Abstract
Nucleic acid-based therapeutics constitutes the next frontier in cardiovascular disease (CVD) management. Recent findings have shown a strong regulatory role of a new class of non-coding RNAs (ncRNAs) known as long ncRNAs (lncRNAs), on biological pathways, cell function, and cardiovascular tissue homeostasis in health and disease. Accumulating studies demonstrate that lncRNAs may regulate protein-coding genes, RNAs, and epigenetic factors in a tight spatial and temporal manner. Herein, we discuss key technical hurdles, current limitations and challenges as well as advantages and disadvantages of emerging experimental methods in the lncRNA field.
LncRNAs are derived from a diverse set of non-coding sequences, display mRNA-like features such as splicing and polyadenylation, and are defined as being longer than 200 nucleotides (nt). LncRNAs are either defined by their function or their genomic locus with respect to protein-coding genes. Whereas the number of ~19,000 human protein-coding genes has plateaued, the number of lncRNAs keeps increasing annually. Based on the NONCODE consortium (http://noncode.org), >100 000 lncRNA genes are predicted to be present in the human genome. Although a fast growing number of studies have begun to elucidate our understanding of their function, only around 200 lncRNAs in humans are functionally characterized. For the vast majority, the precise function remains poorly understood, particularly in CVD.
Versatile genome editing tool CRISPR/Cas9
Clustered regularly interspaced palindromic repeats (CRISPR) /Cas9 provides a precise gene-editing tool for a wide range of applications. Gene editing is more complex for non-coding genes than protein-coding genes, since they are missing an open reading frame. Apart from inherent technical considerations using gene-editing techniques, lncRNAs are not as well-conserved across species as microRNAs for example, which complicates extrapolation of findings from animals to humans, and vice versa.
Advantages and disadvantages of lncRNA genome editing strategies
Deletion of entire locus
Excision of the entire genomic lncRNA locus becomes an attractive choice, since identification of whether the phenotype or functional role is ascribed to the lncRNA itself or its regulatory elements that mediate interactions with DNA, RNA, or protein molecules remains a challenge. Successful examples of whole genomic locus deletion are UCA1, lncRNA-21A, NORAD, and Morrbid using two single guide RNAs (sgRNAs) flanking the first and last exon in combination with paired Cas9 nickase, which induces more favorable non-homologous end joining (NHEJ) relative to homologous recombination (HR) (Supp. Fig. 1A).1,2 One advantage of this approach is its scalability from a few hundred bp up to 106 bp long fragments. Since many lncRNAs are known host genes for microRNAs, snRNAs, snoRNAs, and micropeptides, and lncRNAs may lie in an antisense orientation to regulatory DNA elements, deletion of the entire locus harbors potential risks of misaligning a phenotype to its driver gene. Moreover, transcription of the lncRNA Airn has been described, rather than its RNA product itself, to mediate repression of its antisense counter partner Igfr2.3 This model of transcriptional interference by competing for RNA Pol II highlights the importance for understanding the function of a candidate lncRNA and its silencing mechanism(s).
Deletion of a functional unit
In an elegant study, Xue and colleagues revealed critical elements of the secondary structure of Bvht, which controls cardiac lineage commitment. 4 Notably, they found that a small deletion of 11 nt in the 5’ asymmetric G-rich internal loop motif in the modular secondary structure of Bvht using CRISPR/Cas9-mediated HR in mouse embryonic stem cells prevented its interaction with its binding partner CNBP. In order to increase efficiency of the mutation at designated locus at both alleles, two templates including different selection markers (i.e. puromycin and hygromycin) were used for subsequent dual selection of positive clones following HR. Selection cassettes were removed later by Cre-recombinase of LoxP sites (Supp. Fig. 1B).4 While this strategy has the advantage of understanding structure-function relationships of small domains of lncRNAs, the prediction tools for identifying such small structural domains are fairly nascent and require careful interpretation, often necessitating complementary mutational approaches to map such domains.
Promoter region deletion
Identification of promoter regions is facilitated by RNA-seq data on ENCODE; however, lncRNAs are known for their multiple isoforms often derived from several promoters, which complicates silencing of a single lncRNA. Morrbid promoter deletion was achieved using two sgRNAs targeting the 5’ and 3’ flanking region of its promoter. sgRNAs were cloned into Cas9 vector containing either a GFP or mCherry selection marker, which were subsequently used for sorting of double positive cells (Supp. Fig. 1C).2 The primary challenge that remains is the rather low efficiency of homozygote knockout clones, although deletion frequency is inversely related to deletion size.
