Abstract
Background
Diabetes Mellitus (diabetes) is associated with significantly increased risk of peripheral vascular disease (PVD). Diabetes is classified as a coronary heart disease (CHD) risk equivalent, but it is unknown whether diabetes is a CHD risk equivalent for PVD.
Objectives
To evaluate the odds of peripheral arterial disease (PAD) or carotid artery stenosis (CAS) among participants with diabetes, CHD or both, compared to participants without diabetes or CHD, in a nationwide vascular screening database. We hypothesized that diabetes and CHD would confer similar odds of PAD and CAS.
Methods
A cross-sectional analysis of all eligible Life Line Screening Inc. participants age 30 to 90 years with ankle brachial indices (ABI) for PAD (ABI <0.9 in either leg) and carotid artery duplex ultrasound imaging for CAS (internal carotid artery stenosis ≥50%) were included for analysis, N=3,522,890.
Results
Diabetes and CHD were present in 372,330 (10.7%) and 182,760 (5.8%) of participants, respectively; PAD and CAS were present in 155,000 (4.4%) and 130,347 (3.7%) of participants. After multivariable adjustment, PAD odds were 1.56 (95% C.I. 1.54,1.59) and 1.69 (95% C.I. 1.65,1.73) for participants with diabetes or CHD, respectively. Participants with both diabetes and CHD had 2.75-fold increased odds of PAD (95% C.I. 2.66, 2.85). Findings were similar for CAS; compared with no diabetes or CHD, CAS odds increased for participants with diabetes alone (1.53, 95% C.I. 1.50,1.56), CHD alone (1.72 (95% C.I. 1.68,1.76), and both diabetes and CHD (2.57 95% C.I. 2.49, 2.66). Findings were consistent for women and men.
Conclusion
In a large database of over 3.5 million self-referred participants, diabetes was a CHD risk equivalent for PAD and CAS, and participants with comorbid diabetes and CHD had an especially robust association with PAD and CAS. Counseling regarding screening and prevention of peripheral vascular disease may be useful for patients with diabetes.
Graphical abstract
Peripheral Vascular Disease Risk for Diabetes and Coronary Heart Disease
INTRODUCTION
Diabetes mellitus (diabetes) increases coronary heart disease morbidity and mortality and is considered a coronary heart disease (CHD) risk equivalent (1). Diabetes is also a strong risk factor for peripheral vascular disease, including lower extremity peripheral arterial disease (PAD) (2–4) and carotid artery stenosis (CAS) (4–6). Risk of PAD and CAS is also higher among patients with prior myocardial infarction or CHD (3,6,7). It is unknown whether diabetes and CHD confer similar risks of vascular disease and whether diabetes is a risk-equivalent for peripheral vascular diseases such as PAD or CAS. Moreover, it is unknown whether comorbid diabetes and CHD increase vascular disease risk more than either diabetes or CHD alone. Increased understanding of diabetes as a vascular disease risk equivalent has broad application and importance, as it is estimated that by 2020 more than 30 million people in the United States will have prevalent diabetes (8). Using the Lifeline vascular screening program of more than 3.5 million participants (9), this study sought to evaluate whether diabetic participants without CHD have similar odds of both PAD and CAS as nondiabetic participants with established CHD. This study also investigated the odds of PAD and CAS with concomitant diabetes and CHD.
RESEARCH DESIGN AND METHODS
Study population
The study population has been described previously (4,9,10). The study was based on data provided by Life Line Screening Inc. (Independence, Ohio) to the Society for Vascular Surgery for research purposes. The study cohort consists primarily of self-referred individuals who paid for vascular screening tests out of pocket. Screenings were performed from 2003 to 2008 at more than 20,000 sites nationwide. Before undergoing the screening procedure, individuals completed an extensive questionnaire that included information on demographics, smoking, exercise, cardiovascular risk factors, medical comorbidities, and family history of atherosclerosis and vascular disease. Participants between 30 and 90 years of age were included in this analysis.
Ascertainment of PAD and CAS
Ascertainment of PAD and CAS was determined with simple noninvasive procedures that have high sensitivity and specificity: the ankle brachial index (ABI) and carotid Duplex, respectively (11), as previously described (9). Systolic blood pressure was measured in both arms (brachial arteries) and both ankles (posterior tibial arteries). If a posterior tibial Doppler signal was inaudible, the dorsalis pedis artery signal was measured. Left and right ABI measurements were obtained by dividing the ankle systolic blood pressure by the highest arm pressure. PAD was defined as an ABI <0.90 in either leg, and participants with an ABI >1.4 were excluded because these values may be falsely elevated due to calcification of the arterial wall (12). CAS was defined by carotid artery stenosis ≥50% in either (or both) internal carotid arteries (internal carotid artery peak systolic velocity ≥125 cm/s) (13,14).
