Abstract
N1-methylnicotinamide (1-NMN) has been investigated as an endogenous probe for the renal transporter activity of organic cation transporter 2 (OCT2) and multidrug and toxin extrusion proteins 1 and 2-K (MATE1 and MATE2-K). As pregnancy increased the renal secretion of metformin, a substrate for OCT2, MATE1, and MATE2-K, we hypothesized that the renal secretion of 1-NMN would be similarly affected. Blood and urine samples collected from women prescribed metformin for type 2 diabetes, gestational diabetes, and polycystic ovarian syndrome during early, mid, and late pregnancy (n = 34 visits) and postpartum (n = 14 visits) were analyzed for 1-NMN using liquid chromatography-mass spectrometry. The renal clearance and secretion clearance, using creatinine clearance to correct for glomerular filtration, were estimated for 1-NMN and correlated with metformin renal clearance. 1-NMN renal clearance was higher in both mid (504 ± 293 ml/min, P < 0.01) and late pregnancy (557 ± 305 ml/min, P < 0.01) compared with postpartum (240 ± 106 ml/min). The renal secretion of 1-NMN was 3.5-fold higher in mid pregnancy (269± 267, P < 0.05) and 4.5-fold higher in late pregnancy compared with postpartum (342 ± 283 versus 76 ± 92 ml/min, P < 0.01). Because creatinine is also a substrate of OCT2, MATE1, and MATE2-K, creatinine clearance likely overestimates filtration clearance, whereas the calculated 1-NMN secretion clearance is likely underestimated. Metformin renal clearance and 1-NMN renal clearance were positively correlated (rs = 0.68, P < 0.0001). 1-NMN renal clearance increases during pregnancy due to increased glomerular filtration and net secretion by renal transporters.
Introduction
Renal transporters influence the elimination of many drugs. Cationic drugs as well as endogenous metabolites are taken up by the organic cation transporter 2 (OCT2) on the basolateral membrane in the kidney and excreted into the urine via multidrug and toxin extrusion proteins 1 and 2-K (MATE1 and MATE2-K) in proximal tubule cells (Fujita et al., 2006; Omote et al., 2006). Metformin, a hypoglycemic agent that improves insulin sensitivity, is a well-established probe of OCT2, MATE1, and MATE2-K activity both in vitro and in vivo (Kimura et al., 2005; Tanihara et al., 2007).
Recently, N1-methylnicotinamide (1-NMN) has been proposed as an endogenous probe for OCT2, MATE1, and MATE2-K in vivo (Maïza et al., 1992; Ito et al., 2012; Müller et al., 2015). 1-NMN is formed by methylation of nicotinamide, the amide form of niacin and a precursor for the synthesis of NAD+ and NADP+ by N-methyltransferase (Aksoy et al., 1995, Sauve, 2008). 1-NMN is not bound to plasma proteins (Weber et al., 1991) and has renal clearance that exceeds the estimated glomerular filtration rate (GFR), indicating net secretion in the proximal tubule (Ito et al., 2012). 1-NMN was determined to be a substrate for OCT2, MATE1, and MATE2-K (Ito et al., 2012). Renal clearance of 1-NMN decreased significantly after administration of pyrimethamine, a potent inhibitor of MATE1 and MATE2-K (Ito et al., 2012). Additionally, the renal clearance of 1-NMN and metformin were decreased following administration of trimethoprim, an inhibitor of OCTs and MATEs, in healthy volunteers (Müller et al., 2015).
Renal transporter activity can also be altered by nonpharmacological means. As previously reported, metformin renal clearance increased significantly in mid and late pregnancy compared with postpartum (Eyal et al., 2010). Pregnant women undergo numerous physiologic changes, including increases in renal blood flow and glomerular filtration rate (GFR), that can alter drug disposition and elimination (Davison and Dunlop, 1980; Carlin and Alfirevic, 2008). Although a portion of the higher metformin renal clearance during pregnancy was attributed to higher GFR, tubular section of metformin was also upregulated in pregnancy (Eyal et al., 2010). We hypothesized that if 1-NMN is an endogenous substrate for OCT2, MATE1, and MATE2-K, the renal secretion clearance of 1-NMN in pregnant women would be expected to be higher than postpartum. The objective of the present study was to characterize changes in 1-NMN in women prescribed metformin in early, mid, and late pregnancy.