Introduction of RNA destabilizing elements (RDEs)
A favorable strategy for silencing lncRNA expression is by introducing destabilizing elements such as stop codons. To this end, multiple polyadenlyation signals may be introduced towards the 5’ end of a ncRNA gene, leading to the stabilization of upstream transcripts as well as destabilizing the downstream transcript due to absent 5’-cap structure (Supp. Fig. 1D). Similar to CRISPR/Cas9, zinc finger nucleases (ZFNs) can be used to generate NHEJ or HR to knockout or integrate exogenous elements of interest. For example, ZFNs were used in the biallelic integration of poly(A) signals into the locus of MALAT1.5 The ZFNs integrate RDEs between the TATA box and the transcriptional start site. Complementary experiments are necessary to verify whether the RNA product itself mediates the functional effect or whether transcriptional interference is the dominant mechanism of action. Other than ZFNs, Lee et al. used TALENs to facilitate an HR-mediated recombination of a stop element, which could be later reverted back using Cre recombinase to demonstrate the lncRNA rescue effect.1
Overall, RDEs such as stop elements, miRNA binding sites, RNase P recognition site, self-cleaving ribozymes, or AU-rich elements provide an excellent repertoire of tools for manipulating in an effective manner lncRNA gene expression; however, considerations about transcriptional interference represent a caveat with any of these strategies.5.
Consideration of techniques for transient lncRNA silencing
Transient methods have emerged as powerful, scalable tools to identify potentially functional lncRNAs. RNAi-based techniques such as short interfering RNA oligos (siRNA) or vector-associated short hairpin RNA (shRNA) both utilize the RNA-induced silencing complex (RISC), whereas shRNAs are often integrated into viral backbones for stable knockdown. Depending on the lncRNA, RNAi may not be the method of choice, because the RISC is localized in the cytosol and hence nuclear lncRNA or chromatin-associated lncRNAs may not be subjected to degradation. Recently, nuclear active RNAi factors have been characterized in a subset of human cell lines, which provides new possibilities for knockdown of nuclear-associated lncRNAs.6 In order to target nuclear or chromatin-associated lncRNAs, GapmeRs provide a powerful tool leading to RNAse-H dependent degradation, which is an endogenous enzyme. If localization of the lncRNA is unknown, GapmeRs may still present a reasonable method of choice, since the site of transcription is primarily nuclear. GapmeRs are ~20 nt long LNA-modified antisense oligonucleotide sequences (ASOs) that bind by simple Watson-Crick base-pairing to target RNA-transcripts. Furthermore, GapmeRs are applicable for in vivo studies. For example, administration of GapmeRs against MALAT1 resulted in the inhibition of ischemia-induced angiogenesis in mice, and GapmeRs targeting Chast prevented pathological cardiac remodeling.7,8
To assess the degree of lncRNA knockdown, exon-spanning qPCR primers, which do not overlap with the targeted sequence, should be designed carefully. Furthermore, exon-spanning primers are important for RNA fractionation experiments, especially when using genomic DNA-rich chromatin-associated fractions, to avoid misleading interpretation for lncRNA expression in nuclear vs chromatin fractions (S.H. and M.W.F., personal observations).
Identification of lncRNA interacting binding partners—advantages and disadvantages
The functional diversity of lncRNAs arises either through its architecture and/or its binding affinity in a combinational manner by RNA-RNA, RNA-DNA, or RNA-protein interactions. The RNA-centric methods to identify lncRNA interactors should be chosen carefully with respect to probe specificity, cellular context, and lncRNA secondary structure.
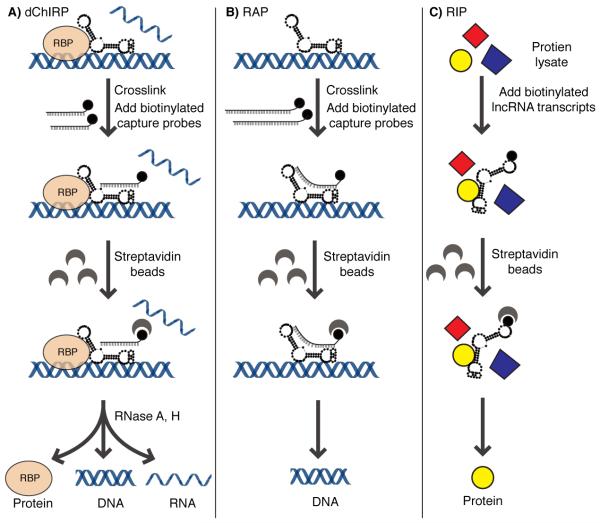
dChIRP
One method of identifying chromatin-associated lncRNA interactors is termed domain-specific chromatin isolation by RNA purification (dChIRP), an improved technique to the initial ChIRP assay (Fig. 1A).9,10 The aim of this method is to dissect functional domains of target RNA within its cellular context. The method is based on biotinylated antisense 20-mer oligos, which are divided into domain-specific pools, instead of “odd” vs “even” pools as described earlier in ChIRP.10 Additional steps are optimized throughout the protocol as described.9
Figure 1. Identification of lncRNA interactors.
