Abstract
IgG4-related disease was only recently discovered, so its description, management, and new discoveries related to its etiology are rapidly evolving. Because IgG4 itself is a unique antibody which is intimately related to the diagnosis of the disease, the role of plasmablasts in the pathophysiology remains an active area of discussion. Recent studies have uncovered a possible role for CD4-positive cytotoxic T lymphocytes, T follicular helper cells, and M2 macrophages. The clinical presentation is variable and can be vague, as this disease affects many organs and new presentations are continuing to be described. The diagnosis depends on clinical and histopathological assessment. The mainstay of treatment is with glucocorticoids, but rituximab has recently shown promise. Monitoring disease activity using imaging modalities (including positron emission tomography) and serum markers is imperative, as relapses are common. IgG4-related disease spans many medical disciplines but is a treatable condition with which all clinicians should be familiar.
Keywords: IgG4, plasmablasts, IgG4 related disease, IgG, Sjogren’s syndrome
Introduction
IgG4-related disease is a recently described entity which is quite rare. Descriptions and recognition of the disease are advancing rapidly. Certainly, the fact that it closely resembles malignancy is one of the many reasons why recognizing this disease is important. Furthermore, it is generally very treatment-responsive, so early diagnosis can contribute to a significant improvement in morbidity for patients. The underlying disease mechanisms are an area of active research, and further understanding will aid in the treatment of patients.
Epidemiology
Because IgG4-related disease was only recently described and mimics other diseases, recognition remains low and a true estimation of prevalence remains elusive. In 2011, the prevalence of autoimmune pancreatitis in Japan was 4.6 per 100,000 population 1, but prevalence data for other organs involved in IgG4-related disease are not well characterized. In Japanese patients with autoimmune pancreatitis as well as Japanese patients with IgG4-related disease of other organ types 2, the patients are predominantly male; there are 3 to 4 males to every 1 female patient. The mean age in both studies was late 60s. In a cohort from Spain with IgG4-related disease, 69.1% of patients were male and the median age at diagnosis was 53 years 3.
Interestingly, a recent review analyzed published pediatric cases of IgG4-related disease and found 25 cases with an average age of 13 and a mild female predominance (64%) 4.
In one small cohort of 25 patients with IgG4-associated cholangitis and autoimmune pancreatitis, 88% reported a history of “blue collar” work, often with exposures to occupational chemicals 5.
Clinical presentation
Patients classically present with subacute, non-specific complaints, which can be general, such as weight loss, or organ-specific complaints. The classic finding is a tumefactive lesion in at least one organ, but the disease can develop over years, adding new organs one at a time, so patients may present with multi-organ disease 6. In the study by Chen et al., the mean number of organs involved was 3.0 ± 1.6 (range of 1–10) 7. If the disease has progressed, organ failure can occur. Ruling out malignancy is crucial when the diagnosis of IgG4-related disease is considered. The differential is broad and is largely organ-specific, but multi-centric Castleman’s disease and other multi-organ autoimmune diseases such as granulomatosis with polyangiitis, Sjögren’s syndrome, eosinophilic granulomatosis with polyangiitis, and sarcoidosis should be considered.
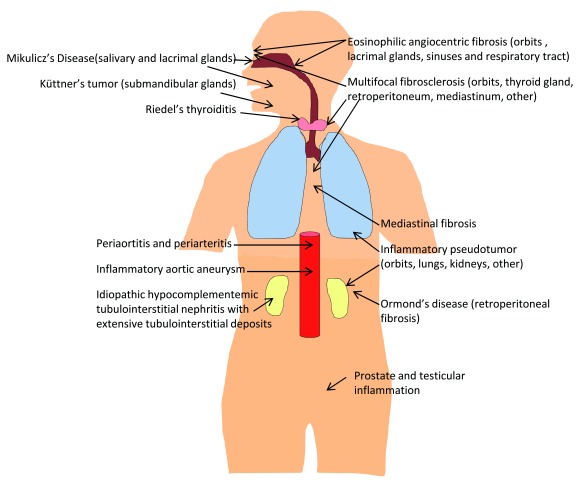
Although the classic organ involved in IgG4-related disease is the pancreas, which presents classically with obstructive jaundice due to damage to the biliary tree, many organs can be involved ( Figure 1). Recent novel reports of IgG4-related disease include disease involving the lung presenting with cough, dyspnea, chest pain, and fever 8; involving the sinuses, which in some cases can be invasive 9– 11; involving the esophagus presenting with dysphagia 12; involving the prostate presenting with urinary retention 13; or involving the testes presenting with scrotal pain 14. Skin manifestations were rare in the series reported by Chen et al. 7. A review of the dermatological literature showed that the most common skin presentations are papules, plaques, and nodules of the head and neck 15. Clinical symptoms of patients were well summarized by Chen et al. ( Table 1) 7. Organ involvement from three case series is summarized in Table 2.
Figure 1. Many organs are involved in IgG4-related disease.
This figure shows diseases that have now been re-classified to be part of IgG4-related disease, including eponymous names.
Table 1. Clinical manifestations of IgG4-related disease, as summarized in Chen et al. (out of 200 cases, 91% Chinese) 7.
| Clinical sign or symptom | Percentage reporting
sign or symptom in Chen et al. 7 |
|---|---|
| Submandibular gland swelling | 51.0% |
| Lacrimal gland swelling | 42.0% |
| Superficial lymph node enlargement | 37.0% |
| Abdominal pain | 35.0% |
| Parotid gland swelling | 24.0% |
| Nasal congestion | 21.5% |
| Jaundice | 14.0% |
| Pruritus | 13.5% |
| Cough | 13.5% |
| Lower back pain | 13.5% |
| Dysuresia | 13.0% |
| Dry mouth/dry eye | 12.5% |
| Nausea or vomiting or both | 12.0% |
| Fever | 9.0% |
| Edema | 8.5% |
| Exophthalmos | 5.5% |
| Arthralgia | 4.0% |
| Thyroid enlargement | 2.5% |
| History of allergy | 59.0% |
| Asymptomatic | 1.0% |
Table 2. Comparison of organ involvement in three large case series.
| Organ | Chen
et al.