Definition of Diabetes Mellitus and Other Variables of Interest
Similar to other studies (3), diabetes was defined as a self-reported physician diagnosis or the use of glucose lowering medication (4,9). Diabetes severity was defined as: 1) diabetes without the use of either oral glucose-lowering agents or insulin; 2) diabetes with oral glucose-lowering agents only or 3) diabetes with use of insulin with or without use of oral glucose-lowering agents. Coronary heart disease was defined as a self-reported prior myocardial infarction or prior coronary revascularization (coronary artery bypass, angioplasty or percutaneous coronary intervention) (15). A diagnosis of hypertension was based on either a self-reported diagnosis of hypertension or the use of antihypertensive medications (15). Hypercholesterolemia was defined as physician diagnosis or medication use. Participants who had smoked 100 cigarettes during their lifetime and were still currently smoking were considered smokers (current) or not currently smoking (former) (4). Participants who reported engagement in some kind of vigorous leisure time exercise at least once per week were considered active and all other participants were considered sedentary (9). Race and ethnicity were self-reported.
Quality control
As described previously (9), all Life Line sites utilize identical protocols and are subject to a quality control program. Included in this program are random monthly audits where ultrasonography images are graded for each team and individual performance is tracked. Physician audits are performed on a quarterly basis. All results are processed by the results center and all outliers are reviewed. Percentage of abnormal findings is tracked per team to identify groups finding abnormally high or low number of disease cases. A clinical leadership team holds monthly review meetings to evaluate performance of teams and individual members. For imaging modalities, annual competencies are performed and all new employees must have demonstrated initial competencies before being allowed to perform testing without supervision.
Statistical analysis
Each individual was assigned a unique identifier, and the investigators had access only to de-identified data. The population of interest was categorized into those with versus those without diabetes, and then sub-grouped by presence or absence of prior CHD. Baseline characteristics and the prevalence of PAD and CAS between participants with and without diabetes and with and without CHD were compared using the χ2 test for proportions and a two-sided independent sample t-test or ANOVA for continuous variables. Using the population without diabetes or CHD as the referent, odds ratios (ORs) and 95% CIs were calculated by logistic regression to examine the risk of PAD and CAS separately for participants with diabetes alone, CHD alone or both diabetes and CHD. In addition to unadjusted OR, model 1 was adjusted for age. Model 2 was adjusted for age, race/ethnicity, BMI, hypertension, current smoking, high cholesterol, exercise and family history. In sex-specific analysis, the strength of association between diabetes, with and without CHD, and PAD and CAS was evaluated using the logistic regression model in model 2. The Wald test was used for testing interaction between the presence of diabetes and CHD for the presence of each vascular disease subtype (PAD and CAS). We also estimated the prevalence and 95% CIs for participants without diabetes or CHD, with diabetes alone, CHD alone and both diabetes and CHD across discrete age groups (40 to 50 years, 51 to 60 years, and so on). Sensitivity analyses were performed across categories of diabetes and CHD. All statistical analyses were performed with PASW (version 18.0; SPSS Inc., Chicago, IL), SAS (version 9.12; SAS Institute Inc., Cary, NC), and the R package (R Development Core Team; available from http://www.r-project.org/).
To estimate the precise relationship between age and different peripheral vascular disease phenotypes, prevalence values from each disease were plotted against age. Prevalence of CAS and PAD was defined as count per 100,000. Prevalence of disease as a function of age was presented using scatter plots. Age was defined on the basis of 6 strata. The first stratum corresponded to participants aged 30. All subsequent strata were on the basis of 10-year age increments beginning with 41 years of age. A linear regression model was employed to examine age-related trends in CAS and PAD prevalence. Because prevalence of PAD and CAS had a right skewed distribution, estimation and inference for PAD and CAS was on the basis of the log scale. Uncertainty associated with the estimated trend was presented using 95% CIs of the regression line.
RESULTS
We analyzed data on 3,522,890 participants participating in the Life Line vascular screening. Overall, 372,330 (10.7%) had diabetes and 182,760 (5.8%) had CHD. Baseline characteristics of the study population stratified by diabetes and CHD are noted in Table 1. As expected, participants with both CHD and diabetes were older with more prevalent cardiovascular risk factors.
Table 1.