Materials and Methods
Chemicals and Materials.
1-Methylnicotinamide chloride (1-NMN) was purchased from Sigma (St. Louis, MO). A labeled internal standard, d3-1-methylnicotinatmide iodide (d3-1-NMN) was purchased from Toronto Research Chemical (Toronto, ON, Canada). High-performance liquid chromatography -grade acetonitrile and water were purchased from Fisher Scientific (Pittsburgh, PA). Synthetic urine was prepared as described in Shmaefsky (1990).
Study Subjects.
This study was a secondary use of samples collected from an opportunistic study of the pharmacokinetics of oral metformin in women with diabetes, gestational diabetes, or polycystic ovarian syndrome during pregnancy and postpartum (Eyal et al., 2010). Oral metformin dosing ranged from 0.5 to 3 g/day given every 8 to 24 hours as fully described in Eyal et al. (2010). In brief, blood and urine samples were collecting during early (10–14 weeks gestation), mid (22–26 weeks gestation), and late pregnancy (34–38 weeks gestation); postpartum (≥ 12 weeks); and postpartum postlactation cessation (≥12 weeks). Plasma and urine samples were stored at −80°C until analysis. The study was approved by the Institutional Review Boards at the University of Washington.
Plasma 1-Methylnicotinamide Analysis.
For each subject, plasma samples from a single dosing interval were analyzed (5 to 8 samples per subject). The method was adapted from Lang et al. (2010). After thawing on ice, 10 μl of plasma was diluted with 90 μl of water and vortexed for 15 seconds. Working standards (3.1–500 nM) and quality control (QC) samples (20 and 200 nM) were prepared with each set of samples by diluting 10 μl of stock solution with 10 μl of synthetic urine and 80 μl of water. The synthetic urine was used to provide a matrix for sample extraction. Extraction efficiency did not differ between plasma and synthetic urine (data not shown). Fifty-microliter aliquots of the diluted working standards, QC, and plasma samples were then mixed with 50 µl of 500 nM deuterated d3-1-NMN and 300 µl of ice-cold acetonitrile. Samples were vortexed for 30 seconds follow by centrifugation at 4°C and 15 g for 20 minutes.
Urine 1-Methylnicotinamide Analysis.
For each subject, urine samples from a single dosing interval were analyzed. Most subjects had a single urine sample for the entirety of the dosing interval. For subjects with multiple urine samples for the dosing interval, a pooled aliquot was created based on a weighted average of the urine volumes collected in each interval. After thawing on ice, 10 µl of urine was diluted with 990 µl of water and vortexed for 15 seconds. Working standards (25–1000 nM) and quality control samples (200 and 800 nM) were prepared with each set of samples by diluting 10 µl of each in 990 µl of a dilute blank urine solution, 10 µl of urine for every 980 µl of water. Fifty-microliter aliquots of the working standards, QC, and urine samples were then mixed with 50 µl of 500 nM d3-1-NMN. This was followed by the addition of ice-cold acetonitrile, vortexing, and centrifugation as described for plasma samples. Samples were transferred to vials for subsequent analysis.
Liquid Chromatography-Tandem Mass Spectrometry Analysis.
Ten microliters of each sample was injected onto the high-performance liquid chromatography system with mass spectrometry detection. The liquid chromatography-tandem mass spectrometry system consisted of a Water’s (Milford, MA) quaternary H Class ultraperformance liquid chromatograph coupled to an API 4000 Qtrap tandem quadrupole mass spectrometer from AB Sciex (Framingham, MA). The column was a Cogent Diamond Hydride (150 mm × 2.1 mm × 4 µm) and was fitted with a Cogent Diamond Hydride precolumn (20 mm × 2.0 mm × 4 µm) from MicroSolv Technology (Eatontown, NJ). The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at a flow rate of 0.3 ml/min. The initial conditions were 30% B, which increased to 50% B between 1 and 4 minutes. At 4 minutes, the gradient rapidly increased to 100% B for 1 minute before returning to the initial conditions for 2 minutes. The total run time was 7 minutes per sample.
