Summary
Life-long homeostatic setpoints for mood-related behaviors emerge during adolescence. Serotonin (5-HT) plays an important role in refining the formation of brain circuits during sensitive developmental periods. In rodents, the role of 5-HT1A receptors in general and autoreceptors in particular has been characterized in anxiety. However, less is known about the role of 5-HT1A receptors in depression-related behavior. Here we show that whole-life suppression of heteroreceptor expression results in a broad depression-like behavioral phenotype accompanied by physiological and cellular changes within mPFC-DRN circuitry. These changes include increased basal 5-HT in a mPFC that is hyporesponsive to stress, and decreased basal 5-HT levels and firing rates in a DRN hyperactivated by the same stressor. Remarkably, loss of heteroreceptors in the PFC at adolescence is sufficient to recapitulate this depression-like behavioral syndrome. Our results suggest that targeting mPFC 5-HT1A heteroreceptors during adolescence in humans may have life-long ramifications for depression and its treatment.
Keywords: Serotonin, 5-HT1A, depression, anxiety, development, adolescence, mPFC, DRN
Graphical Abstract
Introduction
Serotonin (5-HT) is a monoamine neuromodulator that is involved in the regulation of numerous physiological and behavioral functions including mood- and anxiety-related behaviors (Olivier, 2015). In addition to modulating adult affective behaviors, 5-HT also plays an important role in refining the formation of brain circuits during sensitive developmental periods. For example, 5-HT levels are critical for the formation of whisker barrel fields in the somatosensory cortex of the mouse during postnatal development (Erzurumlu and Gaspar, 2012; Fox, 1992). Furthermore, in rodents transiently increasing 5-HT signaling during early postnatal development (P2–P11) results in increased anxiety and depression-related behavior later in life that is associated with anatomical and physiological changes in brain regions that regulate emotion, such as the prefrontal cortex (PFC) (Rebello et al., 2014). While it is clear that manipulations of 5-HT signaling in early postnatal development can impact the formation of cortico-limbic circuits, less is known about the role of 5-HT during adolescent development, a time period of expansive maturation of frontal circuitry as well as vulnerability for the onset of affective disorders.
Brain development in humans continues through adolescence (Arnett, 2000) and during this time the brain is faced with the need to adapt to many social, physical, behavioral, sexual and cognitive experiences that occur during its final transition into maturity (Lee et al., 2014). Amongst these late occurring maturational changes, strengthening of top-down cognitive control serves as a hallmark of this adolescent to adult transition (Casey et al., 2008; Gogtay et al., 2004). Consequently, it is not surprising that the peak age of onset for many psychiatric disorders is adolescence (Paus et al., 2008). To treat these disorders, drugs that target 5-HT system and approved for use in adults are increasingly prescribed to adolescents despite little knowledge about whether or how manipulation of 5-HT signaling in adolescence could lead to long-term changes in the adult brain.
While 5-HT signals through fourteen different receptors, signaling through 5-HT1A receptors has been shown to influence mood-related behavior in adult animals (Garcia-Garcia et al., 2014). 5-HT1A receptors are inhibitory G-protein coupled receptors that exist as either autoreceptors on 5-HT neurons that mediate negative feedback or as heteroreceptors that mediate post-synaptic inhibitory responses to 5-HT in forebrain areas (Garcia-Garcia et al., 2014). In mice, the evidence strongly supports a role of 5-HT1A receptors (autoreceptors) in shaping the circuits that mediate anxiety throughout early postnatal and adolescent development (Donaldson et al., 2014; Garcia-Garcia et al., 2015; Lo Iacono and Gross, 2008; Vinkers et al., 2010). In contrast, despite significant evidence implicating 5-HT1A receptors with depression in humans (Kaufman et al., 2016), how these receptors might influence depression-related behavior in mice is less well understood. Further, when and where these receptors are required for this function remains unknown.
Here, we demonstrate that whole-life suppression of 5-HT1A heteroreceptor expression, in pyramidal cells, results in a broad depression-like phenotype. We further show that this effect can be recapitulated by loss of 5-HT1A these heteroreceptors during adolescence and cannot be rescued by heteroreceptor restoration to normal levels in adulthood. Finally, we show that mPFC pyramidal 5-HT1A heteroreceptors in adolescence can broadly regulate susceptibility to depression-like behaviors. These findings unravel the complicated time and region-dependent roles for 5-HT1A receptors in depression-related behaviors.
