Abstract
Drug-drug interactions (DDIs) occur when the action of one drug interferes with or alters the activity of another drug taken concomitantly. This can lead to decreased drug efficacy or increased toxicity. Because of DDIs, physicians in the clinical practice attempt to avoid potential interactions when multiple drugs are coadministrated; however, there is still a large knowledge gap in understanding how drugs taken in the past can contribute to DDIs in the future. The goal of this study was to investigate the consequence of neonatal drug exposure on efficacy of other drugs administered up through adult life. We selected a mouse model to test phenobarbital exposure at a neonatal age and its impact on efficacy of omeprazole in adult life. The results of our experiment show an observed decrease in omeprazole’s ability to raise gastric pH in adult mice that received single or multiple doses of phenobarbital at a neonatal age. This effect may be associated with the permanent induction of cytochrome P450 enzymes in adult liver after neonatal phenobarbital treatment. Our data indicates that DDIs may result from drugs administered in the past in an animal model and should prompt re-evaluation of how DDIs are viewed and how to avoid long-term DDIs in clinical practice.
Introduction
Polypharmacy-induced drug-drug interactions (DDIs) significantly raise the risks for decreasing therapeutic efficacy and increasing adverse reactions, with particularly higher risks for the elderly, children, and female populations (Sharifi et al., 2014). Approximately 50% of the population aged over 65 years now takes at least five different medications, with 35–60% of these elderly patients exposed to a potential DDI, and 5–15% suffering clinically significant adverse reactions (Magro et al., 2012). Additionally, it is estimated that 49% of hospitalized patients under the age of 21 are exposed to a potential DDI (Feinstein et al., 2015).
On the basis of their mechanisms of actions, DDIs are classified into two main categories: pharmacokinetics and pharmacodynamics. DDIs that are considered in the category of pharmacokinetics occur when one drug alters the absorption, distribution, metabolism, or excretion of another drug in the patient’s body. Altering any of these pharmacokinetic factors can increase or decrease the concentrations of a drug or its metabolites in circulation (Seymour and Routledge, 1998). Certain drugs possess the ability to increase or decrease the rate at which another drug is metabolized, leading to significantly lower or higher serum concentrations of the drug or its metabolites. These types of interactions are common owing to the induction of gene expression of a certain number of cytochrome P450 enzymes (P450s) that are responsible for the biotransformation of 80% prescription drugs (Jana and Paliwal, 2007). Induction of P450s by drugs occurs shortly after the inducing drug is taken, with a delay depending on the half-life of the drug. Induction is considered to be a temporary event in adults, in whom P450 levels will return to normal once the inducing drug is stopped (Lynch and Price, 2007). However, a large body of work from Shapiro’s laboratory has demonstrated that neonatal exposure to phenobarbital can cause a permanent elevation of basal levels of enzyme activities in a variety of P450-mediated drug metabolizing enzymes (Agrawal et al., 1995, Agrawal and Shapiro, 2000, 2005). Recently, research in our laboratory has shown that the permanent elevation of gene expression and enzyme activities of several P450s in adult mouse liver by neonatal treatment with phenobarbital is dependent on two key factors: the age at which the mouse is treated in early life and the dose at which phenobarbital is given (Tien et al., 2015). Phenobarbital is still the first drug of choice for treating acute neonatal seizures, which occur in 2–3 out of 1000 live births (Hellström-Westas et al., 2015), and is still widely administered to babies. It is also a known inducer of the CYP2B6, 2C9, 2C19, and 3A4 in human and CYP2B10, 2C29, and 3A11 in mice (Czekaj, 2000). Treatment at earlier ages with high doses of phenobarbital produces a permanent induction of P450 enzymes at the adult age in mouse liver (Tien et al., 2015). However, whether the permanent induction of P450 enzymes has an effect on the efficacy of other drugs administered to adults was not investigated.