Schematic illustration of lncRNA pulldown methods including (A) dChIRP, (B) RAP, and (C) RIP.
RAP
Engreitz et al. developed a method termed RNA antisense purification (RAP) using biotinylated antisense probes to target endogenous RNA similar to ChIRP. 10,11 120 nt long capture probes spanning the entire transcript separated by every 15 nt are produced through T7-specific in vitro transcription. Cross-linked cell lysate are then purified for biotin-labeled RNA capture probes and eluted DNA was analyzed by subsequent next generation sequencing analysis (Fig. 1B). Control probes designed in the same way, but not complementary to target transcript should bind to the same non-specific double stranded DNA that the lncRNA probe could potentially bind to with the same efficiency, but without hybridizing to target RNA.11
To address the question of direct and indirect RNA-RNA and RNA-protein interactions, Engreitz et al. further developed conventional RAP (i.e. RNA-RAP) in three related protocols: RAP-RNA[AMT], RAP-RNA[FA] and RAP-RNA[FA-DSG]. Those three protocols mainly differ in the chemistry used for cross-linking.12
RIP
In contrast to the classical RNA immunoprecipitation (RIP) assay, the RIP assay for lncRNAs employ target RNAs that are in vitro transcribed using either T7 or SP6 specific polymerase for biotin-labeling and does not use formaldehyde for fixation. Mass spectrometry analyzes the eluted proteins (Fig. 1C).13
The advantage of using dChIRP compared to RAP and RIP is the ability to simultaneously identify RNA, DNA, and protein interactors within its cellular context. The lower resolution limit of dChIRP is proposed by ~200-500 bp to which the identified domains are appropriately dissected. Depending on the reagent used for cross-linking, RAP may also identify RNA, DNA, and protein interactors. However, RAP uses ~120 nt long capture probes versus 20 nt long probes for dChIRP. Longer capture probe increases specificity; however, this may also hinder binding due to lncRNA secondary structure. RIP is independent of biotin-oligonucleotide probes and without any crosslinking and is used primarily for identifying protein interactors. A caveat of in vitro RNA pull-downs is that heating followed by slow cool down may not necessarily fold into the right in vivo structure. Another caution is the high input of biotin-labeled RNA to the lysate compared to low in vivo abundance of lncRNAs transcripts, which may yield artifacts.
Conclusions
In summary, the function and genomic locus of lncRNA critically define the targeting strategy. Combining careful genomic loci analysis with precise genetic editing techniques in consideration with RNA secondary structure will improve our understanding of lncRNAs and their precise mechanism of action with RNAs, DNAs, and interacting proteins as recently demonstrated for the lncRNA Bvht.4 The locus and function of a newly identified lncRNA is important for considering whether large genomic deletions might affect cryptically encoded small RNA classes and/or antisense regulatory elements of protein-coding genes. Another important consideration is whether the lncRNA acts in cis or trans and whether the RNA product itself is essential for fulfilling its function or if its transcription per se that underlies its function. In the case of cis function, targeting may be best using small genetic editing approaches. In contrast, trans functioning lncRNAs are less sensitive to larger genetic deletions. Genetic rescue experiments by either removing the RDE as described for NORAD or by ectopic expression using viral systems are recommended, although the latter is only applicable for trans acting lncRNAs since integration will be random.1
Currently, for therapeutic applications in human subjects, targeting lncRNAs using ASO-based strategies would be more favorable than genomic editing, a field that is still nascent. Furthermore, investigation of human lncRNAs in the context of CVD should not be deterred if a rodent homolog is not identified, and vice versa. While lncRNAs are not well-conserved across species (e.g. rodent Bvht lacks a human homolog), the presence of upstream or downstream ultra-conserved elements for some lncRNAs may provide insights for translating function across species.. Finally, SNPs located in lncRNAs or evolutionarily ultra-conserved regions may bear relevance for CVD susceptibility and may provide insights for an emerging class of new therapeutics.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the National Institutes of Health (HL115141, HL117994, and GM115605 to M.W.F.), the Arthur K. Watson Charitable Trust (to M.W.F.), the Dr. Ralph & Marian Falk Medical Research Trust (to M.W.F.), and a Swiss National Foundation Early Postdoc Mobility Fellowship (SNF# P2BEP3_162063 to S.H).