7,
percentage of 200 cases a |
Fernandez-
Codina et al. 3, percentage of 55 cases b |
Inoue
et al.
2,
percentage of 235 cases c |
|---|---|---|---|
| Lymph nodes | 56.5% | ||
| Adenopathic | 2% | ||
| Salivary glands | 16% | 34% | |
| Submandibular
gland |
51.0% | ||
| Parotid gland | 24.0% | ||
| Pancreas | 38.5% | 16% | 60% c |
| Lung | 32.0% d | 9% | 13% |
| Pleura | 4% | ||
| Sinus | 21.5% | ||
| Maxillary | 15% | ||
| Bile ducts/biliary | 19.0% | 4% | 13% |
| Retroperitoneal
tissue |
18.0% | 27% | 4% |
| Prostate | 13.0% | 0% | |
| Kidney | 10.0% | 7% | 23% |
| Artery | 7.0% | 0% | 4% |
| Gallbladder | 5.5% | 5% | |
| Skin | 5.5% | 0% | |
| Inflammatory
pseudotumor |
5.0% | ||
| Liver | 3.0% | 0% | |
| Orbit | 4% | ||
| Orbitary
pseudotumor |
22% | ||
| Extraocular
muscles |
2% | ||
| Lacrimal gland | 42.0% | 15% | 23% |
| Aorta | 9% | 20% |
Chen et al. had predominantly Chinese cases 7, Fernandez-Codina et al. 3 analyzed patients from a Spanish registry 6, and Inoue et al. studied a Japanese cohort 2. aChen et al. included those who fulfilled 2011 diagnostic criteria for definite, probable, or possible IgG4-related disease 7. bFernandez-Codina et al. used 2012 international diagnostic consensus on IgG4-related disease for their inclusion 6. Fernandez-Codina et al. also reported 2% involvement of the thyroid, breast, pericardium, or mediastinum; 7% involvement of the mesentery; 4% involvement of the pachymeninges; and 0% involvement of the hypophysis. cInoue et al. included patients with a diagnosis of type I autoimmune pancreatitis as well as those with IgG4-related disease on pathology, which is possibly what accounts for a greater number of patients with pancreatic involvement 2. Inoue et al. reported 5% involvement of the paravertebra and 3% other sites. dIn Chen et al., this includes parenchyma, airway, and pleura.
Although most patients present before multi-organ involvement is evident, Higashioka et al. reported a case of a patient who had 10 organs involved in IgG4-related disease at the time of his diagnosis 16.
The clinical presentation of IgG4-related disease often mimics a tumor, which can result in unnecessary surgical intervention. Surgical resection for presumed malignancies occurred in 18 out of 53 patients, about 34%, who were ultimately diagnosed with IgG4-associated cholangitis in the series reported by Ghazale et al. 17. For example, Park and Kim 18 and Wang et al. 19 reported a case in which the discovery of a mass in the renal parenchyma resulted in a patient undergoing nephrectomy, and ultimately the mass was found to be IgG4-related disease. Similarly, Kim et al. presented a case of a patient in whom there was concern for cholangiocarcinoma and who underwent a left trisectionectomy but was ultimately diagnosed with IgG4-related disease 20. IgG4-related disease can also be initially mistaken for lymphoma, as both diseases can present with lymph node swelling as an initial symptom 21, 22. Differentiating IgG4-related disease from cancer is of the utmost importance. The diagnosis of IgG4-related disease is ultimately reliant on diagnostic tools ( Table 3).
Table 3. Evolution of diagnostic criteria for diagnosis of IgG4-related disease.
|
Autoimmune pancreatitis diagnosis
using the HISORt a criteria, 2007 73 |
Obstructive jaundice, pancreatic mass/enlargement, or pancreatitis with
at least one of the HISORt criteria: |
|
| Histology | Lymphoplasmacytic infiltrate with storiform fibrosis or
immunostaining shows abundant IgG4-positive cells. |
|
| Imaging | Diffusely enlarged pancreas and a diffusely irregular,
narrow pancreatic duct |
|
| Serology | Elevated IgG4 levels | |
| Other organ
involvement |
Extrapancreatic manifestations | |
| Response to
therapy |
Manifestations resolve with steroid therapy | |
|
IgG4-associated cholangitis diagnosis
using the HISORt criteria, 2008 17 | ||
| Histology of bile
duct |
Lymphoplasmacytic sclerosing cholangitis on
resection specimens |
|
| Imaging | Strictures of the bile ducts | |
| Serology | Elevated IgG4 levels | |
| Other organ
involvement |
Pancreas, kidney, retroperitoneum, salivary glands | |
| Response to
therapy |
Normalization of liver enzyme levels or resolution of
stricture |
|
|
Comprehensive diagnostic criteria for
IgG4-related disease, 2011 74 | ||
| Definite: 1+2+3. Probable: 1+3. Possible: 1+2. | ||
| 1. Clinical examination showing characteristic diffuse/localized swelling or
masses in single or multiple organs | ||
| 2. Hematological examination shows elevated serum IgG4 concentrations
(135 mg/dL) | ||
| 3. Histopathologic examination shows
(1) Marked lymphocyte and plasmacyte infiltration and fibrosis (2) Infiltration of IgG4 + plasma cells: ratio of IgG4 + to IgG + cells >40% and >10 IgG4 + plasma cells per high-power field | ||
In 2007, Chari published criteria to diagnose autoimmune pacreatitis 73, and Ghazale et al. followed in 2008 with criteria to diagnose IgG4-associated cholangitis 17; both were the Mayo Clinic experiences. Ultimately, in 2011, comprehensive criteria for diagnosing IgG4-related disease were developed by Umehara et al., from Japan 74. aHISORt, histology, imaging, serology, other organ involvement, and response to therapy.