This study aimed to determine whether treatment with the P450-inducing drug phenobarbital early in life can affect the efficacy of a drug taken later in adult life. Omeprazole is a proton-pump inhibitor commonly used to treat stomach ulcers, gastroesophageal reflux disease, and heartburn (Maróstica et al., 2007). Its action involves blocking the release of acid by proton pumps in the stomach so as to raise the pH of the gastric lumen. We chose omeprazole as a model drug to test for changes in efficacy in adult mice after neonatal treatment with phenobarbital. Its efficacy can be tested by measuring pH of gastric juices after daily dosing. Omeprazole is known to be primarily metabolized by CYP2C19 and CYP3A4 in humans (Andersson et al., 1994; Chang et al., 1995; Andersson, 1996). Its metabolism can be altered by coadministration with phenobarbital (Park et al., 2005). Although the primary P450 enzymes metabolizing omeprazole in mice are not defined yet, we select CYP2C29 and CYP3A11 as representative members of CYP2C and CYP3A subfamilies, respectively, in this study. Investigating whether neonatal phenobarbital exposure affects the ability of omeprazole to increase stomach pH in adults can give insight into how drug treatment in early life can impact drug interactions in later life. This knowledge may prompt a re-evaluation of how DDIs in the clinic are viewed and predicted. In order for a drug-drug interaction to occur, it may not be necessary for two drugs to be taken concomitantly.
Materials and Methods
Chemicals.
Phenobarbital and omeprazole were purchased from Sigma-Aldrich (St. Louis, MO).
Animal Treatment with Drugs.
The use of animals in the current study was approved by the Institutional Animal Care and Use Committee at the University of Connecticut. C57BL/6 mice were bred and housed under the standard conditions in the Animal Care Service Facility at the University of Connecticut according to the animal care guidelines provided by the American Association for Laboratory Animal Science. Treatment schedules of phenobarbital or omeprazole in each experiment are outlined in figures. Phenobarbital at a dose of 200 mg/kg or control saline [phosphate-buffered saline (PBS)] was given to the mice via intraperitoneal injection. The selected dose of phenobarbital was proven to be able to permanently induce P450 expression in adult liver when treatment occurs at a neonatal age (Tien et al., 2015). Three consecutive doses of omeprazole at 150 mg/kg per day or control saline (PBS) were given to the mice via intraperitoneal injection. This dose of omeprazole was selected because it has previously been demonstrated to efficiently inhibit the gastric H+/K+-ATPase pump in mice (Aristoteli et al., 2006). Blocking the activity of the gastric proton-pump prevents the release of hydrochloric acid from parietal cells and increases the pH of the stomach as the cavity becomes less acidic (Marostica et al., 2007). Because no difference between male and female was found in a previous study (Tien et al., 2015) on the permanent induction of gene expression of CYP3A11 and CYP2C29 in adult by phenobarbital treatment at early life, only male mice were used in each control and treatment group.
pH Measurement in Gastric Stomach.
One hour after the final omeprazole or control PBS treatment, all adult mice were anesthetized with isoflurane and an incision was made in the stomach. An Orion 9863BN micro pH electrode was placed in the stomach and the pH of gastric liquids was measured by a pH probe using a previous described procedure (Brenneman et al., 2014). Mice were then sacrificed and livers were harvested.
Real-Time Quantitative PCR and Western Blot Analysis.
Total RNAs and proteins were isolated from the harvested livers, and expression of CYP2C29 and CYP3A11 in liver at mRNA level was determined by real-time quantitative polymerase chain reaction (RT-qPCR) and protein level by Western blot with procedures described in a previous study (Tien et al., 2015).
Statistics.
Data are presented as mean ± S.D. with n = 3 mice per group in the control or treatment group. Statistical analyses were performed using GraphPad Prism 6. Comparisons between control and treatment groups were performed using unpaired t test, and a p-value < 0.05 was considered statistically significant. The sample size (n = 3) had a power over 80% to find p < 0.05, if the means of the treatment groups compared with the control group were greater than 2-fold and standard deviations were less than 30%.
Results
Omeprazole Inhibits the Gastric Proton-Pump to Increase pH in the Gastric Stomach in Adult Mice.