The column remained at ambient temperature, and the autosampler tray was maintained at 4°C. The mass spectrometry parameters were set as follows: curtain gas 20, collision activated dissociation gas-2, ion spray potential, collision cell exit potential 15 V, desolvation temperature 500°C, collision gas flow of 50 psi, declustering gas 70. The mass spectrometer was operated in the positive electrospray mode for fragmentation of the parent m/z 137.1 → 94.0 (quantification) and m/z 137.1 → 78.0 (qualification) for 1-NMN and m/z 140.1 → 97.0 (quantification) and m/z 140.1 → 78.0 (qualification) for d3-1-NMN. The dwell times for all fragments was set at 250 ms. The retention time for 1-NMN and d3-1-NMN was 2.4 minutes.
Data Analysis.
Peak integration was performed using Analyst software (version 1.6.2) from AB Sciex. The peak area of 1-NMN was normalized to the d3-1-NMN to determine the peak area ratio. As the 1-NMN calibration curve and QC samples for urine were prepared with blank urine, peak area ratios for the calibration curve and QC samples were corrected for the average endogenous 1-NMN in the blank urine. No baseline correction was required for the plasma samples. The lower limit of quantitation was determined as the lowest calibration point with a back-calculated error ≤15% and was 3.1 and 25 nM for plasma and urine, respectively. A linear equation was used to estimate the relationship between peak area ratios and the concentration of 1-NMN.
Pharmacokinetic Analysis.
Metformin steady-state parameters were estimated using standard noncompartmental techniques, as described previously (Eyal et al., 2010). Creatinine clearance was estimated by CrCL = [(urine creatinine concentration)(urine volume)]/[serum creatinine concentration)(duration of collection interval)]. For subjects with multiple urine samples during a dosing interval, creatinine clearance was calculated using a weighted average of urine creatinine values by urine volume.
1-NMN plasma AUC was calculated using the trapezoidal method. The 1-NMN average plasma steady-state concentration was calculated as Css,ave = (N1-methylnicotinamide plasma AUC)/(duration of collection interval). 1-NMN renal clearance was estimated by CLr = [(N1-methylnicotinamide concentration in urine)(urine volume)]/(N1-methylnicotinamide AUC). 1-NMN net renal secretion clearance was estimated by CLsec = CLR – fu × GFR, where the unbound fraction of 1-NMN in plasma, fu, was set to 1 (Weber et al., 1991) and GFR was estimated using CrCL.
Statistical Analysis.
GraphPad Prism (version 5.04 for Windows, GraphPad Software, San Diego, CA) was used for statistical analyses. A Mann-Whitney test was used to compare the estimated renal clearance parameters for early, mid, and late pregnancy to postpartum study days. A Kruskal-Wallis test was used to compare the renal clearance parameters between nonlactating and lactating postpartum visits, as well as postcessation of lactation to postpartum visits. The Spearman correlation (rs) was used to determine associations. All results are reported as mean ± S.D., unless otherwise indicated. P ≤ 0.05 was considered significant.
Results
A total of 25 pregnant subjects (18 white, 2 black, 1 Asian, 1 Hispanic/Latina, 1 Native American, 1 Pacific Islander, and 1 mixed race) participated in the study. Additional subject characteristics are described in Table 1. Of the subjects who were studied postpartum, six were breastfeeding. Three of these subjects were studied again 4 to 12 weeks after cessation of lactation. 1-NMN renal clearance parameters did not differ significantly between lactation and postcessation of lactation study days. As a result, the data for these subjects were reported as the mean of the parameters obtained from the two study days.
TABLE 1.
Characteristics of study subjects
Results are reported as mean ± S.D. unless otherwise indicated.