Results
Suppression of forebrain pyramidal 5-HT1A heteroreceptors
We have previously described the use of a tTS based system for generating mice in which 5-HT1A heteroreceptor expression can be repressed without affecting autoreceptors (Hetero-KO mice) (Richardson-Jones et al., 2011). Specifically, crossing Htr1Ateto/teto mice to a CamKIIα-tTS line, allows for a doxycycline (DOX) dependent expression of endogenous 5-HT1A heteroreceptors. In the presence of DOX (ON) 5-HT1A receptors are expressed normally (Control). In the absence of DOX (OFF), receptor expression is repressed in the forebrain but not in the raphe nucleus (RN) (Hetero-KO) (Figure 1A). In Hetero-KO mice, tTS expression is directed by promoter elements from the α-CaMKII gene (Mayford et al., 1996), which is widely expressed in forebrain pyramidal neurons but excluded from interneurons (Chen et al., 2010; Xu et al., 2000). To confirm the cell-type specificity of 5-HT1A suppression in our system, we performed patch-clamp recordings to examine the response of mPFC pyramidal and non-pyramidal (presumed GABAergic) neurons to the 5-HT1A receptor full agonist, 5-carboxytryptamine (5-CT). Consistent with what we have previously observed in the hippocampus (HP) (Richardson-Jones et al., 2011), the average current response to 5-CT was greatly diminished in Hetero-KO mice compared to controls (Figure S1A,B). In addition, only 30% of pyramidal mPFC neurons from Hetero-KO mice while 82% of cells from control mice responded above baseline to 5-CT with a significantly hyperpolarizing response (Table 1). In contrast, no difference was observed in non-pyramidal cells, as 43% and 44% of cells from control and Hetero-KO mice respectively respond to 5-CT (Table 1). To ensure that loss of response to 5-CT in the Hetero-KO mice is not due to general alterations in Gαi/o-signaling, we tested the ability of neurons to respond to the selective GABAB agonist baclofen, which also targets the Gαi/o-GIRK pathway. All pyramidal cells from Hetero-KO and control mice respond robustly to baclofen (100% response for both groups) (Table 1), indicating that the downstream Gαi/o-GIRK pathway remains intact. We then directly confirmed the suppression of pyramidal 5-HT1A heteroreceptors by qPCR analysis in the PFC and the brainstem containing the RN. As expected, Hetero-KO mice showed significantly decreased 5-HT1A mRNA in the PFC but not in the RN (Figure S1C).
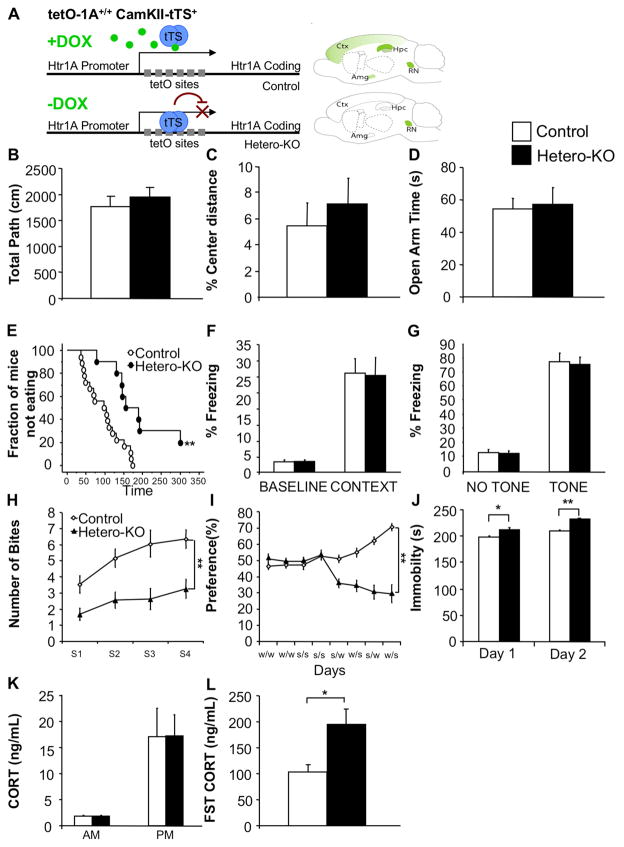
Figure 1. Forebrain suppression of pyramidal 5-HT1A heteroreceptors results in a depression-like phenotype.