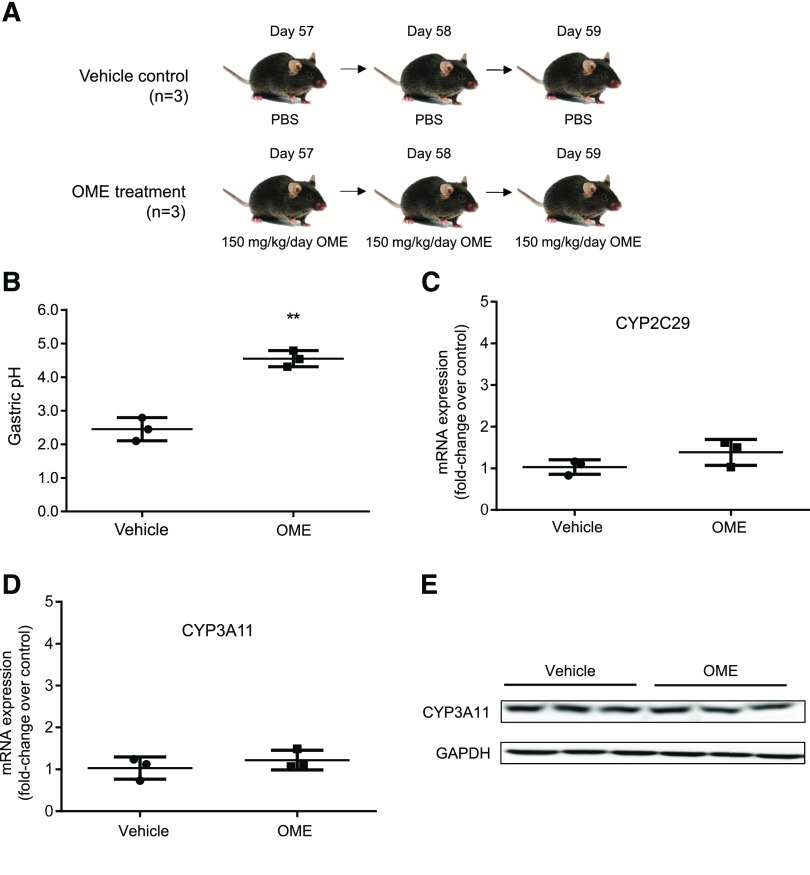
A control experiment illustrated in Fig. 1A was performed to examine efficacy of omeprazole to inhibit proton pumps in adult mouse stomach. The adult mice that received PBS as a control had a gastric stomach pH of 2.4 ± 0.2 in a nonfasting condition (Fig. 1B). The adult mice that received omeprazole for 3 days had a gastric stomach pH of 4.5 ± 0.2 under the nonfasting condition after the final treatment. This indicates that omeprazole efficiently blocked the activity of gastric proton pump to reduce the amount of acids released and significantly raised the pH in the stomach (**p < 0.01). Expression of CYP2C29 and CYP3A11 at mRNA level and CYP3A11 at the protein level in mouse livers was further examined by RT-qPCR and Western blot, respectively. Because no antibody specific against CYP2C29 was available, CYP2C29 protein level was not determined by Western blot in this study. No differences in gene expression of CYP3A11 and CYP2C29 at the mRNA level were noted after treatment with omeprazole compared with the control (Fig. 1, C and D). CYP3A11 expression at the protein level is also consistent between the control and omeprazole treatment (Fig. 1E). These results show that treatment with omeprazole has no effect on the induction of expression of CYP2C29 and CYP3A11.
Fig. 1.
Omeprazole treatment increases gastric pH without altering P450 expression in adult mice. (A) An illustration of animal treatment. The adult mice were treated with either vehicle control (PBS) (n = 3) or omeprazole (OME) at 150 mg/kg per day (n = 3) for three consecutive days at the ages 57, 58, and 59 days after birth. (B) Gastric pH with mean ± S.D. in the mouse stomach at 1 hour after the last treatment of PBS or omeprazole. (C) Relative fold changes of mRNA of CYP2C29, (D) mRNA of CYP3A11, and (E) protein of CYP3A11 in the mouse livers with the control and omeprazole treatment. **p < 0.01. GADPH; glyceraldehyde 3-phosphate dehydrogenase.
