest.
Prior studies have demonstrated that CVRF are associated with an increased risk of cardiovascular disease in HCT survivors8, 36 as well as in the general population.12, 37 Survivors with persistent CVRF at two or more years post- HCT have been shown to have an increased risk for several adverse cardiovascular outcomes, including ischemic heart disease, cardiomyopathy, and all-cause cardiovascular death, when compared to HCT survivors without these risk factors.38 Additionally, prior data have demonstrated that the risk of major cardiac events is potentiated beyond what would be expected in a simple additive model among childhood cancer survivors exposed to chest-directed radiotherapy who also have hypertension, one CVRF of interest.39 In our population of HCT survivors treated with TBI during childhood, the concern for long-term cardiovascular health is especially pronounced given that all individuals received chest-directed radiotherapy and the vast majority received anthracycline chemotherapy as well.
Earlier analyses of metabolic risk in adult survivors of childhood leukemia have demonstrated that those exposed to TBI are more likely to develop CVRF8, 32 and metabolic syndrome 40 when compared to survivors not so treated. In the pediatric age group, however, data are limited. Two cross-sectional analyses have demonstrated that those exposed to TBI during childhood are more likely to manifest multiple cardiometabolic traits, including central adiposity, elevated blood pressure, insulin resistance, and dyslipidemia,23 and/or abnormal glucose tolerance,17 at an early age. Similarly, 17 very young patients treated with HCT at less than three years of age (n=11 treated with TBI) showed that more than half developed dyslipidemia,41 which may be associated with early development of metabolic syndrome.9 In this analysis, we present novel longitudinal follow-up data on the risk of individual CVRF, and CVRF cluster, among a large cohort of childhood HCT survivors uniformly treated with TBI at a single cancer center.
We found a number of treatment factors to be associated with the development of CVRF cluster, and its individual components. Those treated with CRT, in addition to TBI, were at fourfold risk for development of CVRF cluster. Some have theorized that radiation-induced neuroendocrine dysregulation of the hypothalamic-pituitary axis may be linked to metabolic derangements after radiation impacting the brain, either via leptin insensitivity42 or increased expression of hypothalamic inflammatory pathways.43-45 These derangements may explain some of the excess metabolic risk found among those exposed to CRT, in addition to TBI, in our cohort, but the mechanism underlying this association warrants further study.
GH deficiency was also significantly associated with both elevated blood pressure and development of CVRF cluster in our cohort. Both of these associations have been previously documented in the literature.46-49 In addition to its effect on linear growth, GH deficiency is associated with changes in body composition,50 including reduced lean body mass and increased visceral adiposity,51 a phenotype that has been closely linked, in both healthy individuals and childhood cancer survivors, to insulin resistance and glucose intolerance.52-54 While it is plausible that this phenotype, which has also been consistently noted in TBI-exposed childhood cancer survivors,55, 56 is associated with early onset of CVRF and CVRF cluster, it is noteworthy that GH deficiency was not associated with glucose intolerance in the current stud.
Irrespective of GH status, TBI-treated individuals are more likely to be underweight than unexposed individuals,57 despite their adverse cardiometabolic profiles. It is thus not surprising that HCT survivors in our cohort, uniformly exposed to TBI, were less likely to be obese than matched NHANES population-based controls (p=0.009). As noted above, TBI-exposed HCT survivors have been shown to demonstrate a pattern of visceral adiposity and muscle deficits consistent with sarcopenic obesity,56 which has also been demonstrated in the general HCT survivor population.58 Survivors exposed to TBI have also been shown to have increased C– reactive protein and leptin levels, and decreased adiponectin, suggesting increased inflammation in the presence of visceral fat deposition.23 Dysfunctional adipose tissue, rather than obesity per se, may play an important role in the pathogenesis of metabolic syndrome in TBI-exposed HCT survivors.59
Interestingly, exposure to higher doses of doxorubicin was associated with low HDL in our cohort. Prior preclinical studies have demonstrated that doxorubicin administration is associated with glucose intolerance and hyperlipidemia60, 61 through the inhibition of peroxisomal proliferator activated receptor γ (PPARγ), a ligand-activated transcription factor that plays a key role in fat cell differentiation and insulin sensitization.62 One small study of breast cancer patients also found an association between doxorubin and lower HDL with downregulation of PPARγ noted after doxorubicin administration.63 To our knowledge this association has not been noted in childhood transplant survivors and thus requires replication and additional study in the future.