(A) tTS system cartoon. Top: Mice homozygous for the Htr1atetO allele and possessing one copy of the α-CaMKII–tTS transgene express 5-HT1A receptors in normal patterns when maintained on doxycycline (DOX). Bottom: In the absence of DOX, transcription of 5-HT1A is suppressed in the forebrain but not in the raphe nucleus (RN). (B) No differences were detected between Hetero-KO mice and controls in total path (ANOVA for main effect of group: F(1,26)=0.659; p=0.4242) or (C) % center distance in the open-field (ANOVA for main effect of group: F(1,26)=0.322; p=0.5754; n=10–18/group). (D) No differences were detected between groups in time spent in the open arms of the elevated-plus maze (ANOVA for main effect of group: F(1,26)=0.036; p=0.8515; n=10–18/group). (E) Hetero-KO mice displayed increased latency in the NSF (Kaplan-Meier survival analysis with Mantel-Cox: p<0.01; n=10–18/group). (F) No differences were detected between groups in the fear-conditioning paradigm. Percent time freezing at baseline (ANOVA for main effect of group: F(1,17)=0.213; p=0.6505) and during context test (ANOVA for main effect of group: F(1,17)=0.021; p=0.8876). (G) Percent time freezing in novel context in the absence and presence of the tone (ANOVA for main effect of group: F(1,17)=0.056; p=0.8166; n=8–11/group). (H) Hetero-KO mice displayed decreased cookie consumption (ANOVA repeated measures for main effect of group: F(1,23)=37.243; p<0.0001; for main effect of time: F(1,3)=3.345; p<0.05; n=8–17/group) and (I) decreased sucrose preference compared to controls (Two-way ANOVA repeated measures time x group interaction for choice testing days (5–8): F(3,54)=10.065; p<0.0001; main effect of group: F(1,18)=96.561; p<0.0001; n=9–11/group). (J) Hetero-KO mice displayed decreased mobility compared to controls in a 2 day FST (ANOVA for main effect of group: Day 1, F(1,17)=6.389; p<0.05; Day 2, F(1,17)=47.447; p<0.01; n=8–11/group). (K) No difference in CORT levels at the onset of both the light and the dark phase was detected between groups (ANOVA for main effect of group: AM: F(1,14)=0.047; p=0.8309; PM: F(1,16)=0.001; p=0.9758; n=8–9/group). (L) Hetero-KO mice display an enhanced CORT response to a forced-swim stressor compared to controls (ANOVA for main effect of group: F(1,10)=7.306; p=0.0222; n=6/group). Male mice were tested starting at 12–14 weeks of age. Data are represented as mean±SEM. *p≤0.05, **p<0.01. See also Figure S1 and S2.
Table 1. mPFC pyramidal neuron response to 5-HT1A agonists.
Whole cell recordings from mPFC cells showing percentage of cells that show a response to the bath perfused drug. A smaller proportion of Hetero-KO mPFC pyramidal cells responds to 5-CT compared to controls (Control: 9/11;Hetero-KO: 3/10)(Fisher’s: p<0.05; n=10–11/group). No changes were observed in the percentage of non-pyramidal cells. (Control: 3/7;Hetero-KO: 4/9)(Fisher’s: p=1.0)(n=7–9/group). All pyramidal cells respond robustly to Baclofen (Control: 4/4;Hetero-KO: 4/4)(Fisher’s: p=1.0; n=4/group).
| Transgenic | Pyramidal 5-CT | Non-Pyramidal 5-CT | Pyramidal Baclofen |
|---|---|---|---|
| Control | 82% | 43% | 100% |
| Hetero-KO | 30%* | 44% | 100% |
5-HT1A Hetero-KO mice display anhedonia and depressive-like behavior
We have previously shown that whole-life suppression of 5-HT1A heteroreceptors results in increased immobility in the forced-swim test (FST), without changes in the open-field, light-dark and elevated-plus maze tests in male mice (Richardson-Jones et al., 2011). Here, we test whether the forced-swim phenotype is an isolated finding or whether it is part of a larger depression-like syndrome.