| Characteristics | Early Pregnancy (10–14 weeks Gestation) | Mid Pregnancy (22–26 weeks Gestation) | Late Pregnancy (34–38 weeks Gestation) | Postpartum (≥12 weeks) |
|---|---|---|---|---|
| Number of subjects | 6 | 15 | 13 | 14 |
| Age (years) | 31 ± 6 | 33 ± 6 | 31 ± 7 | 33 ± 6 |
| Body weight (kg) | 98.4 ± 21.7 | 110.8 ± 29.2 | 118.8 ± 23.2 | 99.2 ± 18.3 |
| Metformin dosing interval median (range) (hours) | 12 (8–12) | 12 (8–24) | 12 (8–24) | 12 (8–24) |
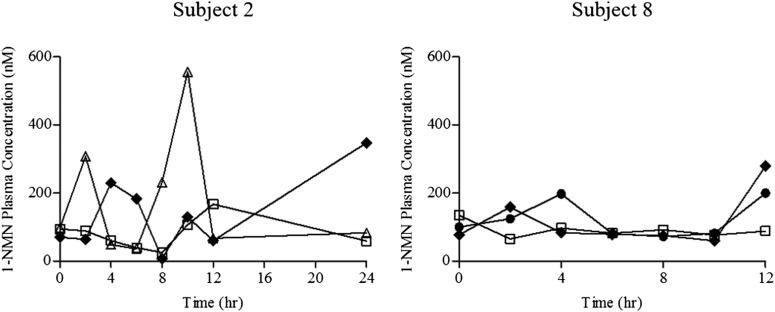
Plasma concentrations of 1-NMN ranged from 8.0 to 1237.0 nM with a median concentration of 165.7 nM. 1-NMN concentrations were highly variable between subjects. Figure 1 shows the plasma concentration versus time profile for two representative subjects. 1-NMN concentrations were variable during each dosing interval with up to 15-fold variation in each patient.
Fig. 1.
1-NMN plasma profiles for two subjects at different gestational stages. Early pregnancy (circles), mid pregnancy (squares), late pregnancy (triangles), postpartum (diamonds).
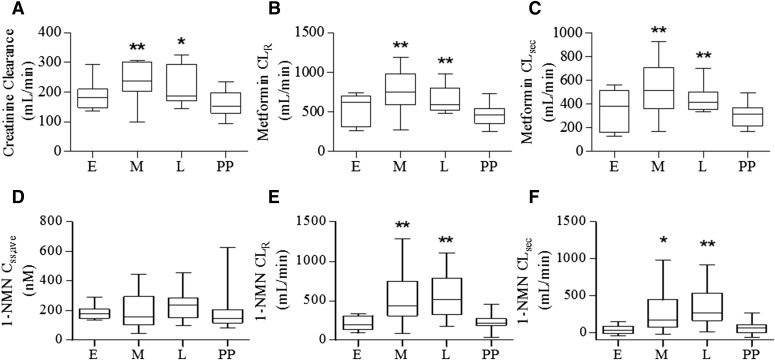
The renal clearances of creatinine, metformin, and 1-NMN are summarized in Table 2. Figure 2 shows the mean and distribution of each of these parameters in early, mid, and late pregnancy and postpartum. As previously reported in Eyal et al. (2010), mean creatinine clearance was 48% higher in mid pregnancy (235 ± 66 ml/min) and 36% higher in late pregnancy (217 ± 63 ml/min) compared with postpartum (159 ± 44 ml/min). Mean metformin renal clearance was 66% and 40% higher in mid and late pregnancy (775 ± 252 and 653 ± 166 ml/min), respectively, compared with postpartum (467 ± 131 ml/min). No significant difference in 1-NMN clearance was found postpartum between lactation, postcessation of lactation, and nonlactation groups (353 ± 212, 188 ± 99, and 213 ml/min, respectively). Mean metformin secretion clearance was 77% higher in mid pregnancy (538 ± 202 ml/min) and 44% higher in late pregnancy (438 ± 110 ml/min) compared with postpartum (304 ± 95 ml/min).
TABLE 2.
Estimated metformin and 1-NMN pharmacokinetic parameters throughout gestation
Results are reported as mean ± S.D. The subject characteristics for early, mid, and late pregnancy were compared with postpartum.
| Early-Pregnancy (10–14 weeks gestation) | Mid-Pregnancy (22–26 weeks gestation) | Late-Pregnancy (34–38 weeks gestation) | Postpartum ≥12 weeks | |
|---|---|---|---|---|
| Number of subjects | 6 | 15 | 13 | 14 |
| Creatinine clearance (ml/min) | 188 ± 55 | 235 ± 66** | 217 ± 63* | 159 ± 44 |
| Metformin | ||||
| Metformin CLR (ml/min) | 542 ± 200 | 775 ± 252** | 653 ± 166** | 467 ± 131 |
| Metformin CLsec (ml/min) | 354 ± 186 | 538 ± 202** | 438 ± 110** | 304 ± 95 |
| 1-NMN | ||||
| 1-NMN Css,ave (nM) | 188 ± 55 | 205 ± 121 | 230 ± 101 | 184 ± 135 |
| 1-NMN CLR (ml/min) | 215 ± 94 | 504 ± 293** | 557 ± 305** | 240 ± 106 |
| 1-NMN CLsec (ml/min) | 42 ± 67 | 269 ± 267* | 342 ± 283** | 76 ± 92 |
CLR, renal clearance; CLsec, renal secretion clearance.