Concurrent Administration of Phenobarbital and Omeprazole Temporarily Reduces Efficacy of Omeprazole in Proton-Pump Inhibition in Adult Mice.
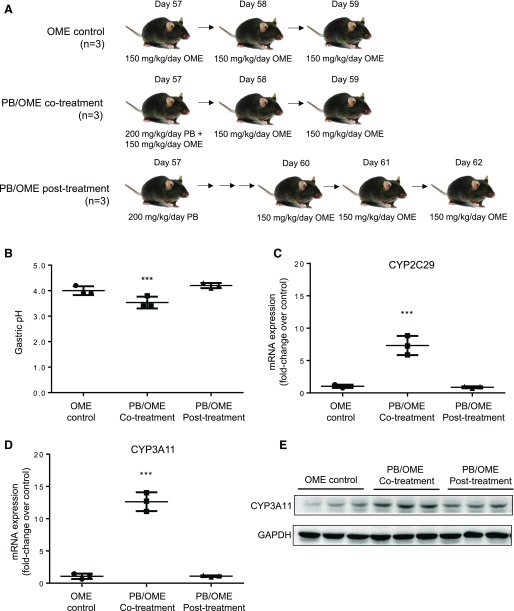
Figure 2A illustrates an experimental design to examine phenobarbital-omeprazole (PB/OME) interaction on efficacy of omeprazole in proton-pump inhibition in adult mice. Mice in the omeprazole control group had a gastric pH of 4.0 ± 0.1, comparable to the result shown in Fig. 1B. Mice in the cotreatment group had a gastric pH of 3.5 ± 0.1, which was significantly lower (***p < 0.001) than the control group. This indicates that administering phenobarbital concurrently with omeprazole in adult mice causes a drug-drug interaction, diminishing the efficacy of omeprazole to increase gastric stomach pH. Mice in the post-treatment group had a gastric pH of 4.2 ± 0.1, which was similar to the omeprazole control mice and showed no statistically significant difference. This indicates that in adult mice, the efficacy of proton-pump inhibition by omeprazole is not affected by a previous administration of phenobarbital. To exclude an effect on the gastric pH by phenobarbital, a group of mice (n = 3) was treated with phenobarbital (200 mg/kg) at day 57 after birth, and following PBS treatment at days 58 and 59, the mice had a gastric pH of 2.7 ± 0.3 in a nonfasting condition, which was not different from the control group, which received PBS treatment at days 57, 58, and 59 (pH = 2.4 ± 0.2).
Fig. 2.
Concurrent administration of phenobarbital (PB) and omeprazole (OME) results in a drug-drug interaction and reduces omeprazole efficacy. (A) An illustration of animal treatment. Adult mice in the OME-control group (n = 3) were treated with three consecutive doses of 150 mg/kg per day of omeprazole at 57, 58, and 59 days after birth. Adult mice in the PB/OME cotreatment group (n = 3) received a single dose of 200 mg/kg phenobarbital together with a dose of 150 mg/kg omeprazole at day 57, followed by 2 days of treatment with just omeprazole at days 58 and 59. Adult mice in the PB/OME post-treatment group (n = 3) received the same single dose of 200 mg/kg phenobarbital at day 57, then the three consecutive doses of 150 mg/kg per day of omeprazole were started 3 days later at days 60, 61, and 62. (B) Gastric pH with mean ± S.D. in the mouse stomach at 1 hour after the last treatment of omeprazole. (C) Relative fold changes of mRNA of CYP2C29, (D) mRNA of CYP3A11, and (E) protein of CYP3A11 in the mouse livers with PB (control, co-, and post-)/OME treatment. ***p < 0.001.