We first confirmed the absence of an anxiety-like behavioral phenotype in the Hetero-KO mice by testing them in several innate conflict based anxiety tasks. As expected, in the open-field test, we saw no differences between male Hetero-KO mice and their controls in total distance travelled or percentage distance travelled in the center (Figure 1B,C). Likewise, no differences were detected between Hetero-KO mice and their controls in time spent in the open arms in the elevated-plus maze (Figure 1D).
In the novelty-suppressed feeding paradigm (NSF) (Bodnoff et al., 1988; Samuels, 2011), Hetero-KO mice showed an increased latency to feed relative to their controls (Figure 1E), with no changes in home cage consumption (Figure S2A). Unlike other conflict-based tasks, the NSF test contains an appetitive dimension (food in food deprived animals). As such, it is possible that Hetero-KO mice increased latency can represent either increased fear/apprehension of the arena or decreased motivation (hedonic drive) to access the food.
Thus, to assess whether suppression of 5-HT1A heteroreceptor signaling disrupts fear-related behaviors, we performed cued and contextual fear-conditioning experiments. No differences were detected between groups in either baseline freezing before tone-shock pairing, (Figure 1F) or in freezing to the training context after the training (Figure 1F). Similarly, no differences between groups in response to the paired tone were detected in an alternate context (Figure 1G).
Having observed no changes in fear and no changes in two innate anxiety tests, we hypothesized that the NSF deficit we observed might be related to anhedonia, a core symptom of depression. Interestingly, unlike the other conflict based anxiety tests assessed, the NSF test is sensitive to chronic treatment with antidepressants suggesting that it might rely on distinct circuitry (Dulawa and Hen, 2005). We thus explored this possibility by assessing the effect of suppression of 5-HT1A heteroreceptors on appetitive driven behavior in the Cookie test (Surget et al., 2011). Unlike the NSF test, this test is done with sated animals and is dependent on the increased appetitive value of a chocolate cookie, which is in conflict with the neophobic tendencies of the mouse. A reduction of the cookie consumption may therefore be interpreted as anhedonia, a habituation deficit or a combination of both effects. While repeated sessions resulted in a progressive increase in cookie consumption in both groups, Hetero-KO mice consistently take fewer bites per session compared to their respective controls (Figure 1H). We next tested whether this behavioral difference might reflect anhedonia by performing a sucrose preference test. Here we found that while control mice develop a preference for sucrose over time, Hetero-KO mice fail to do so, resulting in a significant difference between groups (Figure 1I). Taken together, our results indicate that the observed behavioral differences are not due to increased fear or anxiety in the testing arenas but rather may reflect decreased motivation or anhedonia consistent with a depression-like syndrome.
Diverse evidence has linked 5-HT1A receptors to depression and the response to stress and suppression of 5-HT1A heteroreceptors results in increased immobility in the FST (Richardson-Jones et al., 2011). We thus decided to query the HPA axis stress reactivity by measuring serum corticosterone (CORT) after the FST. In this cohort, we find increased immobility on both days of a 2-day FST in Hetero-KO mice (Figure 1J). In addition, these mice showed increased CORT levels elicited by FST when compared to controls (Figure 1L). No differences in baseline CORT levels were detected at either the dark/light or light/dark transitions (Figure 1K). Thus, the increased latency in the NSF, decreased consumption in the cookie test, failure to develop sucrose preference, increased immobility in the FST and increased physiologic reactivity to stress demonstrates that Hetero-KO mice have a broad depression-related phenotype.
Altered stress induced c-fos immunoreactivity in the mPFC-DRN pathway
In order to identify cellular correlates of behavior, we mapped induction of c-fos expression after a swim-stressor in areas known to express 5-HT1A receptors. In the forebrain, the number of c-fos positive cells in the prelimbic (PrL) area of the mPFC was significantly lower in Hetero-KO mice when compared to their controls. Fewer c-fos positive cells were also observed in the neighboring infralimbic area (IL), but these differences were not significant. In addition, no significant differences were detected in the dentate gyrus (DG) or the basolateral amygdala (BLA). In the raphe, no significant differences were observed in either the lateral wings (LW) of the dorsal raphe, the dorsal raphe proper (DRN), or the medial raphe nucleus (MRN) (Figure 2A).