Demographics and Medical History by Diabetes and Prior Coronary Heart Disease*
| No Diabetes Mellitus (n = 3,150,560) |
Diabetes Mellitus (n = 372,330) |
|||||
|---|---|---|---|---|---|---|
| Total | No Prior CHD (n = 2,967,800) |
Prior CHD (n = 182,760) |
No Prior CHD (n = 323,027) |
Prior CHD (n = 49,303) |
P | |
| Demographics | ||||||
| Age (± SD) | 62.81(10.45) | 69.27(9.85) | 65.61(9.58) | 69.78(8.69) | <0.0001 | |
| Male (%) | 33.26 | 57.12 | 37.61 | 58.85 | <0.0001 | |
| Black | 2.9 | 2.26 | 5.86 | 4 | <0.0001 | |
| Hispanic | 2.41 | 1.69 | 3.62 | 2.54 | ||
| Asian | 1.97 | 1.35 | 2.93 | 2.17 | ||
| Native American | 2.65 | 3.83 | 3.43 | 4.18 | ||
| Other | 0.62 | 0.61 | 0.81 | 0.8 | ||
| Caucasian | 89.46 | 90.25 | 83.35 | 86.3 | ||
| BMI | 27.38(5.55) | 27.57(5.18) | 30.71(6.76) | 30.35(6.28) | <0.0001 | |
| Medical history | ||||||
| Hypertension | 43.17 | 66.55 | 73.09 | 82.8 | <0.0001 | |
| High cholesterol | 48.73 | 75.71 | 70.96 | 85.4 | <0.0001 | |
| Smoking | <0.0001 | |||||
| Current | 24.41 | 28.64 | 25.14 | 29.17 | ||
| Former | 23.24 | 30.42 | 25.2 | 31.78 | ||
| Never | 52.35 | 40.94 | 49.66 | 39.05 | ||
| Exercise | 63.23 | 63.35 | 56.74 | 55.11 | <0.0001 | |
| Family history of CVD | 22.55 | 49.54 | 25.93 | 55.31 | <0.0001 | |
CHD, coronary heart disease; SD, standard deviation; CVD, cardiovascular disease
Values are percent or mean (SD)
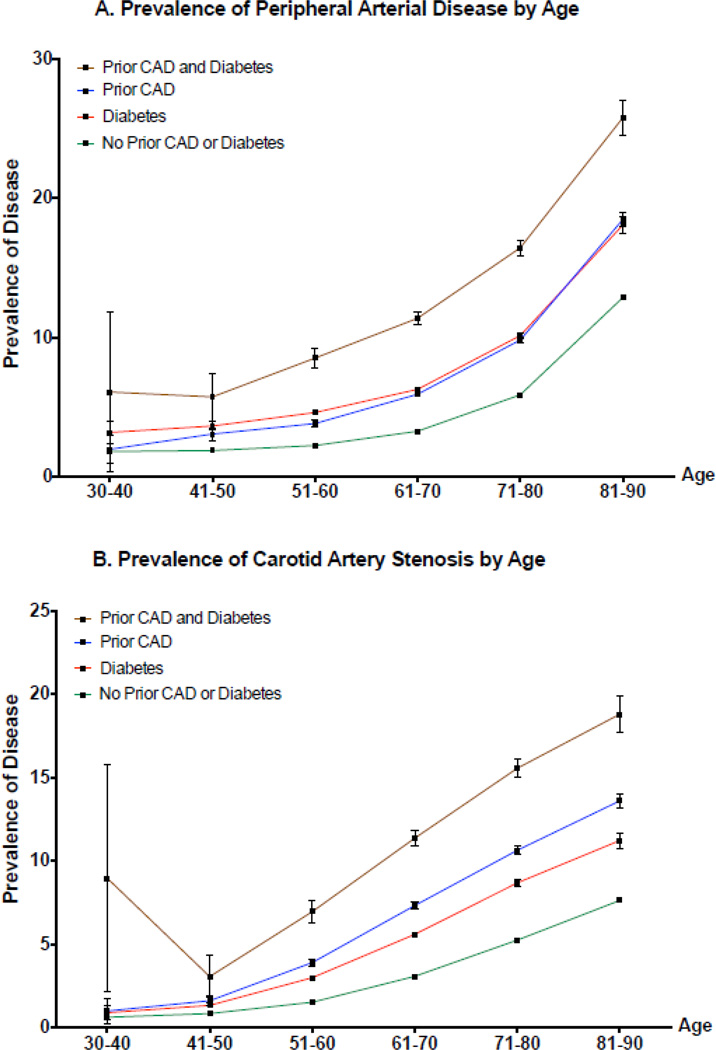
Peripheral arterial disease and CAS were identified in 4.4% and 3.7% of the population, respectively. The unadjusted prevalence of PAD and CAS are shown in Table 2. Compared to participants with no diabetes or CHD, participants with either CHD or diabetes had an increased prevalence of PAD and CAS, respectively (Table 2). The prevalence of PAD and CAS was greatest for men and women with both prior CHD and diabetes. Similar observations were noted across 10-year age groups (Figure 1), and across 10-year age groups stratified by gender (eFigures 1–2).