Gene expression of CYP2C29 and CYP3A11 at the mRNA level was also determined by RT-qPCR in the livers collected after the completion of omeprazole treatment (Fig. 2, C and D). Compared with the omeprazole control group, expression of both CYP2C29 and CYP3A11 was significantly induced to 7.3 ± 0.9– (***p < 0.001) and 12.6 ± 0.8– (***p < 0.001) fold higher, respectively, in the PB/OME cotreatment mice. Mice that received phenobarbital 3 days prior to beginning omeprazole treatment had no significant changes in either CYP3A11 or 2C29 expression after the last dose of omeprazole. Similar changes at protein level for CYP3A11 were observed in Fig. 2E. These results indicate that phenobarbital-mediated P450 induction in adult mice is not long-term, and induced levels of enzymes will return to normal at 6 days after phenobarbital treatment is stopped.
Here we demonstrated that induction of the expression of CYP2C29 and CYP3A11 by phenobarbital was associated with the decreased efficacy of omeprazole in proton-pump inhibition shown as a decreased rise in mouse gastric pH when phenobarbital and omeprazole were coadministrated, but such effect was temporary and was not observed in a treatment in which omeprazole administration was 3 days later than the phenobarbital treatment.
Neonatal Administration of Phenobarbital Reduces the Efficacy of Omeprazole in Proton-Pump Inhibition in Adult Mice.
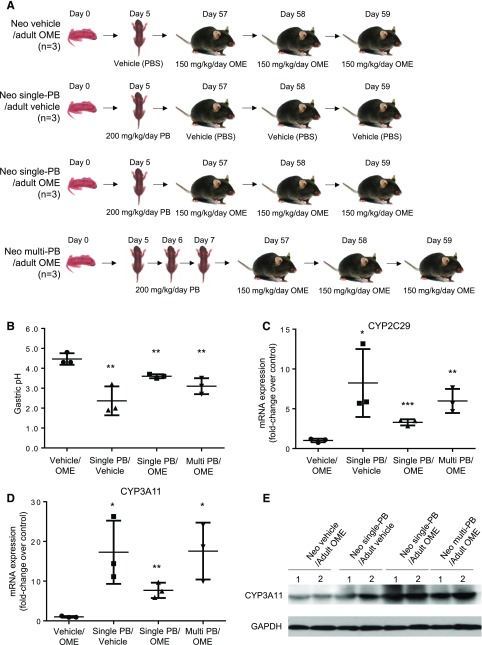
An experimental design is illustrated in Fig. 3A. In the Neo vehicle/adult OME control group, omeprazole treatment at the adult age repeatedly showed efficient proton-pump inhibition to raise gastric pH to 4.5 ± 0.2. In the Neo single-PB/adult vehicle (PBS) group, no omeprazole was administered at the adult age; therefore, no gastric pH was increased (pH = 2.3 ± 0.8). Mice in the Neo single-PB/adult OME group had a significantly lower pH level, 3.6 ± 0.1, than the control mice that were given PBS at day 5 (**p < 0.01). Mice in the Neo multi-PB/adult OME group experienced an even lower gastric pH of 3.1 ± 0.2 (**p < 0.01). These results suggest that phenobarbital exposure at a neonatal age could result in a long-term interaction with omeprazole to lower efficacy in proton-pump inhibition in adult stomach. To ensure that phenobarbital treatment has no effect on gastric stomach pH, we also included a group of mice (n = 3) receiving a single dose of 200 mg/kg per day phenobarbital at day 5 and three consecutive doses of PBS vehicle at 57, 58, and 59 days after birth. Their gastric pH values were comparable to a normal physiologic level at 2.4 ± 0.4, indicating phenobarbital treatment at day 5 had no effect on gastric stomach pH.
Fig. 3.