Monitoring and following disease
Serum studies
Although IgG4 levels are classically elevated in IgG4-related disease (Japanese criterion is more than 135 mg/dL), this finding is not necessary to make the diagnosis—which is based on histopathological findings when histopathology is available, as obtaining specimens (for example, in the pancreas and biliary tract) is not without risk—and is not specific to the disease 23. Indeed, approximately 30% of patients with IgG4-related disease have normal IgG4 serum levels 23 (the upper limit of normal for serum IgG4 is 135 mg/dL 6), but the greater the number of organs involved in the disease, the higher the elevation in the serum IgG4 level 24. An elevated IgG4 level is also not specific for the disease, as there are many diseases associated with this laboratory abnormality; it similarly does not have a good positive predictive value 25. Importantly, Oseini et al. showed that elevated IgG4 can be seen in patients with cholangiocarcinoma 24, and Ghazale et al. showed that elevated IgG4 cannot be used to distinguish autoimmune pancreatitis from pancreatic cancer, thus elevated serum IgG4 levels do not exclude these deadly differential diagnoses 26. Patients with primary sclerosing cholangitis can also have elevated IgG4 levels 27, 28. However, 65 out of 72 patients with IgG4-related disease had elevated IgG4 levels, yielding a sensitivity of 90% according to a study by Carruthers et al. 25.
With glucocorticoid treatment, elevated IgG4 levels can decrease but may remain above the upper limit of normal 23. In one study of the treatment of autoimmune pancreatitis, the IgG4 level remained above the upper limit of normal in 115 out of 182 patients (63%) treated with glucocorticoids 29. Furthermore, despite elevated levels, patients may remain in remission; in this study, only 30% of patients with persistent elevation of serum IgG4 had a relapse 29. Although an increase in IgG4 level may be a marker of relapse in some patients, for 10% of the patients in this study, relapses also occurred without an increase in the serum concentration of IgG4 29.
Although it is not yet clinically available, quantitation of circulating plasmablasts using flow cytometry is more sensitive than serum IgG4 levels, so this measurement could emerge as a better test for the diagnosis and monitoring of IgG4-related disease in the future 30, 31.
IgG4/IgG RNA ratio is a novel, highly accurate marker for IgG4-related disease and has been validated for IgG4-associated cholangitis and autoimmune pancreatitis 32. The clinician should be aware that very high serum IgG4 levels may lead to errors in the measurement of IgG4 serum concentrations, resulting in reporting of spuriously low values, a type of “prozone” effect 33.
In conclusion, IgG4 levels alone are insufficient to make a diagnosis of IgG4-related disease or to monitor patients in follow-up. Elevations in eosinophils and C-reactive protein can also be observed in patients with IgG4-related disease, but these are non-specific 6.
Imaging
As already stated, histopathology is an integral part of the diagnosis, but imaging can aid in defining the disease involvement and in follow-up. A recent study showed the efficacy of using positron emission tomography/computed tomography (PET/CT) with the glucose analogue 2-[(18)F]-fluoro-2-deoxy-d-glucose (FDG) in patients diagnosed with one-organ involvement of IgG4-related disease to assess for the involvement of other organs 34. In this study, Zhang et al. reported that 9 out of 10 patients initially enrolled in the study with single-organ disease were ultimately found to have multi-organ disease 34. PET/CT is also useful for monitoring disease progression and identifying sites for biopsy 35. CT and magnetic resonance imaging (MRI) can also be used to identify organ enlargement, and lesions identified by CT typically generate low signal intensity in T2-weighted MRI 36, 37.
Histopathology of IgG4-related disease
Histopathology is the gold standard for diagnosis of IgG4-related disease, since the disease cannot be diagnosed on the basis of IgG4 levels in the serum or in tissue samples on pathology, although these findings are suggestive of the disease. The classic findings are dense lymphoplasmacytic infiltrate, storiform fibrosis, and obliterative phlebitis (which means partial or complete obliteration of a vein) 38. The presence of at least two of these findings in combination with infiltration of IgG4-positive plasma cells is highly suggestive of a diagnosis of IgG4-related disease, with the cutoff value for the number of IgG4-positive plasma cells being defined for each organ. Eosinophils are commonly present. IgG4-positive plasma cells are distributed diffusely throughout the tissue, even in patients who have normal levels of serum IgG4. This finding can help to differentiate IgG4-related disease from other diseases characterized by plasma cell infiltration but is not sufficient to make the diagnosis. The cutoff value for the number of IgG4-positive plasma cells required to make a diagnosis is specific to the organ, but the ratio of IgG4 to IgG cells must be greater than 40% 38.
It is worth mentioning that the clinician must be cautious to not over-emphasize the role of histopathology, especially IgG4 immunostaining, when making a diagnosis of IgG4-related disease. Strehl et al. highlighted this nicely: they analyzed 121 randomly selected pathology specimens with lymphoplasmacytic infiltrates 39. Interestingly, variably high numbers of IgG4-positive plasma cells were found in diverse samples, particularly in rheumatoid synovitis, carcinoma-associated inflammation, and sclerosing sialadenitis 39. This article thus highlights the fact that IgG4 can be elevated in inflammation unrelated to IgG4-related disease 39. Cheuk and Chan also warned of the danger of over-interpretation of lymphadenopathy with positive immunostaining for IgG4 in a patient with no other clinical or laboratory signs of IgG4-related disease 40.
Treatment options
Watchful waiting
Although organ involvement in IgG4-related disease requires treatment, less aggressive management is appropriate if this is not the case. For example, in asymptomatic lymphadenopathy, watchful waiting is acceptable.
Glucocorticoids
The first-line treatment of IgG4-related disease is glucocorticoids 29, and the first prospective, randomized, placebo-controlled trial examining their efficacy for the treatment of autoimmune pancreatitis was just published by Masamune et al. 41. In this study, remission was induced with prednisolone in both groups and then withdrawn at 26 weeks in 19 of the patients versus continued for 3 years in 30 patients 41. The authors found that the relapse rate was significantly lower in the maintenance group, where 7 out of 30 patients (23.3%) relapsed compared with the cessation group, where 11 out of 19 (57.9%) relapsed ( P = 0.011) 41.