Allogeneic HCT recipients with grades II-IV aGVHD were also at 4.3-fold increased risk for elevated blood pressure, and 4.2-fold increased risk for CVRF cluster, in multivariate analysis. While this risk may have resulted from prolonged and intensified use of immunosuppressive agents, it is noteworthy that 65.3% of our allogeneic HCT recipients received T cell-depleted HCTs, and thus had reduced exposure to GVHD medications. Additionally, none of the subjects in the current study was on glucocorticoids at the time of their first long-term follow-up appointment, which coincides with the time at which monitoring for CVRF would have commenced. The precise relationship between GVHD prophylactic medication and subsequent risk for cardiometabolic derangements warrants further study.
In our cohort of HCT survivors exposed to TBI during childhood, individuals were more likely to have hypertriglyceridemia and low HDL but not glucose intolerance, elevated blood pressure, or CVRF cluster, when compared to matched population controls. The similar prevalence of glucose intolerance among HCT survivors and population controls is of particular interest given the well-established link between TBI exposure and diabetes mellitus.15, 53, 64 It is possible that these derangements were not yet apparent in our relatively young cohort (median age: 20.3) and will become manifest with longer follow-up. In conventionally treated childhood cancer survivors exposed to abdominal radiation, for instance, there appears to be a minimum latency of 20 years between radiation therapy and onset of diabetes mellitus;65 a similarly prolonged clinically silent period may precede the onset of CVRF, including glucose intolerance, in HCT survivors exposed to TBI during childhood.
A number of limitations must be considered when interpreting the results of this study. Importantly, given the retrospective nature of this analysis, waist circumferences were not routinely collected and we thus relied on BMI as an indicator of obesity, which is known to correlate poorly with true adiposity in childhood cancer survivors,66 and in the TBI-exposed population in particular.23 Additionally, formal GH stimulation testing was only performed in those with evidence of poor linear growth, so the true prevalence of GH deficiency in this cohort may have been under-estimated. Reliable data on lifestyle behaviors or family history were also lacking. Nevertheless, this analysis has a number of related strengths, including comprehensive exposure data and follow-up details on a large number of patients who were treated on protocol-driven studies with intensive TBI-based therapy at a young age, and then followed over time in specialized survivorship clinics.
In summary, individuals exposed to TBI during childhood were more likely than population controls to develop hypertriglyceridemia and low HDL at a young age. These data emphasize the importance of routine screening for CVRF in this high risk population starting in childhood and adolescence. Future studies are needed to clarify the mechanisms underlying these derangements and the impact of primary and secondary prevention on ultimate cardiovascular morbidity and mortality in childhood HCT survivors.
Highlights.