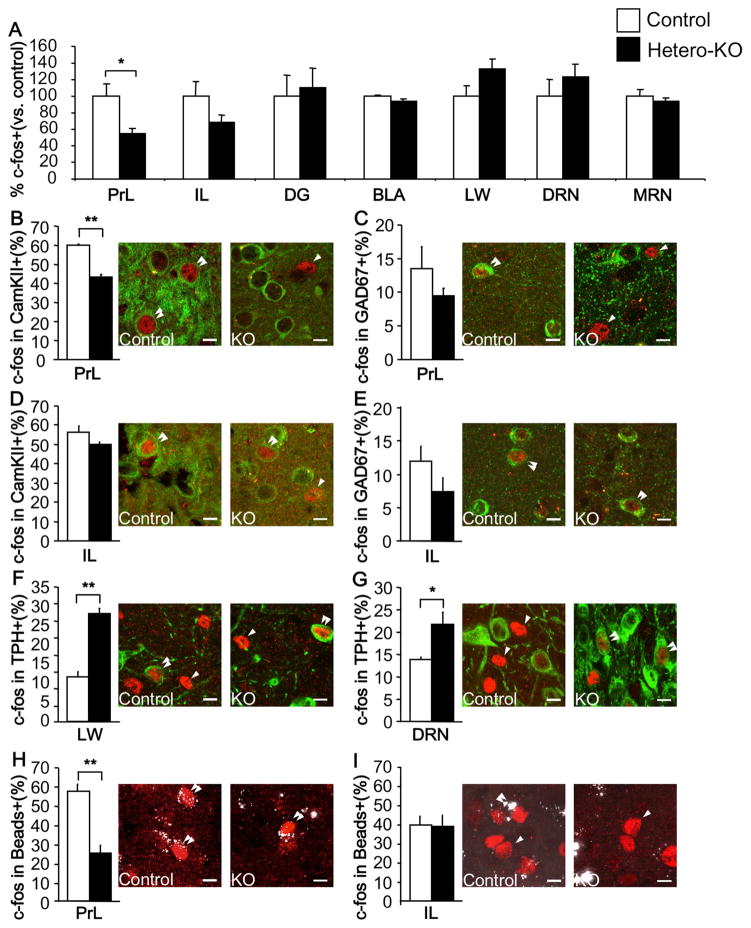
Figure 2. Region specific changes in c-fos activation after a swim-stressor in Hetero-KO mice.
(A) % c-fos expressing cells normalized to controls. Note decreased c-fos+ cells in the prelimbic (PrL) area (ANOVA for main effect of group: F(1,6)=7.893; p<0.05), with no differences in the infralimbic area (IL)(ANOVA for main effect of group: F(1,6)=2.585; p=0.1590), dentate gyrus (DG)(ANOVA for main effect of group: F(1,6)=0.085; p=0.7810), basolateral amygdala (BLA)(ANOVA for main effect of group: F(1,6)=4.105; p=0.0891), lateral wings of the raphe (LW)(ANOVA for main effect of group: F(1,7)=3.311; p=0.1116), dorsal (DRN)(ANOVA for main effect of group: F(1,9)=0.636; p=0.4457) or in the medial raphe nucleus (MRN)(ANOVA for main effect of group: F(1,6)=0.424; p=0.5393) in Hetero-KO mice compared to controls. (B,C) In the PrL, compared to controls, Hetero-KO mice displayed a decreased percentage of CamKII+ cells that are c-fos+ (ANOVA for main effect of group: F(1,6)=84.768; p<0.01); no change was detected in the percent of GAD67+ cells that also are also c-fos+ (ANOVA for main effect of group: F(1,6)=1.352; p=0.2891). (D,E) In the IL, no significant differences were detected between groups in percentage of pyramidal or GABAergic cells that were also c-fos+ (ANOVA for main effect of group: F(1,6)=4.602; p=0.0756, F(1,6)=2.336; p=0.1772, respectively). (F,G) Percentage of TPH+ cells that co-localize with c-fos is increased in the lateral wings (ANOVA for main effect of group: F(1,7)=58.577; p<0.01) and DRN (ANOVA for main effect of group: F(1,10)=8.121; p<0.05) of Hetero-KO mice compared to controls. (H,I) Fewer bead labeled cells co-localize with c-fos in the PrL (ANOVA for main effect of group: F(1,6)=34.336; p<0.01), with no changes in the IL (ANOVA for main effect of group: F(1,6)=0.002; p=0.9679). Single arrow depicts a single labeled cells and double arrow depicts double-labeled cells. Scale bar=10μm. (n=4–5/group). Data are represented as mean±SEM. *p≤0.05, **p<0.01. See also Figure S3.