Table 2.
Prevalence of Peripheral Arterial Disease and Carotid Artery Stenosis by Sex, Diabetes and Coronary Heart Disease
| Percent (95% CI) |
||||
|---|---|---|---|---|
| No Diabetes Mellitus |
Diabetes Mellitus |
|||
| No Prior CHD | Prior CHD | No Prior CHD | Prior CHD | |
| PAD overall | 3.70 (3.67,3.72) | 8.36 (8.23,8.49) | 7.33 (7.23,7.42) | 14.3 (13.97,14.62) |
| Men | 2.61 (2.58,2.64) | 7.00 (6.83,7.16) | 5.61 (5.47,5.74) | 12.19 (11.79,12.60) |
| Women | 4.22 (4.19,4.25) | 10.04 (9.82,10.26) | 8.32 (8.20,8.45) | 17.01 (16.46,17.55) |
| CAS overall | 2.96 (2.94,2.98) | 8.53 (8.39,8.66) | 5.83 (5.75,5.91) | 13.03 (12.73,13.33) |
| Men | 3.19 (3.15,3.22) | 8.79 (8.61,8.97) | 6.10 (5.97,6.24) | 12.66 (12.26,13.06) |
| Women | 2.83 (2.81,2.86) | 8.13 (7.93,8.33) | 5.64 (5.54,5.74) | 13.47 (12.98,13.96) |
PAD, peripheral arterial disease; CAS, carotid artery stenosis; CHD, coronary heart disease; CI, confidence interval
Figure 1.
Figure 1a
Prevalence of Peripheral Arterial Disease by Age
Abbreviations: CAD, coronary artery disease
Figure 1b
Prevalence of Carotid Artery Stenosis by Age
Abbreviations: CAD, coronary artery disease
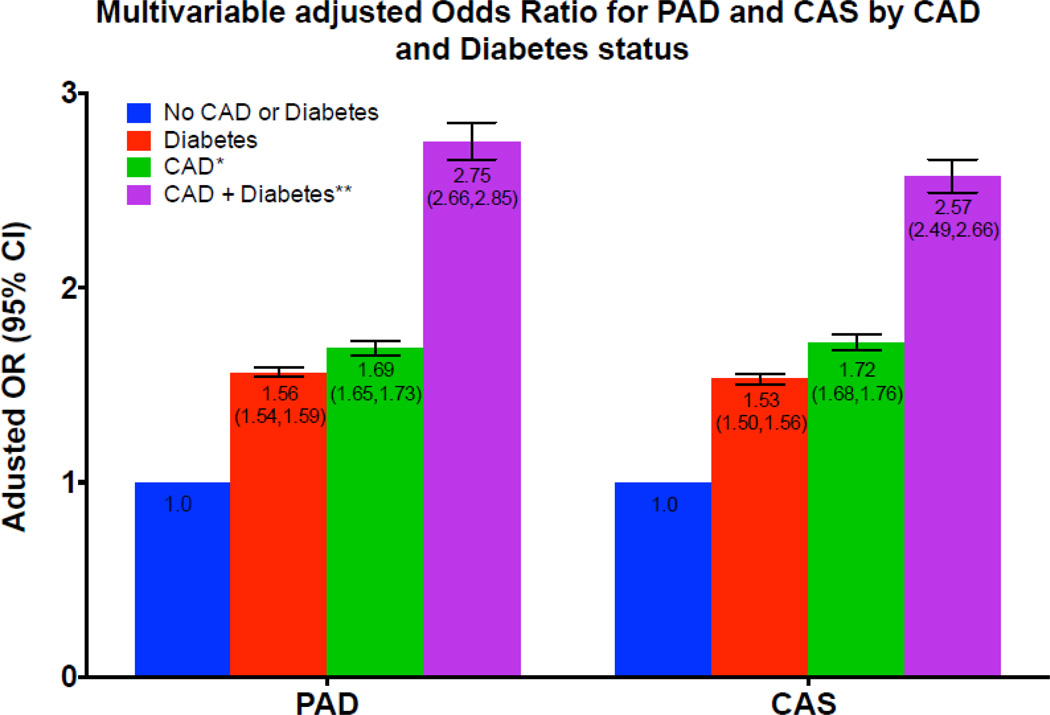
Table 3 presents age- and multivariable adjusted odds of PAD and CAS for CHD alone, diabetes alone and diabetes plus CHD. In reference to the no CHD or diabetes group, participants with CHD had a 1.71-fold increased odds of PAD (95% confidence interval [CI], 1.68 to 1.74) whereas diabetic participants had 1.83-fold increased odds (95% CI, 1.80 to 1.85). Participants with both CHD and diabetes had 3.15-fold increased odds of PAD (95% CI, 3.07 to 3.24). The odds of PAD with CHD or diabetes alone or as comorbid conditions were consistent for men and women (Table 3). The findings for CAS were similar to PAD. In reference to the no CHD or diabetes group, participants with CHD had a 2.19-fold increased odds of CAS (95% CI, 2.15 to 2.23) whereas diabetic participants had 1.79-fold increased odds of CAS (95% CI, 1.76 to 1.82). Participants with both CHD and diabetes had 3.54-fold increased odds of CAS (95% CI, 3.44 to 3.64). The odds of CAS with CHD or diabetes alone or as comorbid conditions were consistent for men and women (Table 3). Further adjustment for race/ethnicity, BMI, hypertension, smoking status, hyperlipidemia, physical activity and family history somewhat attenuated the odds of PAD and CAS. Figure 2 illustrates the multivariable-adjusted odds of PAD or CAS between participants with CHD alone, diabetes alone or CHD plus diabetes for these peripheral vascular phenotypes.