The initial treatment for autoimmune pancreatitis is typically prednisolone 0.6 mg/kg for 2 to 4 weeks, which is then tapered every 2 to 4 weeks to 5 mg/day over the subsequent 3 to 6 months, and then 2.5 to 5 mg/day is continued for 3 years 42, 43. Notably, low doses of prednisone may be as effective as higher doses for induction therapy: a retrospective review found that outcomes were not significantly different in patients who received prednisone in the range of 10 to 60 mg per day 44. Clinical symptoms, imaging, and blood tests are used to guide the taper 42. Many patients have an effective, rapid response to glucocorticoids, and an alternative diagnosis should be considered if this rapid response is not seen 42, 45. Advanced fibrosis, however, is a poor prognostic sign 42.
A recent systematic review by Brito-Zeron et al. reflects the common paradigm with treatment with glucocorticoids: 1,186 out of 1,220 patients (97%) who received monotherapy with glucocorticoids as their first-line drug had a therapeutic response, but the response was classified as complete in only 84 out of 130 patients (65%) in whom this information could be extracted 45. This study also discussed the management of relapses, where glucocorticoids were most commonly used but immunosuppressive agents were used in 149 cases (39%) (azathioprine in 126 out of these 149 cases) and rituximab in 9 cases (2%) 45. The treatment was reported to be effective to treat the relapses for 219 out of 231 cases (95%) treated with glucocorticoids, 56 out of 69 patients (81%) treated with azathioprine, 16 out of 22 patients (72%) treated with other immunosuppressives, and 9 out of 9 patients (100%) treated with rituximab 45.
Alternatives to glucocorticoids
Steroid-sparing treatments, such as azathioprine, mycophenolate mofetil, and methotrexate, are used to allow respite from the side effects of glucocorticoids and in order to maintain remission, but evidence for their effectiveness is lacking 42. A more recent, promising option is treatment with rituximab; the mechanism is via the depletion of CD20-positive plasmablast precursors— with fewer plasmablasts, IgG4 production decreases 46. Yamamoto et al. recently published a case report of successful treatment with abatacept (an inhibitor of T-cell activation), but treatment with this is in its infancy 47.
Alternatives to medication
Surgery and radiotherapy have been reported to treat tumefactive masses in the pancreas, kidneys, or other organs in cases where the diagnosis of IgG4-RD may not have occurred until after histopathologic analysis was performed. However, these modalities have had some role in treating patients with specific involvements, such as infiltrative masses of tubular structures, such as the biliary tract, lymphadenopathy, or masses in other solid organs. In the recent systematic review of treatment of IgG4-related disease, out of 1,952 patients, 1,437 (74%) were treated with glucocorticoids as first-line, but 213 patients (11%) were treated with surgery or radiotherapy and 38 (2%) were treated with other options 45. Surgery was considered effective in 14 out of 17 patients (82%), and radiation was considered effective in 9 out of 12 patients (75%) 45. Combination surgery and glucocorticoids were effective in 20 out of 22 patients (91%) 45.
Mortality
In a recent systematic review of treatment of IgG4-related disease, Brito-Zeron et al. compiled the mortality data from 7 studies for 294 patients and a mean follow-up of 29.2 months 45. In this group, mortality was reported in 26 patients (8.8%). Four patients died of pulmonary disease, 1 died of an aneurysm, 1 of cholangitis, 1 of renal failure, 7 of cancer, 4 of cardiovascular disease, 3 of infection, and 5 of unknown or other causes 45.
Pathophysiology of IgG4
IgG4 molecule
The immunoglobulin IgG4 isotype accounts for less than 5% of the total IgG in healthy patients and is the least abundant of the IgG subclasses 48. It is unique among the immunoglobulin isotypes in that it undergoes a process known as fragment antigen-binding (Fab)-arm exchange. The disulfide bonds between the heavy chains of the IgG4 molecules are unstable, so dissociation of the heavy chains occurs, leading to random recombination. The result is antibodies with two different antigen-binding specificities that are unable to cross-link antigen or form immune complexes in a manner similar to that of IgG1, IgG2, and IgG3 antibodies. IgG4 antibodies are also unable to fix complement 42, 48, 49. IgG4 has traditionally been regarded as anti-inflammatory.
Another unique property of IgG4 is that it sometimes binds the Fc region of IgG, producing a rheumatoid factor (RF), but the physiologic relevance of this is unclear 50. RF was reported to be positive in 20% of patients (50 out of 255) with IgG4-related disease 6. The question of whether IgG4 itself is pathogenic remains to be fully elucidated. Shiokawa et al. injected Balb/c mice with IgGs from patients with IgG4-related disease in order to explore this question 51. Pancreatic injury was seen with both IgG1 and IgG4, but more so from IgG1. Interestingly, patient IgG1-induced damage to the mouse pancreas was somewhat attenuated with the addition of patient IgG4.
Genetics
There is much still to be learned about the genes involved in IgG4-related disease. Genetic susceptibility has been associated with certain human leukocyte antigen (HLA) haplotypes in Japanese and Korean populations 49. More recent studies have identified genes involved in pancreatic cell injury 52, and cytotoxic T-lymphocyte antigen 4 (CTLA-4) gene polymorphisms have been associated with IgG4-related disease in the pancreas 53.
Autoimmunity
Autoimmunity has long been hypothesized as a pathologic mechanism in IgG4-related disease, but studies have failed to identify a clear autoantigen specific to the disease that selectively induces the production of IgG4 autoantibodies 49. It stands to reason that the target of the antibody would be specific to the organs commonly affected in IgG4-related disease. Indeed, a recent study which sought to identify autoantigens in IgG4-related disease identified only one candidate: prohibitin 54. As prohibitin is involved in anti-proliferation, the authors hypothesize that antibodies against prohibitin would result in tissue growth and the presentation of IgG4-related disease 54. Another recent study showed that anti-nuclear antibody (ANA) in IgG4-related disease was not of the IgG4 subclass 55. ANA was reported to be positive in 32% of patients (168 out of 524 patients) 6.