Neonatal administration of phenobarbital (PB) causes a drug-drug interaction and reduces efficacy of omeprazole (OME) in adult mice. (A) An illustration of animal treatment. The mice in the Neo vehicle/adult OME control group (n = 3) received vehicle (PBS) at a neonatal age of day 5 and three consecutive doses of 150 mg/kg per day of omeprazole at adult ages of 57, 58, and 59 days after birth. The mice in the Neo single-PB/adult vehicle group (n = 3) received a single dose of 200 mg/kg per day phenobarbital at day 5 and three consecutive treatments of vehicle (PBS) at 57, 58, and 59 days after birth. The mice in the Neo single-PB/adult OME group (n = 3) received a single dose of 200 mg/kg per day phenobarbital at day 5 and three consecutive doses of 150 mg/kg per day of omeprazole at 57, 58, and 59 days after birth. The mice in the Neo multi-PB/adult OME group (n = 3) received three consecutive doses of 200 mg/kg per day phenobarbital at day 5, 6, and 7 and three consecutive doses of 150 mg/kg per day of omeprazole at 57, 58, and 59 days after birth. (B) Gastric pH with mean ± S.D. in the mouse stomach at 1 hour after the last treatment of omeprazole. (C) Relative fold changes of mRNA of CYP2C29, (D) mRNA of CYP3A11, and (E) protein of CYP3A11 in mouse livers with the neonatal PB (control, single-, and multiple-)/adult OME treatment. *p < 0.05 and **p < 0.01.
After the measurement of gastric pH, livers of all the mice were collected for further analysis of gene expression of CYP2C29 and CYP3A11 at mRNA level (Fig. 3, C and D) and CYP3A11 at protein level (Fig. 3E). Compared with the Neo vehicle/adult OME control group, phenobarbital treatment at the neonatal age with either single or multiple doses resulted in a long-term elevation of mRNA expression of CYP2C29 and CYP3A11 in adult life. Phenobarbital treatment at the neonatal ages also resulted in increases of CYP3A11 at protein level (Fig. 3E).
Discussion
Phenobarbital, along with several other first-generation antiepileptic and sedative drugs, is known to cause induction of several different P450 enzymes (Perucca, 2006). Because of this, physicians are currently aware of the risk of DDIs with many common medications in patients prescribed phenobarbital (Lebowitz et al., 2016). However, the current clinical practice only takes the DDIs into consideration when multiple drugs are administrated at the same time. Historical usage of drugs in previous weeks, months, or years has not been a consideration for DDIs, as in most cases induction of P450 expression by a drug is a temporary event and will disappear in a short period after administration of the inducer drug is stopped. This is true for adults; however, our study may have a significant impact and lead to a change in the concept of DDIs for people who received drug treatment at neonatal and infant ages.
Studies from Shapiro’s laboratory have demonstrated that permanent induction of P450 expression in adult liver can be achieved when neonates and infants are exposed to phenobarbital (Agrawal et al., 1995; Agrawal and Shapiro, 2000, 2005). Our previous study further illustrated that the permanent induction is dose- and age-dependent (Tien et al., 2015). In the current study, we further demonstrated that the permanent induction of P450 expression in adult mice by neonatal phenobarbital exposure may be associated with a significant decrease in the efficacy of inhibition of proton pumps by omeprazole in adult life. Although previous studies have shown that neonatal treatment with phenobarbital can decrease sleep time in vivo in adult rats treated with hexobarbital (Agrawal and Shapiro, 2000, 2005), and neonatal treatment with TCPOBOP (1,4-bis[2-(3,5-dichloropyridyloxy)]benzene) can decrease paralysis time in adult mice treated with zoxazolamine (Chen et al., 2012), neither hexobarbital nor zoxazolamine are commonly used at this time. In the current study in an animal model, by using the commonly used clinical drugs omeprazole and phenobarbital, we have shown a long-term impact on therapeutic efficacy of a drug that is metabolized by P450s when neonatal exposure to another drug that can induce P450 expression has occurred. However, this concept of long-term drug-drug interactions needs to be further confirmed in animal models with more drugs such as midazolam, that are primarily metabolized by the inducible P450 enzymes in adults, and by more drugs such as phenytoin and dexamethasone, which are capable of inducing P450 expression in adults who were exposed at neonatal ages. Dose ranges and exposure-sensitivity windows need to be established for each inducible drug. Furthermore, the underlying molecular mechanisms also need to be explored for explanation of the permanent induction of P450 expression by neonatal drug exposure. Epigenetic mechanisms, including DNA methylation, histone modifications, and microRNAs have all been implicated in regulating the expression of drug-metabolizing enzymes in both neonatal and adult livers (Kacevska et al., 2012, Ingelman-Sundberg et al., 2013, Bonder et al., 2014). Nuclear receptors are also known to control the induction and expression of many hepatic P450 enzymes and transporter genes (Chai et al., 2013, Kandel et al., 2016) and probably play a role in causing their permanent induction during the neonatal developmental period (Chen et al., 2012). Further mechanistic studies for this phenomenon can give greater insight into factors responsible for permanent induction of drug-metabolizing enzymes by other drugs, environmental toxins, and nutritive components.