P < 0.05, **P < 0.01 for comparisons to postpartum.
Fig. 2.
Creatinine clearance (A), metformin renal clearance (B), metformin renal secretion clearance (C), 1-NMN average steady-state concentration (D), 1-NMN renal clearance (E), and 1-NMN renal secretion clearance (F) during early, mid, and late pregnancy and postpartum. (*P < 0.05, **P < 0.01 compared with the postpartum group).
1-NMN renal clearance was 2.1- and 2.3-fold higher in mid and late pregnancy (504 ± 293 and 557 ± 305 ml/min), respectively, compared with postpartum (240 ± 106 ml/min). 1-NMN secretion clearance was 3.5- and 4.5-fold higher in mid and late pregnancy (269 ± 267 and 342 ± 283 ml/min), respectively, compared with postpartum (76 ± 92 ml/min). Of the 25 subjects genotyped for OCT2 G808T, 19 women were G/G, 5 women were G/T, and 1 woman was T/T. The increase in metformin and 1-NMN clearance during mid and late pregnancy was not affected by OCT2 genotype (data not shown). In general, the relative change in 1-NMN renal clearance was higher than metformin renal clearance.
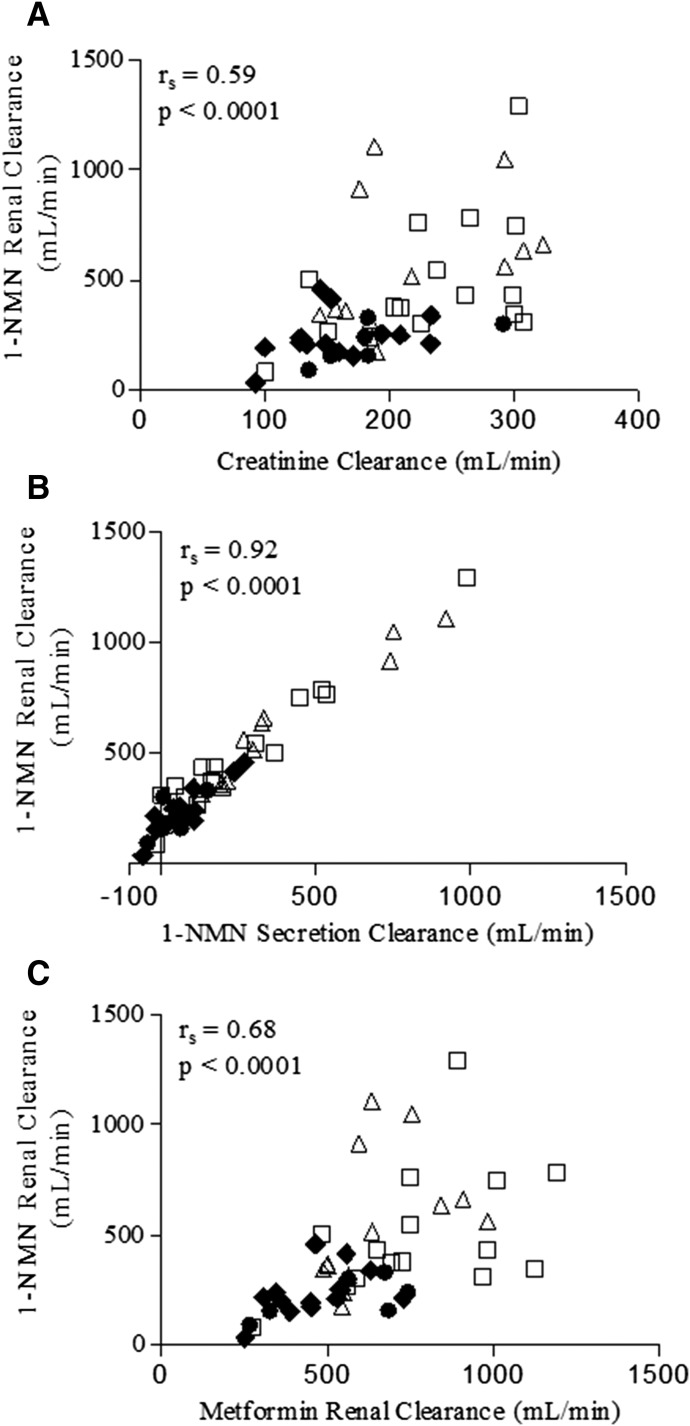
1-NMN renal clearance, for all gestational stages, was correlated with creatinine clearance (r = 0.59, P < 0.001) (Fig. 3A). As expected, 1-NMN renal clearance was highly correlated with 1-NMN secretion clearance (r = 0.92, P < 0.001) (Fig. 3B). Metformin renal clearance and 1-NMN renal clearance were moderately correlated (r = 0.68, P < 0.001) (Fig. 3C). The dose of metformin, metformin dosing interval, and OCT2 genotype did not affect the correlation (data not shown).