Innate immunity
Evidence is beginning to accumulate that there is a role for innate immunity in the mechanism of IgG4-related disease. Nakajima et al. used DNA microarray analysis to show that the expression of genes associated with allergy or innate immunity is lower in the peripheral blood mononuclear cells of patients with IgG4-related disease compared with healthy controls (HCs) and that these levels were increased with steroid therapy 56. Furthermore, Watanabe et al. recently postulated that since activation of the innate immune system leads to the production of IgG4, this pathway could be the target of future treatments for IgG4-related disease 57.
Hypocomplementemia
A feature that is important in IgG4-related disease is hypocomplementemia. The etiology of this was studied by Sugimoto et al., who showed that patients with hypocomplementemia in IgG4-related disease have a high serum level of C1q-binding IgG4, thus implying that IgG4 participates in the activation of complement in these patients by an unknown mechanism 58.
Role of T cells (Th2, Treg, Tfh, and CD4 + CTL)
It has been observed that CD4 T cells are present at the sites of inflammation in IgG4-related disease. Similar to IgE, class switching to IgG4 depends on interleukin-4 (IL-4) and IL-13. These interleukins are considered part of the type 2 helper T (Th2) immune response. Messenger RNA levels of these Th2 cytokines are higher in organs affected by IgG4-related disease in comparison with other classic autoimmune conditions 59, 60. The peripheral blood lymphocytes of patients with IgG4-related disease also express elevated levels of Th2 cytokines 60. A subset of patients also has elevated eosinophils and IgE, suggestive of an underlying disease mediated by Th2 cytokines.
Allergens and nematodes that induce IgE also induce IgG4. However, a so-called “modified Th2 response” refers to the scenario in which IgG4 antibody is produced without IgE antibody production, which seems to be the desired, healthy response to innocuous antigens, as it occurs during occupational exposures to antigens such as in beekeepers 48. Allergen-specific IgG4 antibodies are also induced during allergen immunotherapy and accompany clinical improvement 61.
A role for elevated levels of regulatory T (Treg) cells in IgG4-related disease has also been suggested 62. This is in contrast to most autoimmune conditions, in which a deficiency in Treg cells is present and thought to promote autoimmunity. In IgG4-related disease, it has been hypothesized that collagen deposition occurs through the production of cytokines produced by Th2 and Treg cells and thus that these cells could act synergistically to induce tissue fibrosis, a prominent feature of the disease 63.
Recent studies 64, 65 suggest that the role of Th2 immune responses in IgG4-related disease may be overstated and may, in fact, be a reflection of atopy, which affects approximately 40% 66 of patients with IgG4-related disease, which is similar to the prevalence in the general US population. Instead, evidence is accumulating for a role of CD4-positive cytotoxic T lymphocytes (CTLs). Maehara et al. have shown over-expression of genes associated with CD4-positive CTLs in the salivary glands of patients with IgG4-related disease compared with HCs and compared with patients with Sjögren’s syndrome and chronic sialoadenitis 65. Furthermore, CD4-positive CTLs, which produce IL-1β, transforming growth factor-beta 1 (TGF-β1), and interferon-gamma (IFN-γ), are found in affected tissue. IL-1β and TGF-β1 have been shown to be involved in the pathology of fibrosis 67, an integral feature of IgG4-related disease, thus further strengthening the putative role of CD4-positive CTLs. Finally, rituximab-mediated B-cell depletion results in both clinical improvement of patients’ IgG4-related disease and a decrease in the number of CD4-positive CTLs 64. This new research is exciting, as CD4-positive CTLs may ultimately prove to be the major mechanism of pathogenesis of IgG4-related disease.
The role of circulating follicular helper T (Tfh) cells in IgG4-related disease is an active area of research. Akiyama et al. have published two articles implicating a role for these cells in IgG4-related disease 68, 69. They showed that in patients with untreated IgG4-RD compared with those with primary Sjögren’s syndrome, those with multi-centric Castleman’s disease, and HCs, the number of circulating Tfh2 cells (defined as CD3 +CD4 +CD45RA −CXCR5 +CXCR3 −CCR6 −) was significantly increased. Furthermore, “activated” Tfh2 cells (defined by the additional markers CCR7 lowPD-1 high) were shown to induce IgG4 production and to decrease in response to treatment with glucocorticoids in parallel with decreased disease activity 68. Activated circulating Tfh1 cells were also found to correlate with disease activity but not with serum IgG4 levels 68.
Pathogenic effects of B cells and IgG4
Maillette de Buy Wenniger et al. showed that IgG4-positive B cells were clonally expanded in patients with IgG4-associated cholangitis and decreased when the patients were treated with glucocorticoids 70. The role of the B cells in IgG4-related disease is especially interesting given the utility of rituximab-mediated CD20 + B-cell depletion in treating these patients 46. Recent studies have shown that circulating plasmablasts are increased in patients with IgG4-related disease and that their levels reflect disease activity 30, 31. The plasmablasts are oligoclonal, which suggests that specific antigen(s) may be driving their production 30. In B-cell development, plasmablast differentiation occurs between activated B cells and plasma cells.
Role of M2 macrophages and MARCO
Returning to the issue of the IgG4-related disease being mainly a Th2-mediated disease, a possible disease mechanism could be mediated by M2 macrophages, which are defined as alternatively activated macrophages stimulated by Th2 response. IL-10, IL-13, and CCL18 produced by M2 macrophages induce fibrosis, and a recent study 71 showed that there are increased numbers of M2 macrophages in submandibular glands affected by IgG4-related dacryoadenitis and sialoadenitis and that fibrosis is associated with an increased number of M2 macrophages. Macrophage receptor with collagenous structure (MARCO) is a pattern-recognition receptor with an expression pattern similar to that of the M2 macrophage marker CD163. It has been speculated that MARCO may be involved in the initiation of IgG4-related disease 72. Future research is needed to ascertain whether there is any interaction between CD4-positive CTL or Th2 Tfh cells and M2 macrophages in promoting IgG4-related disease.