Risk for cardiovascular risk factors (CVRF) increases over time after TBI exposure (84 characters)
Survivors have an increased risk of high triglycerides and low HDL versus controls (84 characters)
Data highlight the importance of CVRF screening starting in childhood after TBI (82 characters)
Acknowledgments
This work was supported by the MSK Cancer Center Support Grant/Core Grant (P30 CA008748) and the Clinical and Translational Science Center at Weill Cornell (KL2 TR000458; DNF); KCO is supported in part by the National Institutes of Health (K05 CA160724)
Footnotes
Financial disclosure statement: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copelan EA. Hematopoietic Stem-Cell Transplantation. New England Journal of Medicine. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia S, Davies SM, Scott Baker K, Pulsipher MA, Hansen JA. NCI, NHLBI first international consensus conference on late effects after pediatric hematopoietic cell transplantation: etiology and pathogenesis of late effects after HCT performed in childhood--methodologic challenges. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1428–1435. doi: 10.1016/j.bbmt.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139. doi: 10.1182/blood-2009-06-229369. quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun CL, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19:1073–1080. doi: 10.1016/j.bbmt.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011;118:1413–1420. doi: 10.1182/blood-2011-01-331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 10.Wilhelmsson M, Vatanen A, Borgstrom B, et al. Adverse health events and late mortality after pediatric allogeneic hematopoietic SCT-two decades of longitudinal follow-up. Bone marrow transplantation. 2015;50:850–857. doi: 10.1038/bmt.2015.43. [DOI] [PubMed] [Google Scholar]
- 11.Wu SH, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25:375–384. doi: 10.1007/s10654-010-9459-z. [DOI] [PubMed] [Google Scholar]
- 12.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. The American jo0urnal of medicine. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Airaghi L, Usardi P, Forti S, et al. A comparison between metabolic syndrome post-hematopoietic stem cell transplantation and spontaneously occurring metabolic syndrome. Journal of endocrinological investigation. 2011;34:e6–11. doi: 10.1007/BF03346702. [DOI] [PubMed] [Google Scholar]
- 14.Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone marrow transplantation. 2012;47:619–625. doi: 10.1038/bmt.2011.118. [DOI] [PubMed] [Google Scholar]
- 15.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakker B, Oostdijk W, Bresters D, Walenkamp MJE, Vossen JM, Wit JM. Disturbances of growth and endocrine function after busulphan-based conditioning for haematopoietic stem cell transplantation during infancy and childhood. Bone marrow transplantation. 2004;33:1049–1056. doi: 10.1038/sj.bmt.1704481. [DOI] [PubMed] [Google Scholar]
- 17.Bizzarri C, Pinto RM, Ciccone S, Brescia LP, Locatelli F, Cappa M. Early and progressive insulin resistance in young non-obese cancer survivors treated with hematopoietic stem cell transplantation. Pediatric blood & cancer. 2015;62:1650–1655. doi: 10.1002/pbc.25603. [DOI] [PubMed] [Google Scholar]
- 18.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone marrow transplantation. 2009;43:49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tauchmanova L, Selleri C, Rosa GD, et al. High prevalence of endocrine dysfunction in long-term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer. 2002;95:1076–1084. doi: 10.1002/cncr.10773. [DOI] [PubMed] [Google Scholar]
- 20.Shalitin S, Phillip M, Stein J, Goshen Y, Carmi D, Yaniv I. Endocrine dysfunction and parameters of the metabolic syndrome after bone marrow transplantation during childhood and adolescence. Bone marrow transplantation. 2006;37:1109–1117. doi: 10.1038/sj.bmt.1705374. [DOI] [PubMed] [Google Scholar]
- 21.Bajwa R, Skeens M, Garee A, et al. Metabolic syndrome and endocrine dysfunctions after HSCT in children. Pediatric transplantation. 2012;16:872–878. doi: 10.1111/petr.12002. [DOI] [PubMed] [Google Scholar]
- 22.Paris C, Yates L, Lama P, Zepeda AJ, Gutierrez D, Palma J. Evaluation of metabolic syndrome after hematopoietic stem cell transplantation in children and adolescents. Pediatric blood & cancer. 2012;59:306–310. doi: 10.1002/pbc.24104. [DOI] [PubMed] [Google Scholar]