Table 3.
Age and Multivariable-Adjusted Models Stratified by Sex for Peripheral Arterial Disease or Carotid Artery Stenosis in Relation to Diabetes and prior Coronary Heart Disease
| OR (95% CI) |
||||
|---|---|---|---|---|
| No prior CHD No Diabetes Mellitus |
Prior CHD | Diabetes Mellitus | Prior CHD and Diabetes Mellitus |
|
| Age-adjusted | ||||
| PAD overall | 1.00 | 1.71 (1.68,1.74) | 1.83 (1.80,1.85) | 3.15 (3.07,3.24) |
| Men | 1.86 (1.80,1.91) | 1.92 (1.86,1.97) | 3.51 (3.37,3.66) | |
| Women | 1.89 (1.84,1.94) | 1.85 (1.81,1.88) | 3.46 (3.33,3.61) | |
| CAS overall | 1.00 | 2.19 (2.15,2.23) | 1.79 (1.76,1.82) | 3.54 (3.44,3.64) |
| Men | 2.00 (1.95,2.05) | 1.71 (1.67,1.76) | 3.05 (2.94,3.17) | |
| Women | 2.23 (2.16,2.29) | 1.82 (1.78,1.86) | 3.91 (3.74,4.08) | |
| Multi-variable | ||||
| Adjusted* | ||||
| PAD overall | 1.00 | 1.69 (1.65,1.73) | 1.56 (1.54,1.59) | 2.75 (2.66,2.85) |
| Men | 1.68 (1.62,1.74) | 1.72 (1.67,1.78) | 2.99 (2.85,3.14) | |
| Women | 1.66 (1.61,1.71) | 1.50 (1.47,1.53) | 2.57 (2.46,2.70) | |
| CAS overall | 1.00 | 1.72 (1.68,1.76) | 1.53 (1.50,1.56) | 2.57 (2.49,2.66) |
| Men | 1.63 (1.58,1.68) | 1.51 (1.47,1.56) | 2.36 (2.26,2.47) | |
| Women | 1.83 (1.77,1.89) | 1.54 (1.51,1.58) | 2.94 (2.80,3.10) | |
PAD, peripheral arterial disease; CHD, coronary heart disease; CAS, carotid artery stenosis
Adjusted for age and the variables in Table 1 (race/ethnicity, BMI, hypertension, current smoking, high cholesterol, exercise and family history of cardiovascular disease
Figure 2.
Multivariable adjusted Odds Ratio for PAD and CAS by CAD and Diabetes status
Abbreviations: CAD, coronary artery disease; CAS, carotid artery stenosis; OR, odds ratio
When analyzed in a multivariable logistic regression model including terms for diabetes, CHD and diabetes × CHD, there was a significant interaction between diabetes and CHD for risk of PAD (multivariable adjusted P = 0.04), indicating that comorbid diabetes and CHD act synergistically to increase PAD risk. The interaction term between diabetes and CHD for risk of CAS was non-significant (multivariable adjusted P = 0.25).
To assess the robustness of our findings, the analyses of PAD and CAS risk were repeated across categories of increasing diabetes severity, defined as: neither oral glucose-lowering agents nor insulin; oral glucose-lowering agents but no insulin; or insulin with or without oral glucose-lowering agents. The multivariable adjusted odds of PAD or CAS with CHD or diabetes alone or as comorbid conditions remained consistent for men and women across categories of increasing diabetes severity (eTables 1–3). When insulin use defined diabetes, the association between diabetes and vascular disease subtypes was numerically higher than for CHD (eTable 3). When the definition of CHD was restricted to prior myocardial infarction, with or without revascularization, the relationships between diabetes, CHD and vascular disease subtype were unchanged (data not shown).