Conclusions
IgG4-related disease, though uncommon, is an important disease entity to consider in the differential of a patient presenting with tumefactive lesions. Current research into the underlying pathophysiology is focused on improved understanding of the T-cell and B-cell compartments. Future studies to improve the prognosis of this condition will focus not only on arresting the abnormal immunologic response but also on treating fibrosis.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Mitsuhiro Kawano, Division of Rheumatology, Department of Internal Medicine, Kanazawa University Graduate School of Medicine, Kanazawa, Japan
Rohit K. Katial, National Jewish Health, Denver, USA
Tsutomu Takeuchi, Division of Rheumatology, Department of Internal Medicine, Keio University School of Medicine, Tokyo, Japan
Motohisa Yamamoto, Department of Gastroenterology, Rheumatology and Clinical Immunology, Sapporo Medical University School of Medicine, Sapporo, Japan
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 4 approved]
References
- 1. Kanno A, Masamune A, Okazaki K, et al. : Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2011. Pancreas. 2015;44(4):535–9. 10.1097/MPA.0000000000000325 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Inoue D, Yoshida K, Yoneda N, et al. : IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore). 2015;94(15):e680. 10.1097/MD.0000000000000680 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Fernández-Codina A, Martínez-Valle F, Pinilla B, et al. : IgG4-Related Disease: Results From a Multicenter Spanish Registry. Medicine (Baltimore). 2015;94(32):e1275. 10.1097/MD.0000000000001275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Karim F, Loeffen J, Bramer W, et al. : IgG4-related disease: a systematic review of this unrecognized disease in pediatrics. Pediatr Rheumatol Online J. 2016;14(1):18. 10.1186/s12969-016-0079-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. de Buy Wenniger LJ, Culver EL, Beuers U: Exposure to occupational antigens might predispose to IgG4-related disease. Hepatology. 2014;60(4):1453–4. 10.1002/hep.26999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stone JH, Brito-Zerón P, Bosch X, et al. : Diagnostic Approach to the Complexity of IgG4-Related Disease. Mayo Clin Proc. 2015;90(7):927–39. 10.1016/j.mayocp.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Zhao J, Feng R, et al. : Types of Organ Involvement in Patients with Immunoglobulin G4-related Disease. Chin Med J (Engl). 2016;129(13):1525–32. 10.4103/0366-6999.184459 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Campbell SN, Rubio E, Loschner AL: Clinical review of pulmonary manifestations of IgG4-related disease. Ann Am Thorac Soc. 2014;11(9):1466–75. 10.1513/AnnalsATS.201403-128FR [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Chen BN: IgG4-related disease presenting with destructive sinonasal lesion mimicking malignancy. Eur Arch Otorhinolaryngol. 2016;273(11):4027–9. 10.1007/s00405-016-4033-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Song BH, Baiyee D, Liang J: A rare and emerging entity: Sinonasal IgG4-related sclerosing disease. Allergy Rhinol (Providence). 2015;6(3):151–7. 10.2500/ar.2015.6.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inoue A, Wada K, Matsuura K, et al. : IgG4-related disease in the sinonasal cavity accompanied by intranasal structure loss. Auris Nasus Larynx. 2016;43(1):100–4. 10.1016/j.anl.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 12. Oh JH, Lee TH, Kim HS, et al. : Esophageal Involvement of Immunoglobulin G4-Related Disease: A Case Report and Literature Review. Medicine (Baltimore). 2015;94(50):e2122. 10.1097/MD.0000000000002122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li D, Kan Y, Fu F, et al. : IgG4-related prostatitis progressed from localized IgG4-related lymphadenopathy. Int J Clin Exp Pathol. 2015;8(9):11747–52. [PMC free article] [PubMed] [Google Scholar]
- 14. de Buy Wenniger LM, Scheltema JM, Verheij J, et al. : Testicular inflammation as a new manifestation of IgG4-associated disease. Urology. 2013;82(2):e15–6. 10.1016/j.urology.2013.04.046 [DOI] [PubMed] [Google Scholar]
- 15. Charrow A, Imadojemu S, Stephen S, et al. : Cutaneous manifestations of IgG4-related disease (RD): A systematic review. J Am Acad Dermatol. 2016;75(1):197–202. 10.1016/j.jaad.2016.01.046 [DOI] [PubMed] [Google Scholar]
- 16. Higashioka K, Yoshida K, Oryoji K, et al. : A Case of Immunoglobulin G4-Related Disease with Extensive Multiorgan Involvements. Case Rep Rheumatol. 2015;2015: 392893. 10.1155/2015/392893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghazale A, Chari ST, Zhang L, et al. : Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134(3):706–15. 10.1053/j.gastro.2007.12.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Park HG, Kim KM: IgG4-related inflammatory pseudotumor of the renal pelvis involving renal parenchyma, mimicking malignancy. Diagn Pathol. 2016;11:12. 10.1186/s13000-016-0460-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Chen X, Luo R, et al. : IgG4-related systemic disease mimicking renal pelvic cancer: a rare case. World J Surg Oncol. 2014;12:395. 10.1186/1477-7819-12-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim S, Bae H, Choi M, et al. : Isolated Mass-Forming IgG4-Related Cholangitis as an Initial Clinical Presentation of Systemic IgG4-Related Disease. J Pathol Transl Med. 2016;50(4):300–5. 10.4132/jptm.2015.12.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Xue F, Yang J, et al. : Immunoglobulin G4-related disease mimicking lymphoma in a Chinese patient. Rheumatol Int. 2015;35(10):1749–52. 10.1007/s00296-015-3259-4 [DOI] [PubMed] [Google Scholar]