Footnotes
Disclosures
None
REFERENCES
- 1.Lee S, Kopp F, Chang T-C, Sataluri A, Chen B, Sivakumar S, Yu H, Xie Y, Mendell JT. Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell. 2016;164:69–80. doi: 10.1016/j.cell.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotzin JJ, Spencer SP, McCright SJ, Kumar DBU, Collet MA, Mowel WK, Elliott EN, Uyar A, Makiya MA, Dunagin MC, Harman CCD, Virtue AT, Zhu S, Bailis W, Stein J, Hughes C, Raj A, Wherry EJ, Goff LA, Klion AD, Rinn JL, Williams A, Flavell RA, Henao-Mejia J. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537:239–243. doi: 10.1038/nature19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latos PA, Pauler FM, Koerner MV, Şenergin HB, Hudson QJ, Stocsits RR, Allhoff W, Stricker SH, Klement RM, Warczok KE, Aumayr K, Pasierbek P, Barlow DP. Airn transcriptional overlap, but not its lncRNA products, induces imprinted Igf2r silencing. Science (New York, NY) 2012;338:1469–1472. doi: 10.1126/science.1228110. [DOI] [PubMed] [Google Scholar]
- 4.Xue Z, Hennelly S, Doyle B, Gulati AA, Novikova IV, Sanbonmatsu KY, Boyer LA. A G-Rich Motif in the lncRNA Braveheart Interacts with a Zinc-Finger Transcription Factor to Specify the Cardiovascular Lineage. Molecular cell. 2016 doi: 10.1016/j.molcel.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutschner T, Baas M, Diederichs S. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome Res. 2011;21:1944–1954. doi: 10.1101/gr.122358.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gagnon KT, Li L, Chu Y, Janowski BA, Corey DR. RNAi factors are present and active in human cell nuclei. Cell Rep. 2014;6:211–221. doi: 10.1016/j.celrep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michalik KM, You X, Manavski Y, Doddaballapur A, Zörnig M, Braun T, John D, Ponomareva Y, Chen W, Uchida S, Boon RA, Dimmeler S. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res. 2014;114:1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 8.Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, Just A, Fendrich J, Scherf K, Bolesani E, Schambach A, Weidemann F, Zweigerdt R, de Windt LJ, Engelhardt S, Dandekar T, Batkai S, Thum T. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med. 2016;8:326ra22–326ra22. doi: 10.1126/scitranslmed.aaf1475. [DOI] [PubMed] [Google Scholar]
- 9.Quinn JJ, Ilik IA, Qu K, Georgiev P, Chu C, Akhtar A, Chang HY. Revealing long noncoding RNA architecture and functions using domain-specific chromatin isolation by RNA purification. Nature Biotechnology. 2014;32:933–940. doi: 10.1038/nbt.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Molecular cell. 2011;44:667–678. doi: 10.1016/j.molcel.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engreitz JM, Pandya-Jones A, McDonel P, Shishkin A, Sirokman K, Surka C, Kadri S, Xing J, Goren A, Lander ES, Plath K, Guttman M. The Xist lncRNA exploits three-dimensional genome architecture to spread across the X chromosome. Science (New York, NY) 2013;341:1237973–1237973. doi: 10.1126/science.1237973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engreitz JM, Sirokman K, McDonel P, Shishkin AA, Surka C, Russell P, Grossman SR, Chow AY, Guttman M, Lander ES. RNA-RNA interactions enable specific targeting of noncoding RNAs to nascent Pre-mRNAs and chromatin sites. Cell. 2014;159:188–199. doi: 10.1016/j.cell.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, Hu X, Zhang Y, Zhang D, Li C, Zhang L. Methods for the study of long noncoding RNA in cancer cell signaling. Methods Mol Biol. 2014;1165:115–143. doi: 10.1007/978-1-4939-0856-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.