- 23.Chow EJ, Simmons JH, Roth CL, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:1674–1681. doi: 10.1016/j.bbmt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulcahy Levy JM, Tello T, Giller R, et al. Late effects of total body irradiation and hematopoietic stem cell transplant in children under 3 years of age. Pediatric blood & cancer. 2013;60:700–704. doi: 10.1002/pbc.24252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, Impaired Glucose Tolerance and Diabetes Mellitus in Survivors of Childhood Cancer: Prevalence and Risk Factors. Journal of Clinical Endocrinology & Metabolism. 2006;91:4401–4407. doi: 10.1210/jc.2006-0128. [DOI] [PubMed] [Google Scholar]
- 26.Chow EJ, Simmons JH, Roth CL, et al. Increased Cardiometabolic Traits in Pediatric Survivors of Acute Lymphoblastic Leukemia Treated with Total Body Irradiation. Biology of Blood and Marrow Transplantation. 2010;16:1674–1681. doi: 10.1016/j.bbmt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisk P, Rossner SM, Norgren S, Arvidson J, Gustafsson J. Glucose metabolism and body composition in young adults treated with TBI during childhood. Bone marrow transplantation. 2011;46:1303–1308. doi: 10.1038/bmt.2010.307. [DOI] [PubMed] [Google Scholar]
- 28.Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers Children's Oncology Group. 2013 [Google Scholar]
- 29.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 30.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Seminars in hematology. 1991;28:250–259. [PubMed] [Google Scholar]
- 31.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic medicine : a journal of the British Diabetic Association. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 32.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer epidemiology biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:170–181. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Ibaragi S, Zhu T, et al. Nicotine promotes mammary tumor migration via a signaling cascade involving protein kinase C and cdc42. Cancer Research. 2008;68:8473–8481. doi: 10.1158/0008-5472.CAN-08-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The fourth report on the diagnosis evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 35.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. John Wiley & Sons; 2011. [Google Scholar]
- 36.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:191–198. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 38.Chow EJ, Wong K, Lee SJ, et al. Late cardiovascular complications after hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:794–800. doi: 10.1016/j.bbmt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable Risk Factors and Major Cardiac Events Among Adult Survivors of Childhood Cancer. Journal of Clinical Oncology. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oudin C, Simeoni MC, Sirvent N, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood. 2011;117:4442–4448. doi: 10.1182/blood-2010-09-304899. [DOI] [PubMed] [Google Scholar]
- 41.Perkins JL, Kunin-Batson AS, Youngren NM, et al. Long-term follow-up of children who underwent hematopoeitic cell transplant (HCT) for AML or ALL at less than 3 years of age. Pediatric blood & cancer. 2007;49:958–963. doi: 10.1002/pbc.21207. [DOI] [PubMed] [Google Scholar]
- 42.Brennan BM, Rahim A, Blum WF, Adams JA, Eden OB, Shalet SM. Hyperleptinaemia in young adults following cranial irradiation in childhood: growth hormone deficiency or leptin insensitivity? Clinical endocrinology. 1999;50:163–169. doi: 10.1046/j.1365-2265.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- 43.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective Loss of Leptin Receptors in the Ventromedial Hypothalamic Nucleus Results in Increased Adiposity and a Metabolic Syndrome. Endocrinology. 2008;149:2138–2148. doi: 10.1210/en.2007-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xue J, Ideraabdullah FY. An assessment of molecular pathways of obesity susceptible to nutrient, toxicant and genetically induced epigenetic perturbation. The Journal of Nutritional Biochemistry. 2016;30:1–13. doi: 10.1016/j.jnutbio.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmeister PA, Hingorani SR, Storer BE, Baker KS, Sanders JE. Hypertension in long-term survivors of pediatric hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16:515–524. doi: 10.1016/j.bbmt.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 48.Oudin C, Auquier P, Bertrand Y, et al. Metabolic syndrome in adults who received hematopoietic stem cell transplantation for acute childhood leukemia: an LEA study. Bone marrow transplantation. 2015;50:1438–1444. doi: 10.1038/bmt.2015.167. [DOI] [PubMed] [Google Scholar]