- 22. Hiyoshi Y, Oki E, Zaitsu Y, et al. : IgG4-related disease of the ileocecal region mimicking malignancy: A case report. Int J Surg Case Rep. 2014;5(10):669–72. 10.1016/j.ijscr.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sah RP, Chari ST: Serologic issues in IgG4-related systemic disease and autoimmune pancreatitis. Curr Opin Rheumatol. 2011;23(1):108–13. 10.1097/BOR.0b013e3283413469 [DOI] [PubMed] [Google Scholar]
- 24. Oseini AM, Chaiteerakij R, Shire AM, et al. : Utility of serum immunoglobulin G4 in distinguishing immunoglobulin G4-associated cholangitis from cholangiocarcinoma. Hepatology. 2011;54(3):940–8. 10.1002/hep.24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carruthers MN, Khosroshahi A, Augustin T, et al. : The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis. 2015;74(1):14–8. 10.1136/annrheumdis-2013-204907 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Ghazale A, Chari ST, Smyrk TC, et al. : Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102(8):1646–53. 10.1111/j.1572-0241.2007.01264.x [DOI] [PubMed] [Google Scholar]
- 27. Boonstra K, Culver EL, de Buy Wenniger LM, et al. : Serum immunoglobulin G4 and immunoglobulin G1 for distinguishing immunoglobulin G4-associated cholangitis from primary sclerosing cholangitis. Hepatology. 2014;59(5):1954–63. 10.1002/hep.26977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mendes FD, Jorgensen R, Keach J, et al. : Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101(9):2070–5. 10.1111/j.1572-0241.2006.00772.x [DOI] [PubMed] [Google Scholar]
- 29. Kamisawa T, Shimosegawa T, Okazaki K, et al. : Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58(11):1504–7. 10.1136/gut.2008.172908 [DOI] [PubMed] [Google Scholar]
- 30. Mattoo H, Mahajan VS, Della-Torre E, et al. : De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG 4-related disease. J Allergy Clin Immunol. 2014;134(3):679–87. 10.1016/j.jaci.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wallace ZS, Mattoo H, Carruthers M, et al. : Plasmablasts as a biomarker for IgG4-related disease, independent of serum IgG4 concentrations. Ann Rheum Dis. 2015;74(1):190–5. 10.1136/annrheumdis-2014-205233 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Doorenspleet ME, Hubers LM, Culver EL, et al. : Immunoglobulin G4 + B-cell receptor clones distinguish immunoglobulin G 4-related disease from primary sclerosing cholangitis and biliary/pancreatic malignancies. Hepatology. 2016;64(2):501–7. 10.1002/hep.28568 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; F1000 Recommendation
- 33. Khosroshahi A, Cheryk LA, Carruthers MN, et al. : Brief Report: spuriously low serum IgG4 concentrations caused by the prozone phenomenon in patients with IgG4-related disease. Arthritis Rheumatol. 2014;66(1):213–7. 10.1002/art.38193 [DOI] [PubMed] [Google Scholar]
- 34. Zhang J, Chen H, Ma Y, et al. : Characterizing IgG4-related disease with 18F-FDG PET/CT: a prospective cohort study. Eur J Nucl Med Mol Imaging. 2014;41(8):1624–34. 10.1007/s00259-014-2729-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaidyanathan S, Patel CN, Scarsbrook AF, et al. : FDG PET/CT in infection and inflammation--current and emerging clinical applications. Clin Radiol. 2015;70(7):787–800. 10.1016/j.crad.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 36. Toyoda K, Oba H, Kutomi K, et al. : MR imaging of IgG4-related disease in the head and neck and brain. AJNR Am J Neuroradiol. 2012;33(11):2136–9. 10.3174/ajnr.A3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fujita A, Sakai O, Chapman MN, et al. : IgG4-related disease of the head and neck: CT and MR imaging manifestations. Radiographics. 2012;32(7):1945–58. 10.1148/rg.327125032 [DOI] [PubMed] [Google Scholar]
- 38. Deshpande V, Zen Y, Chan JK, et al. : Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–92. 10.1038/modpathol.2012.72 [DOI] [PubMed] [Google Scholar]
- 39. Strehl JD, Hartmann A, Agaimy A: Numerous IgG4-positive plasma cells are ubiquitous in diverse localised non-specific chronic inflammatory conditions and need to be distinguished from IgG4-related systemic disorders. J Clin Pathol. 2011;64(3):237–43. 10.1136/jcp.2010.085613 [DOI] [PubMed] [Google Scholar]
- 40. Cheuk W, Chan JK: Lymphadenopathy of IgG4-related disease: an underdiagnosed and overdiagnosed entity. Semin Diagn Pathol. 2012;29(4):226–34. 10.1053/j.semdp.2012.07.001 [DOI] [PubMed] [Google Scholar]
- 41. Masamune A, Nishimori I, Kikuta K, et al. : Randomised controlled trial of long-term maintenance corticosteroid therapy in patients with autoimmune pancreatitis. Gut. 2017;66(3):487–494. 10.1136/gutjnl-2016-312049 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Kamisawa T, Zen Y, Pillai S, et al. : IgG4-related disease. Lancet. 2015;385(9976):1460–71. 10.1016/S0140-6736(14)60720-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Kamisawa T, Okazaki K, Kawa S, et al. : Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45(5):471–7. 10.1007/s00535-010-0221-9 [DOI] [PubMed] [Google Scholar]
- 44. Buijs J, van Heerde MJ, Rauws EA, et al. : Comparable efficacy of low- versus high-dose induction corticosteroid treatment in autoimmune pancreatitis. Pancreas. 2014;43(2):261–7. 10.1097/MPA.0000000000000044 [DOI] [PubMed] [Google Scholar]
- 45. Brito-Zerón P, Kostov B, Bosch X, et al. : Therapeutic approach to IgG4-related disease: A systematic review. Medicine (Baltimore). 2016;95(26):e4002. 10.1097/MD.0000000000004002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Carruthers MN, Topazian MD, Khosroshahi A, et al. : Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74(6):1171–7. 10.1136/annrheumdis-2014-206605 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Yamamoto M, Takahashi H, Takano K, et al. : Efficacy of abatacept for IgG4-related disease over 8 months. Ann Rheum Dis. 2016;75(8):1576–8. 10.1136/annrheumdis-2016-209368 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Aalberse RC, Stapel SO, Schuurman J, et al. : Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39(4):469–77. 10.1111/j.1365-2222.2009.03207.x [DOI] [PubMed] [Google Scholar]