We next determined whether the observed changes in neuronal activation in the mPFC were cell-type specific. Interestingly, we found that the decrease in the number of c-fos positive cells in the PrL area was selective for pyramidal (Figure 2B), but not GABAergic (Figure 2C) cells. In contrast, no significant differences were observed in pyramidal or GABAergic cells in the IL of Hetero-KO mice when compared to their controls (Figure 2D,E). Interestingly, in the raphe, we observed that more TPH (5-HT cells marker) positive cells expressed c-fos in both the LW of the DRN and the DRN itself (Figure 2F,G), with no changes in the MRN (Data not shown).
As some pyramidal cells in the mPFC project to the DRN and have been implicated in mediating behavioral control of stress (Amat et al., 2005; Warden et al., 2012), we asked whether the decrease in stress-induced c-fos in the mPFC was in neurons projecting to the DRN. To this end, adult mice were injected with retrobeads into the DRN and allowed to recover for 15 days (Figure S3A). Mice then underwent a swim-stressor for c-fos induction. We observed retrobeads in both the PrL and IL areas of the mPFC (Figure S3A). There was an almost three-fold decrease in the number of DRN-projecting PrL neurons that expressed c-fos in Hetero-KO mice when compared to controls. No such changes within the DRN-projecting IL neurons were detected (Figure 2H,I). The decrease in PrL double-labeled cells in the Hetero-KO mice cannot be accounted for by the 65% decrease in c-fos+ cells in the PrL. This suggests that the decrease in neurons activating c-fos in the Hetero-KO group is predominantly due to changes in neurons that project to the DRN (Figure S3B–E). In sum, loss of 5-HT1A heteroreceptors leads to decreased activation of mPFC cells that project to the DRN and higher activation of DRN 5-HT cells in response to a stressor.
Altered 5-HT tone in mice lacking pyramidal 5-HT1A heteroreceptors
Given the evidence suggesting that postsynaptic 5-HT1A heteroreceptors in the mPFC participate in feedback control of basal DRN 5-HT activity (Hajos et al., 1998; Martin-Ruiz and Ugedo, 2001), we tested whether the lack of 5-HT1A heteroreceptors influences the basal firing rate of DRN 5-HT neurons by performing in vivo anesthetized electrophysiological studies. Indeed, we found that the basal firing rates of DRN 5-HT neurons in Hetero-KO mice are decreased compared to their respective controls (Figure 3A,B).
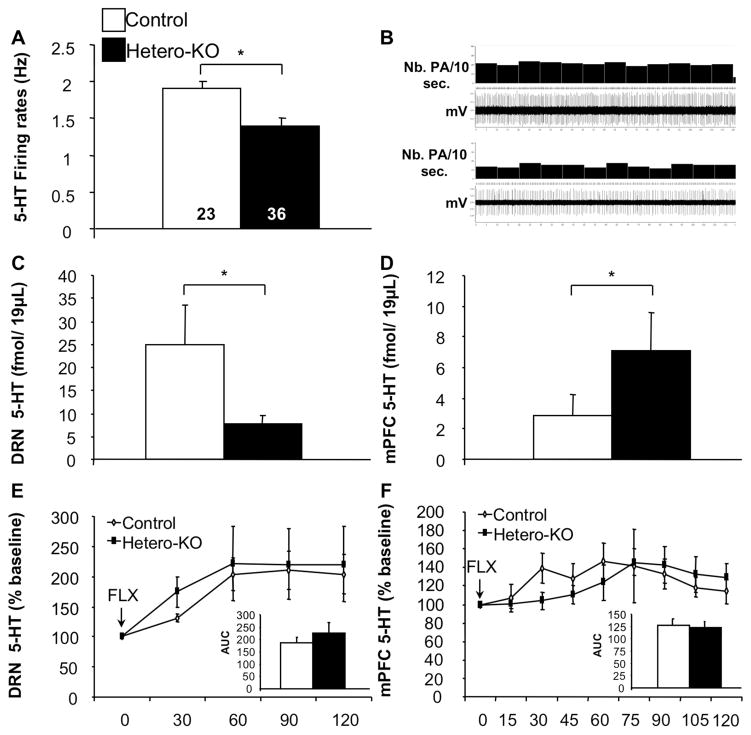
Figure 3. Suppression of 5-HT1A heteroreceptors results in altered 5-HT tone.