- 49.Taskinen M, Lipsanen-Nyman M, Tiitinen A, Hovi L, Saarinen-Pihkala UM. Insufficient growth hormone secretion is associated with metabolic syndrome after allogeneic stem cell transplantation in childhood. Journal of pediatric hematology/oncology. 2007;29:529–534. doi: 10.1097/MPH.0b013e3180f61b67. [DOI] [PubMed] [Google Scholar]
- 50.Talvensaari KK, Lanning M, Tapanainen P, Knip M. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. Journal of Clinical Endocrinology & Metabolism. 1996;81:3051–3055. doi: 10.1210/jcem.81.8.8768873. [DOI] [PubMed] [Google Scholar]
- 51.Attallah H, Friedlander AL, Hoffman AR. Visceral obesity, impaired glucose tolerance metabolic syndrome and growth hormone therapy. Growth hormone & IGF research : official journal of the Growth Hormone Research Society and the International IGF Research Society. 2006;16(A):S62–67. doi: 10.1016/j.ghir.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Ascaso JF, Romero P, Real JT, Lorente RI, Martinez-Valls J, Carmena R. Abdominal obesity, insulin resistance, and metabolic syndrome in a southern European population. European journal of internal medicine. 2003;14:101–106. doi: 10.1016/S0953-6205(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 53.Wei C, Thyagiarajan MS, Hunt LP, Shield JP, Stevens MC, Crowne EC. Reduced insulin sensitivity in childhood survivors of haematopoietic stem cell transplantation is associated with lipodystropic and sarcopenic phenotypes. Pediatric blood & cancer. 2015;62:1992–1999. doi: 10.1002/pbc.25601. [DOI] [PubMed] [Google Scholar]
- 54.Demerath EW, Reed D, Rogers N, et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. The American Journal of Clinical Nutrition. 2008;88:1263–1271. doi: 10.3945/ajcn.2008.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mostoufi-Moab S, Ginsberg JP, Bunin N, et al. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. The Journal of pediatrics. 2012;160:122–128. doi: 10.1016/j.jpeds.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mostoufi-Moab S, Magland J, Isaacoff EJ, et al. Adverse Fat Depots and Marrow Adiposity Are Associated With Skeletal Deficits and Insulin Resistance in Long-Term Survivors of Pediatric Hematopoietic Stem Cell Transplantation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30:1657–1666. doi: 10.1002/jbmr.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103:1730–1739. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 58.Inaba H, Yang J, Kaste SC, et al. Longitudinal changes in body mass and composition in survivors of childhood hematologic malignancies after allogeneic hematopoietic stem-cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3991–3997. doi: 10.1200/JCO.2011.40.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turcotte LM, Yingst A, Verneris MR. Metabolic Syndrome after Hematopoietic Cell Transplantation: At the Intersection of Treatment Toxicity and Immune Dysfunction. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016;22:1159–1166. doi: 10.1016/j.bbmt.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arunachalam S, Tirupathi Pichiah PB, Achiraman S. Doxorubicin treatment inhibits PPARgamma and may induce lipotoxicity by mimicking a type 2 diabetes-like condition in rodent models. FEBS letters. 2013;587:105–110. doi: 10.1016/j.febslet.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 61.de Lima Junior EA, Yamashita AS, Pimentel GD, et al. Doxorubicin caused severe hyperglycaemia and insulin resistance mediated by inhibition in AMPk signalling in skeletal muscle. Journal of cachexia, sarcopenia and muscle. 2016 doi: 10.1002/jcsm.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism differentiation, and cell growth. The Journal of biological chemistry. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 63.Sharma M, Tuaine J, McLaren B, et al. Chemotherapy Agents Alter Plasma Lipids in Breast Cancer Patients and Show Differential Effects on Lipid Metabolism Genes in Liver Cells. PloS one. 2016;11:e0148049. doi: 10.1371/journal.pone.0148049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Archives of internal medicine. 2009;169:1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Vathaire F, El-Fayech C, Ben Ayed FF, et al. Radiation dose to the pancreas and risk of diabetes mellitus in childhood cancer survivors: a retrospective cohort study. The Lancet Oncology. 2012;13:1002–1010. doi: 10.1016/S1470-2045(12)70323-6. [DOI] [PubMed] [Google Scholar]
- 66.Karlage RE, Wilson CL, Zhang N, et al. Validity of anthropometric measurements for characterizing obesity among adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. Cancer. 2015;121:2036–2043. doi: 10.1002/cncr.29300. [DOI] [PMC free article] [PubMed] [Google Scholar]