Fig. 3.
Correlation between 1-NMN renal clearance and creatinine clearance (A), secretion clearance (B), and metformin renal clearance (C). CLR, renal clearance; CLsec, renal secretion clearance. Early pregnancy (circles), mid pregnancy (squares), late pregnancy (triangles), postpartum (diamonds).
Discussion
1-NMN has been proposed as an endogenous probe for OCT2, MATE1, and MATE2-K activity in the kidney (Ito et al., 2012; Müller et al., 2015). The renal clearance of 1-NMN was studied in healthy volunteers as a probe for potential drug-drug interactions including MATE inhibition by trimethoprim and pyrimethamine (Ito et al., 2012; Müller et al., 2015). In this study, we looked at changes in 1-NMN clearance across gestation, as well as correlation between 1-NMN and metformin renal clearance in pregnant women. Although the positive correlation between 1-NMN renal clearance and metformin renal clearance was observed previously (Ito et al., 2012; Müller et al., 2015), our data suggest a similar relationship exists in pregnant women across gestational ages.
We found that plasma concentrations of 1-NMN ranged between 8.0 to 1237.0 nM, which is similar to previously published ranges (Somogyi et al., 1990; Musfeld et al., 2001). Up to 15-fold changes in 1-NMN plasma concentrations were observed during a single metformin dosing interval (between 8 and 24 hours). Variations throughout the day have been observed in previous 1-NMN studies, but not to such a large extent (Okamoto et al., 2003). These changes in 1-NMN plasma levels may result from niacin consumption in food, because peaks in 1-NMN levels appeared to occur after meals or snacks in some patients (data not shown).
1-NMN and metformin renal clearance are significantly higher in mid and late pregnancy compared with postpartum. During pregnancy, renal blood flow increases by 35 to 60% with a resulting 40 to 50% increase in GFR by the end of the first trimester (Carlin and Alfirevic, 2008). The higher filtration clearance for 1-NMN and metformin is not due to lower protein binding in pregnancy because 1-NMN and metformin have negligible protein binding in plasma (Weber et al., 1991; Scheen, 1996). Additionally, 1-NMN and metformin have a high pKa, which is likely to limit tubular reabsorption (1-NMN pKa = 12.2; metformin pKa=11.5). Because the higher renal clearance of 1-NMN and metformin exceeded the increase in creatinine clearance, a proxy for renal filtration clearance, the secretion clearances of 1-NMN and metformin are higher during pregnancy.
Changes in secretion may be attributed to increased renal blood flow or to increased expression of OCT2, MATE1, or MATE2-K during pregnancy. The secretion clearance seems to peak in mid pregnancy for metformin and late pregnancy for 1-NMN. However, the data are variable. Moreover, several (5 out of 48) of the 1-NMN secretion clearances were calculated to be less than zero. These negative secretion clearance values may be due to difficulty in estimating an accurate 1-NMN AUC because of sparse sampling at later time points, extreme fluctuations in 1-NMN concentrations, or creatinine clearance overestimating glomerular filtration, with up to 10 to 40% being actively secreted in proximal tubule cells (Levey et al., 1988; Breyer and Qi, 2010). Moreover, creatinine has been shown to be a substrate of OCT2 and MATEs and may not be a suitable marker for glomerular filtration, especially during pregnancy (Chu et al., 2016). Alternative exogenous and endogenous markers of GFR have been reported in the literature (e.g., inulin, iohexol, cystatin C, etc.). Further studies should be conducted to evaluate the suitability of these markers, instead of creatinine clearance, to estimate GFR in pregnant women.