- 49. Stone JH, Zen Y, Deshpande V: IgG4-related disease. N Engl J Med. 2012;366(6):539–51. 10.1056/NEJMra1104650 [DOI] [PubMed] [Google Scholar]
- 50. Kawa S, Kitahara K, Hamano H, et al. : A novel immunoglobulin-immunoglobulin interaction in autoimmunity. PLoS One. 2008;3(2):e1637. 10.1371/journal.pone.0001637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shiokawa M, Kodama Y, Kuriyama K, et al. : Pathogenicity of IgG in patients with IgG4-related disease. Gut. 2016;65(8):1322–32. 10.1136/gutjnl-2015-310336 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Chang MC, Jan IS, Liang PC, et al. : Human cationic trypsinogen but not serine peptidase inhibitor, Kazal type 1 variants increase the risk of type 1 autoimmune pancreatitis. J Gastroenterol Hepatol. 2014;29(12):2038–42. 10.1111/jgh.12649 [DOI] [PubMed] [Google Scholar]
- 53. Chang MC, Chang YT, Tien YW, et al. : T-cell regulatory gene CTLA-4 polymorphism/haplotype association with autoimmune pancreatitis. Clin Chem. 2007;53(9):1700–5. 10.1373/clinchem.2007.085951 [DOI] [PubMed] [Google Scholar]
- 54. Du H, Shi L, Chen P, et al. : Prohibitin Is Involved in Patients with IgG4 Related Disease. PLoS One. 2015;10(5):e0125331. 10.1371/journal.pone.0125331 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Kiyama K, Yoshifuji H, Kandou T, et al. : Screening for IgG4-type anti-nuclear antibodies in IgG4-related disease. BMC Musculoskelet Disord. 2015;16:129. 10.1186/s12891-015-0584-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Nakajima A, Masaki Y, Nakamura T, et al. : Decreased Expression of Innate Immunity-Related Genes in Peripheral Blood Mononuclear Cells from Patients with IgG4-Related Disease. PLoS One. 2015;10(5):e0126582. 10.1371/journal.pone.0126582 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Watanabe T, Yamashita K, Kudo M: IgG4-Related Disease and Innate Immunity. Curr Top Microbiol Immunol. 2016. 10.1007/82_2016_42 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Sugimoto M, Watanabe H, Asano T, et al. : Possible participation of IgG4 in the activation of complement in IgG4-related disease with hypocomplementemia. Mod Rheumatol. 2016;26(2):251–8. 10.3109/14397595.2015.1076924 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Zen Y, Fujii T, Harada K, et al. : Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45(6):1538–46. 10.1002/hep.21697 [DOI] [PubMed] [Google Scholar]
- 60. Miyake K, Moriyama M, Aizawa K, et al. : Peripheral CD4+ T cells showing a Th2 phenotype in a patient with Mikulicz's disease associated with lymphadenopathy and pleural effusion. Mod Rheumatol. 2008;18(1):86–90. 10.1007/s10165-007-0010-3 [DOI] [PubMed] [Google Scholar]
- 61. Akdis M, Akdis CA: Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. 2014;133(3):621–31. 10.1016/j.jaci.2013.12.1088 [DOI] [PubMed] [Google Scholar]
- 62. Zen Y, Kawakami H, Kim JH: IgG4-related sclerosing cholangitis: all we need to know. J Gastroenterol. 2016;51(4):295–312. 10.1007/s00535-016-1163-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Della-Torre E, Lanzillotta M, Doglioni C: Immunology of IgG4-related disease. Clin Exp Immunol. 2015;181(2):191–206. 10.1111/cei.12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mattoo H, Mahajan VS, Maehara T, et al. : Clonal expansion of CD4 + cytotoxic T lymphocytes in patients with IgG 4-related disease. J Allergy Clin Immunol. 2016;138(3):825–38. 10.1016/j.jaci.2015.12.1330 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Maehara T, Mattoo H, Ohta M, et al. : Lesional CD4 + IFN-γ + cytotoxic T lymphocytes in IgG4-related dacryoadenitis and sialoadenitis. Ann Rheum Dis. 2017;76(2):377–85. 10.1136/annrheumdis-2016-209139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Della Torre E, Mattoo H, Mahajan VS, et al. : Prevalence of atopy, eosinophilia, and IgE elevation in IgG4-related disease. Allergy. 2014;69(2):269–72. 10.1111/all.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wynn TA, Ramalingam TR: Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Akiyama M, Yasuoka H, Yamaoka K, et al. : Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther. 2016;18:167. 10.1186/s13075-016-1064-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Akiyama M, Suzuki K, Yamaoka K, et al. : Number of Circulating Follicular Helper 2 T Cells Correlates With IgG4 and Interleukin-4 Levels and Plasmablast Numbers in IgG4-Related Disease. Arthritis Rheumatol. 2015;67(9):2476–81. 10.1002/art.39209 [DOI] [PubMed] [Google Scholar]
- 70. Maillette de Buy Wenniger LJ, Doorenspleet ME, Klarenbeek PL, et al. : Immunoglobulin G4+ clones identified by next-generation sequencing dominate the B cell receptor repertoire in immunoglobulin G4 associated cholangitis. Hepatology. 2013;57(6):2390–8. 10.1002/hep.26232 [DOI] [PubMed] [Google Scholar]
- 71. Furukawa S, Moriyama M, Tanaka A, et al. : Preferential M2 macrophages contribute to fibrosis in IgG4-related dacryoadenitis and sialoadenitis, so-called Mikulicz's disease. Clin Immunol. 2015;156(1):9–18. 10.1016/j.clim.2014.10.008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Ohta M, Moriyama M, Maehara T, et al. : DNA Microarray Analysis of Submandibular Glands in IgG4-Related Disease Indicates a Role for MARCO and Other Innate Immune-Related Proteins. Medicine (Baltimore). 2016;95(7):e2853. 10.1097/MD.0000000000002853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chari ST: Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J Gastroenterol. 2007;42(Suppl 18):39–41. 10.1007/s00535-007-2046-8 [DOI] [PubMed] [Google Scholar]
- 74. Umehara H, Okazaki K, Masaki Y, et al. : Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22(1):21–30. 10.1007/s10165-011-0571-z [DOI] [PubMed] [Google Scholar]