CONCLUSION
The principal finding of the current study is that diabetes mellitus and prevalent CHD significantly increased the odds of different peripheral vascular disease phenotypes compared to participants with no history of CHD or diabetes. Compared to participants with CHD, the odds of PAD and CAS among participants with diabetes were numerically lower (Figure 2). While statistically significant, this difference is unlikely to be of clinical significance.
Our findings are consistent with previous studies demonstrating that diabetes and prior CHD are independent risk factors for PAD and CAS. Prospective cohort and cross-sectional studies (16–18) demonstrated approximately two-fold increased risk of PAD in diabetic compared to non-diabetic participants, along with a similar risk of PAD (adjusted OR 2.03 95% CI 1.91, 3.46) for participants with versus without prior CHD (18). Diabetes and prior CHD are also independent risk factors for CAS, although the risk has been less well defined (6). However, most prior studies of vascular risk from diabetes and prior CHD did not include participants ≥75 years of age and were limited in power. Because diabetes and vascular disease such as PAD and CAS are both strongly associated with age (9), our study has the advantage of including older participants to examine age-related trends of PAD and CAS associated with diabetes and prior CHD.
Since the Adult Treatment Panel III guidelines, diabetes has been classified as a CHD risk equivalent (1). Contemporary studies continue to support this classification of the cardiovascular risk associated with diabetes. In a recent analysis of follow-up from ≈1.2 million participants in population cohort studies, diabetic participants without prior myocardial infarction and nondiabetic participants with a prior myocardial infarction experienced a doubling in mortality compared to participants with neither diabetes nor CHD (19). Although diabetes is considered a CHD risk equivalent, a gradient of CHD risk exists within cohorts of patients with diabetes (20). Patients with more severe diabetes by increased glycated hemoglobin (21), diabetes duration (22), or use of insulin (23), have an increased risk of cardiovascular disease, including PAD (21), compared to patients with less severe diabetes. In the current study, use or non-use of glucose-lowering medications or insulin was a proxy for diabetes severity. Participants with diabetes using insulin had the strongest odds of PAD and CAS. However, the association with PAD and CAS was apparent regardless of diabetes definition used. Our results indicate a gradient of association with peripheral vascular disease may exist for patients with diabetes of increasing severity. Future studies should use well-defined measures of diabetes severity to further refine the association of diabetes with peripheral vascular disease.
We also found that comorbid diabetes and CHD have a particularly high-risk for peripheral vascular disease such as PAD and CAS, presumably due to greater baseline comorbidity (Table 1). Prior studies have demonstrated that risk factors such as smoking, hypertension, hypercholesterolemia and diabetes additively increase the risk of PAD, and that PAD risk increases with the presence of each risk factor (2). Diabetes potentiates the risk of PAD and CAS among participants with prior CHD, and confers nearly equivalent risk of PAD among participants without prior CHD.
We observed a difference in the effect of comorbid diabetes and CHD on risk of PAD vs. CAS. Together, CHD and diabetes synergistically increased the odds of PAD: participants with both had odds of PAD greater than the product of their individual odds-ratios. In contrast, comorbid diabetes and CHD had a multiplicative (additive on log-OR scale) effect on the odds of CAS. It is unknown whether the difference in interaction for PAD vs. CAS is due to lack of statistical power for the smaller group with CAS (most likely) or to underlying differences in disease pathophysiology by vascular disease subtype.
This study may have clinical implications. Our findings indicate that counseling patients with diabetes about peripheral vascular disease prevention and screening may be beneficial, particularly for those patients requiring insulin. Current guidelines recommend the use of resting ankle-brachial index for patients with suspected peripheral arterial disease, such as individuals age 50 and older with diabetes (24). Future studies might consider whether prevention and screening for peripheral vascular disease can improve cardiovascular outcomes among patients with diabetes.