Recently, several groups have investigated how 1-NMN serum levels and renal excretion differs between healthy controls compared with patients with obesity or type 2 diabetes. It has been shown that expression of nicotinamide-N-methyltransferase in white adipose tissue is approximately twofold higher in patients with insulin resistance and type 2 diabetes (Kannt et al., 2015). In diabetic patients, increased expression of nicotinamide-N-methyltransferase correlated with increased plasma levels of 1-NMN (Kannt et al., 2015). Higher serum levels of 1-NMN were correlated with increased body mass index and higher association for diabetes in Chinese individuals (Liu et al., 2015). Because metformin is prescribed to improve insulin sensitivity or for polycystic ovary syndrome, the plasma levels and renal clearance of 1-NMN may not reflect those that are observed in women without diabetes or polycystic ovarian syndrome. More work should be done in future studies to determine if the increase in 1-NMN renal clearance and net secretion clearance are observed in a healthy pregnant population and other factors that impact 1-NMN disposition.
In conclusion, we found that 1-NMN renal clearance and secretion clearance was higher mid and late pregnancy in women receiving metformin relative to the nonpregnant state. Although some of the elevation in 1-NMN renal clearance is likely explained by higher renal filtration, induced renal transport activity of OCT2, MATE1, and MATE2-K likely contributes to the higher secretion clearance. Further work should explore the mechanisms by which OCT2, MATE1, and MATE2-K increased activity in pregnancy and whether 1-NMN is a specific and robust endogenous biomarker for these transporters.
Acknowledgments
The authors acknowledge Tauri Senn for assistance with development of the N1-methylnicotinamide assay.
Abbreviations
- AUC
area under the concentration-time curve
- GFR
glomerular filtration rate
- MATE1
multidrug and toxin extrusion proteins 1
- MATE2-K
multidrug and toxin extrusion proteins 2-K
- 1-NMN
N1-methylnicotinamide
- OCT2
organic cation transporter 2
- QC
quality control
Authorship Contributions:
Participated in research design: Hebert, Easterling, and Lin.
Conducted experiments: Bergagnini-Kolev, Hebert, and Easterling.
Performed data analysis: Bergagnini-Kolev, Hebert, and Lin.
Wrote or contributed to the writing of the manuscript: Bergagnini-Kolev, Hebert, Easterling, and Lin.
Footnotes
This work was supported in part by the National Institutes of Health (NIH) Eunice Kennedy Shriver National Institute of Child Health & Human Development [Grant U10HD047892]; NIH National Center for Advancing Translational Sciences through the Clinical and Translational Science Awards Program (CTSA) [Grant UL1TR000423]; and the NIH National Institute of General Medical Sciences [Grant T32GM007750].
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
References
- Aksoy S, Brandriff BF, Ward A, Little PFR, Weinshilboum RM. (1995) Human nicotinamide N-methyltransferase gene: molecular cloning, structural characterization and chromosomal localization. Genomics 29:555–561. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Qi Z. (2010) Better nephrology for mice--and man. Kidney Int 77:487–489. [DOI] [PubMed] [Google Scholar]
- Carlin A, Alfirevic Z. (2008) Physiological changes of pregnancy and monitoring. Best Pract Res Clin Obstet Gynaecol 22:801–823. [DOI] [PubMed] [Google Scholar]
- Chu X, Bleasby K, Chan GH, Nunes I, Evers R. (2016) The complexities of interpreting reversible elevated serum creatinine levels in drug development: Does a correlation with inhibition of renal transporters exist? Drug Metab Dispos 44:1498–1509. [DOI] [PubMed] [Google Scholar]
- Davison JM, Dunlop W. (1980) Renal hemodynamics and tubular function normal human pregnancy. Kidney Int 18:152–161. [DOI] [PubMed] [Google Scholar]
- Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GDV, Clark SM, Risler L, Wang J, Kelly EJ, et al. (2010) Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos 38:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T, Urban TJ, Leabman MK, Fujita K, Giacomini KM. (2006) Transport of drugs in the kidney by the human organic cation transporter, OCT2 and its genetic variants. J Pharm Sci 95:25–36. [DOI] [PubMed] [Google Scholar]
- Ito S, Kusuhara H, Kumagai Y, Moriyama Y, Inoue K, Kondo T, Nakayama H, Horita S, Tanabe K, Yuasa H, et al. (2012) N-methylnicotinamide is an endogenous probe for evaluation of drug-drug interactions involving multidrug and toxin extrusions (MATE1 and MATE2-K). Clin Pharmacol Ther 92:635–641. [DOI] [PubMed] [Google Scholar]
- Kannt A, Pfenninger A, Teichert L, Tönjes A, Dietrich A, Schön MR, Klöting N, Blüher M. (2015) Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia 58:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Okuda M, Inui K. (2005) Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm Res 22:255–259. [DOI] [PubMed] [Google Scholar]
- Lang R, Wahl A, Skurk T, Yagar EF, Schmiech L, Eggers R, Hauner H, Hofmann T. (2010) Development of a hydrophilic liquid interaction chromatography-high-performance liquid chromatography-tandem mass spectrometry based stable isotope dilution analysis and pharmacokinetic studies on bioactive pyridines in human plasma and urine after coffee consumption. Anal Chem 82:1486–1497. [DOI] [PubMed] [Google Scholar]
- Levey AS, Perrone RD, Madias NE. (1988) Serum creatinine and renal function. Annu Rev Med 39:465–490. [DOI] [PubMed] [Google Scholar]
- Liu M, Li L, Chu J, Zhu B, Zhang Q, Yin X, Jiang W, Dai G, Ju W, Wang Z, et al. (2015) Serum N(1)-methylnicotinamide is associated with obesity and diabetes in Chinese. J Clin Endocrinol Metab 100:3112–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maïza A, Waldek S, Ballardie FW, Daley-Yates PT. (1992) Estimation of renal tubular secretion in man, in health and disease, using endogenous N-1-methylnicotinamide. Nephron 60:12–16. [DOI] [PubMed] [Google Scholar]
- Müller F, Pontones CA, Renner B, Mieth M, Hoier E, Auge D, Maas R, Zolk O, Fromm MF. (2015) N(1)-methylnicotinamide as an endogenous probe for drug interactions by renal cation transporters: studies on the metformin-trimethoprim interaction. Eur J Clin Pharmacol 71:85–94. [DOI] [PubMed] [Google Scholar]
- Musfeld C, Biollaz J, Bélaz N, Kesselring UW, Decosterd LA. (2001) Validation of an HPLC method for the determination of urinary and plasma levels of N1-methylnicotinamide, an endogenous marker of renal cationic transport and plasma flow. J Pharm Biomed Anal 24:391–404. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Ishikawa A, Yoshitake Y, Kodama N, Nishimuta M, Fukuwatari T, Shibata K. (2003) Diurnal variations in human urinary excretion of nicotinamide catabolites: effects of stress on the metabolism of nicotinamide. Am J Clin Nutr 77:406–410. [DOI] [PubMed] [Google Scholar]
- Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y. (2006) The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci 27:587–593. [DOI] [PubMed] [Google Scholar]
- Sauve AA. (2008) NAD+ and vitamin B3: from metabolism to therapies. J Pharmacol Exp Ther 324:883–893. [DOI] [PubMed] [Google Scholar]
- Scheen AJ. (1996) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 30:359–371. [DOI] [PubMed] [Google Scholar]
- Shmaefsky BR. (1990) Artificial urine for laboratory testing. The American Biology Teacher 52:170–172. [Google Scholar]
- Somogyi A, Siebert D, Bochner F. (1990) Determination of endogenous concentrations of N1-methylnicotinamide in human plasma and urine by high-performance liquid chromatography. Anal Biochem 187:160–165. [DOI] [PubMed] [Google Scholar]
- Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. (2007) Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol 74:359–371. [DOI] [PubMed] [Google Scholar]
- Weber W, Toussaint S, Looby M, Nitz M, Kewitz H. (1991) System analysis in multiple dose kinetics: evidence for saturable tubular reabsorption of the organic cation N1-methylnicotinamide in humans. J Pharmacokinet Biopharm 5:553–574. [DOI] [PubMed] [Google Scholar]