Our study, although performed in a mouse model, can prompt a re-evaluation of how DDIs are presently viewed and predicted. Further translational studies in human subjects will need to be completed to prove that this concept can make a clinical impact. Historical usage of drugs, particularly during neonatal and infant ages, may serve as a consideration factor for predicting drug response.
Abbreviations
- DDI
drug-drug interaction
- PB/OME
phenobarbital-omeprazole
- P450
cytochrome P450
- PBS
phosphate-buffered saline
- RT-qPCR
real-time quantitative polymerase chain reaction
- TCPOBOP
1,4-bis[2-(3,5-dichloropyridyloxy)]benzene
Authorship Contributions
Participated in research design: Tien, Piekos, Pope, Zhong.
Conducted experiments: Tien, Piekos, Pope.
Performed data analysis: Tien, Zhong
Wrote or contributed to the writing of the manuscript: Tien, Piekos, Pope, Zhong.
Footnotes
This study was supported by the National Institutes of Health National Institute of General Medical Sciences [Grants R01GM-087376 and R01GM-118367] and the National Institute for Environmental Health Sciences [Grant R01ES-019487]. This study was also partially supported by the Institute for System Genomics at the University of Connecticut.
References
- Agrawal AK, Pampori NA, Shapiro BH. (1995) Neonatal phenobarbital-induced defects in age- and sex-specific growth hormone profiles regulating monooxygenases. Am J Physiol 268:E439–E445. [DOI] [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. (2000) Latent overexpression of hepatic CYP2C7 in adult male and female rats neonatally exposed to phenobarbital: a developmental profile of gender-dependent P450s. J Pharmacol Exp Ther 293:1027–1033. [PubMed] [Google Scholar]
- Agrawal AK, Shapiro BH. (2005) Neonatal phenobarbital imprints overexpression of cytochromes P450 with associated increase in tumorigenesis and reduced life span. FASEB J 19:470–472. [DOI] [PubMed] [Google Scholar]
- Andersson T. (1996) Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin Pharmacokinet 31:9–28. [DOI] [PubMed] [Google Scholar]
- Andersson T, Miners JO, Veronese ME, Birkett DJ. (1994) Identification of human liver cytochrome P450 isoforms mediating secondary omeprazole metabolism. Br J Clin Pharmacol 37:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aristoteli LP, O’Rourke JL, Danon S, Larsson H, Mellgard B, Mitchell H, Lee A. (2006) Urea, fluorofamide, and omeprazole treatments alter helicobacter colonization in the mouse gastric mucosa. Helicobacter 11:460–468. [DOI] [PubMed] [Google Scholar]
- Bonder MJ, Kasela S, Kals M, Tamm R, Lokk K, Barragan I, Buurman WA, Deelen P, Greve JW, Ivanov M, et al. (2014) Genetic and epigenetic regulation of gene expression in fetal and adult human livers. BMC Genomics 15:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman KE, Willingham C, Kilbourne JA, Curtiss R, 3rd, Roland KL. (2014) A low gastric pH mouse model to evaluate live attenuated bacterial vaccines. PLoS One 9:e87411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X, Zeng S, Xie W. (2013) Nuclear receptors PXR and CAR: implications for drug metabolism regulation, pharmacogenomics and beyond. Expert Opin Drug Metab Toxicol 9:253–266. [DOI] [PubMed] [Google Scholar]