Limitations and strengths
There are several limitations to this study. First, this was a cross-sectional, retrospective observational study design with inherent limitations in study design, analysis and interpretation. Second, our population database is a self-referred population who paid out-of-pocket for their screening tests, which may limit the generalizability of our findings. However, as reported previously, the prevalence of cardiovascular disease risk factors in this population database is similar to that reported in the general U.S. population (9,25). Moreover, the prevalence of PAD and CAS in the Life Line Survey was similar to other cohorts (25), demonstrating good external validity for the Life Line Survey population. Third, diabetes and CHD were self-reported and not verified with laboratory testing or in the medical record. However, as we report previously, the prevalence of diabetes in this population database is similar to that reported in other larger cohorts (4), suggesting that self-reported diabetes status accurately reflects the burden of diabetes in this population database. Nevertheless, we cannot rule-out misclassification in which nondiabetic participants mistakenly self-reported having diabetes. It is also possible that some participants had prevalent diabetes that had not yet been diagnosed; similar limitations may exist in regards to self-reported CHD. We also lack information on the duration and percent glycated hemoglobin of participants with diabetes, both of which have been associated with increased cardiovascular disease risk (22,26). We therefore may have diluted the actual effect of diabetes on PAD or CAS by including a relatively lower-risk diabetes population. In fact, sensitivity analyses presented in etables 1–3 demonstrate that the relationship between diabetes and PAD or CAS increased when diabetes was defined separately as oral glucose-lowering medications alone (etable 2) or insulin, with or without oral glucose-lowering medications (etable 3). Both misclassification of prevalent diabetes and a lower-risk diabetes population would have likely biased towards a null result. Fourth, PAD was assessed by ABI using the posterior tibial artery; the dorsalis pedis was used only when the posterior tibial artery was inaudible. Traditionally the highest of either the dorsalis pedis or the posterior tibial pressures is used for ABI calculations (12). However, a recent report compared this traditional method with an alternative method using the lower of the two ankle artery pressures, and both methods had similar diagnostic and predictive accuracy for all-cause and cardiovascular mortality, suggesting the utility of the alternate method in identifying a clinically meaningful population with PAD (27). Finally, patients with an ABI >1.4 were excluded due to arterial wall calcification (12), and the toe-brachial index was not available to confirm the presence of PAD in patients with a high ABI, a significant fraction of whom may have diabetes (24).
The present study also has several strengths, including a large number of women and men; the inclusion of a broad age range; and numerically large numbers of participants with different race/ethnicities across the United States. The large number of participants with PAD and CAS allows excellent power and facilitates adjustment for multiple covariates. Standardized assessment of ankle brachial indices and duplex carotid artery ultrasound was performed in every subject, thereby using a simple noninvasive validated procedure for the diagnosis of PAD and CAS that have high sensitivity and specificity.
In a large database of over 3.5 million self-referred participants we conclude that diabetes appears to be a coronary heart disease risk equivalent for PAD and CAS, and that participants with comorbid diabetes and CHD have an especially robust association with PAD and CAS. Our findings suggest that counseling regarding screening and prevention of peripheral vascular disease may be beneficial for patients with diabetes.
Supplementary Material
Acknowledgments
This work has used computing resources at the High Performance Computing Facility of the Center for Health Informatics and Bioinformatics at New York University Langone Medical Center. We gratefully acknowledge the participation and generosity of Life Line Screening (Cleveland, OH), who provided these data free of charge for the purposes of research and with no restrictions on its use for research or resultant publications.
Financial Support:
Dr. Newman was partially funded by the National Heart, Lung, and Blood Institute (NHBLI) of the National Institute of Health (NIH, K23HL125991) American Heart Association Mentored Clinical and Population Research Award (15MCPRP24480132). Dr. Berger was partially funded by the NHLBI of the NIH (HL114978). Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Relationships with Industry: None
Abbreviations
- diabetes
Diabetes mellitus
- PVD
Peripheral vascular disease
- CHD
Coronary heart disease
- PAD
Peripheral arterial disease
- CAS
Carotid artery stenosis
- ABI
Ankle brachial index
- ORs
Odds ratios
- CIs
Confidence intervals
- BMI
Body mass index
- SD
Standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 2.Joosten MM, Pai JK, Bertoia ML, Rimm EB, Spiegelman D, Mittleman MA, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012 Oct 24;308(16):1660–1667. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004 Aug 10;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 4.Shah B, Rockman CB, Guo Y, Chesner J, Schwartzbard AZ, Weintraub HS, et al. Diabetes and vascular disease in different arterial territories. Diabetes Care. American Diabetes Association. 2014 Jun;37(6):1636–1642. doi: 10.2337/dc13-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Göksan B, Erkol G, Bozluolcay M, Ince B. Diabetes as a determinant of high-grade carotid artery stenosis: evaluation of 1,058 cases by Doppler sonography. J Stroke Cerebrovasc Dis. 2001 Nov;10(6):252–256. doi: 10.1053/jscd.2001.123773. [DOI] [PubMed] [Google Scholar]
- 6.Inchiostro S, Dalfollo M, Marzano A, Citroni N, Peccatori S, Fait D, et al. Prevalence of diabetes and/or ischaemic heart disease in classes of increasing carotid artery atherosclerosis: an ultrasonographic study. Diabet Med. 2003 Aug;20(8):670–676. doi: 10.1046/j.1464-5491.2003.01016.x. [DOI] [PubMed] [Google Scholar]
- 7.Jones WS, Patel MR, Rockman CB, Guo Y, Adelman M, Riles T, et al. Association of the ankle-brachial index with history of myocardial infarction and stroke. American College of Cardiology. 2014 Apr;167(4):499–505. doi: 10.1016/j.ahj.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the Future Diabetes Population Size and Related Costs for the U.S. Diabetes Care. 2009 Nov 25;32(12):2225–2229. doi: 10.2337/dc09-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savji N, Rockman CB, Skolnick AH, Guo Y, Adelman MA, Riles T, et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. Journal of the American College of Cardiology. 2013 Apr 23;61(16):1736–1743. doi: 10.1016/j.jacc.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 10.Rockman CB, Hoang H, Guo Y, Maldonado TS, Jacobowitz GR, Talishinskiy T, et al. The prevalence of carotid artery stenosis varies significantly by race. J Vasc Surg. 2013 Feb;57(2):327–337. doi: 10.1016/j.jvs.2012.08.118. [DOI] [PubMed] [Google Scholar]
- 11.Criqui MH, Alberts MJ, Fowkes FGR, Hirsch AT, O’Gara PT, Olin JW, et al. Atherosclerotic Peripheral Vascular Disease Symposium II: screening for atherosclerotic vascular diseases: should nationwide programs be instituted? 2008:2830–2836. doi: 10.1161/CIRCULATIONAHA.108.191172. [DOI] [PubMed] [Google Scholar]
- 12.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012 Dec 11;126(24):2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 13.Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, Bluth EI, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. 2003:340–346. doi: 10.1148/radiol.2292030516. [DOI] [PubMed] [Google Scholar]
- 14.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS: Guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive Summary. Circulation. 2011;124:489–532. doi: 10.1161/CIR.0b013e31820d8d78. [DOI] [PubMed] [Google Scholar]
- 15.Hiramoto JS, Katz R, Weisman S, Conte M. Gender-Specific Risk Factors for Peripheral Artery Disease in a Voluntary Screening Population. Journal of the American Heart Association. 2014 Mar 4;3(2):e000651–e000661. doi: 10.1161/JAHA.113.000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004 Jul;27(7):1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 17.Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, et al. Ethnic-Specific Prevalence of Peripheral Arterial Disease in the United States. American Journal of Preventive Medicine. 2007 Apr;32(4):328–333. doi: 10.1016/j.amepre.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004 Aug 10;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 19.Schramm TK, Gislason GH, Køber L, Rasmussen S, Rasmussen JN, Abildstrøm SZ, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008 Apr 15;117(15):1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 20.Howard BV, Best LG, Galloway JM, Howard WJ, Jones K, Lee ET, et al. Coronary heart disease risk equivalence in diabetes depends on concomitant risk factors. Diabetes Care. American Diabetes Association. 2006 Feb;29(2):391–397. doi: 10.2337/diacare.29.02.06.dc05-1299. [DOI] [PubMed] [Google Scholar]
- 21.Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004 Sep 21;141(6):421–431. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 22.Fox CS, Sullivan L, D’Agostino RB, Wilson PWF. Framingham Heart Study. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care. 2004 Mar;27(3):704–708. doi: 10.2337/diacare.27.3.704. [DOI] [PubMed] [Google Scholar]
- 23.Boyne MS, Saudek CD. Effect of insulin therapy on macrovascular risk factors in type 2 diabetes. Diabetes Care. 1999 Apr;22(Suppl 3):C45–C53. [PubMed] [Google Scholar]
- 24.2011 WRITING GROUP MEMBERS, 2005 WRITING COMMITTEE MEMBERS, ACCF/AHA TASK FORCE MEMBERS. 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;124:2020–2045. doi: 10.1161/CIR.0b013e31822e80c3. [DOI] [PubMed] [Google Scholar]
- 25.Stein RA, Rockman CB, Guo Y, Adelman MA, Riles T, Hiatt WR, et al. Association between physical activity and peripheral artery disease and carotid artery stenosis in a self-referred population of 3 million adults. Arteriosclerosis, Thrombosis, and Vascular Biology. Lippincott Williams & Wilkins. 2015 Jan;35(1):206–212. doi: 10.1161/ATVBAHA.114.304161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh A, Donnino R, Weintraub H, Schwartzbard A. Effect of strict glycemic control in patients with diabetes mellitus on frequency of macrovascular events. Am J Cardiol. 2013 Oct 1;112(7):1033–1038. doi: 10.1016/j.amjcard.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 27.Nead KT, Cooke JP, Olin JW, Leeper NJ. Alternative ankle-brachial index method identifies additional at-risk individuals. Journal of the American College of Cardiology. 2013 Aug 6;62(6):553–559. doi: 10.1016/j.jacc.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.