- Chang M, Tybring G, Dahl ML, Götharson E, Sagar M, Seensalu R, Bertilsson L. (1995) Interphenotype differences in disposition and effect on gastrin levels of omeprazole--suitability of omeprazole as a probe for CYP2C19. Br J Clin Pharmacol 39:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WD, Fu X, Dong B, Wang YD, Shiah S, Moore DD, Huang W. (2012) Neonatal activation of the nuclear receptor CAR results in epigenetic memory and permanent change of drug metabolism in mouse liver. Hepatology 56:1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czekaj P. (2000) Phenobarbital-induced expression of cytochrome P450 genes. Acta Biochim Pol 47:1093–1105. [PubMed] [Google Scholar]
- Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C. (2015) Potential drug-drug interactions in infant, child, and adolescent patients in children’s hospitals. Pediatrics 135:e99–e108. [DOI] [PubMed] [Google Scholar]
- Hellström-Westas L, Boylan G, Ågren J. (2015) Systematic review of neonatal seizure management strategies provides guidance on anti-epileptic treatment. Acta Paediatr 104:123–129. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Zhong XB, Hankinson O, Beedanagari S, Yu AM, Peng L, Osawa Y. (2013) Potential role of epigenetic mechanisms in the regulation of drug metabolism and transport. Drug Metab Dispos 41:1725–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S, Paliwal J. (2007) Molecular mechanisms of cytochrome p450 induction: potential for drug-drug interactions. Curr Protein Pept Sci 8:619–628. [DOI] [PubMed] [Google Scholar]
- Kacevska M, Ivanov M, Ingelman-Sundberg M. (2012) Epigenetic-dependent regulation of drug transport and metabolism: an update. Pharmacogenomics 13:1373–1385. [DOI] [PubMed] [Google Scholar]
- Kandel BA, Thomas M, Winter S, Damm G, Seehofer D, Burk O, Schwab M, Zanger UM. (2016) Genomewide comparison of the inducible transcriptomes of nuclear receptors CAR, PXR and PPARα in primary human hepatocytes. Biochim Biophys Acta 1859:1218–1227. [DOI] [PubMed] [Google Scholar]
- Lebowitz MB, Olson KL, Burns M, Harper MB, Bourgeois F. (2016) Drug-Drug Interactions Among Hospitalized Children Receiving Chronic Antiepileptic Drug Therapy. Hosp Pediatr 6:282–289. [DOI] [PubMed] [Google Scholar]
- Lynch T, Price A. (2007) The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am Fam Physician 76:391–396. [PubMed] [Google Scholar]
- Magro L, Moretti U, Leone R. (2012) Epidemiology and characteristics of adverse drug reactions caused by drug-drug interactions. Expert Opin Drug Saf 11:83–94. [DOI] [PubMed] [Google Scholar]
- Maróstica M, Arçari DP, Bartchewsky W, Jr, Trevisan M, Ribeiro ML, Pedrazzoli J, Jr, Hoehr NF, Gambero A. (2007) Effects of a one-week treatment with acid gastric inhibitors on Helicobacter pylori-infected mice. Scand J Gastroenterol 42:1404–1412. [DOI] [PubMed] [Google Scholar]
- Park EJ, Cho HY, Lee YB. (2005) Effect of Cimetidine and Phenobarbital on metabolite kinetics of Omeprazole in rats. Arch Pharm Res 28:1196–1202. [DOI] [PubMed] [Google Scholar]
- Perucca E. (2006) Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 61:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour RM, Routledge PA. (1998) Important drug-drug interactions in the elderly. Drugs Aging 12:485–494. [DOI] [PubMed] [Google Scholar]
- Sharifi H, Hasanloei MA, Mahmoudi J. (2014) Polypharmacy-induced drug-drug interactions; threats to patient safety. Drug Res (Stuttg) 64:633–637. [DOI] [PubMed] [Google Scholar]
- Tien YC, Liu K, Pope C, Wang P, Ma X, Zhong XB. (2015) Dose of Phenobarbital and Age of Treatment at Early Life are Two Key Factors for the Persistent Induction of Cytochrome P450 Enzymes in Adult Mouse Liver. Drug Metab Dispos 43:1938–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]