(A) Hetero-KO mice displayed lower basal 5-HT firing rates compared to controls (ANOVA for main effect of group: F(1,57)=4.647; p<0.05; n=23–36/group). (B) Representative recordings of DRN 5-HT neurons obtained in control (Top) and Hetero-KO (Bottom). (C) Decreased basal 5-HT levels in the DRN in Hetero-KO mice (ANOVA for main effect of group: F(1,14)=4.865; p<0.05)(D) along with increased basal 5-HT levels in the mPFC in Hetero-KO mice (ANOVA for main effect of group: F(1,14)=4.629; p<0.05). (E) Area under the curve analysis, revealed no significant effect of group in the DRN (ANOVA for main effect of group: F(1,14)=0.144, p=0.708) but a significant effect of time (ANOVA repeated measures for main effect of time: DRN: F(1,14)=6.215, p<0.01) in 5-HT levels following injection of FLX. (F) 5-HT measured by area under the curve analysis, revealed no significant effect of group in the mPFC (ANOVA for main effect of group: F(1,14)=0.432, p=0.521) but a significant effect of time (ANOVA repeated measures for main effect of time: F(1,14)=3.387, p<0.01) measured after FLX. (n=7–9/group). Data are represented as mean±SEM. *p≤0.05, **p<0.01.
Having observed differences in DRN basal firing rates in Hetero-KO mice, we next determined whether these differences are reflected at the neurochemical level by using in vivo microdialysis locally in the DRN and in the mPFC. We observed significantly decreased basal 5-HT levels in the DRN (Figure 3C) while finding increased mPFC 5-HT levels in Hetero-KO when compared to control mice (Figure 3D). We also assessed whether 5-HT1A heteroreceptor suppression-induced changes in 5-HT tone results from altered 5-HT uptake. To do so, the 5-HT reuptake inhibitor fluoxetine (FLX) was injected in both groups of mice. Both groups show a similar increase 5-HT in response to FLX, (Figure 3E,F), suggesting that the neurochemical changes in the DRN and mPFC are not related to changes in SERT expression/function.
5-HT1A heteroreceptor levels during adolescence regulates lifelong mood set-points
Global disruption of 5-HT1A receptors in adolescence, but not early adulthood, results in an anxious phenotype (Garcia-Garcia et al., 2015). We next tested whether the Hetero-KO phenotype requires normal 5-HT1A heteroreceptor function in adolescence. First, we assessed whether the our system would allow for testing an adolescent time window. To this end, Htr1atetO/tetO-αCamKII-tTS+ male mice were raised on DOX containing chow until P28 when it was replaced with control chow. Using I125-MPPI autoradiography, we then compared the density of 5-HT1A receptors binding sites in the PFC, dorsal HP (dHP) and DRN at P35 and P43 compared to control mice that had remained on DOX chow (Figure S4A,B). To assess whether the suppression of 5-HT1A receptors was reversible, one group of mice had DOX chow removed between P28 and P43. 5-HT1A binding was then assessed at P50 (Figure S4C). In mice that had DOX removed at P28, we detected a 77.7% reduction in 5-HT1A receptor binding sites in the PFC, a 67.8% reduction in the dHP and no changes in the DRN by P35 when compared to their respective controls (Figure S4D,G). By P43, these mice display an 85.6% reduction in 5-HT1A binding sites in the PFC, 79.7% in the dHP and no changes in the DRN (Figure S4E,H). Importantly, when DOX chow was returned on P43, 5-HT1A receptor levels in these regions were indistinguishable between groups by P50 (Figure S4F,I).
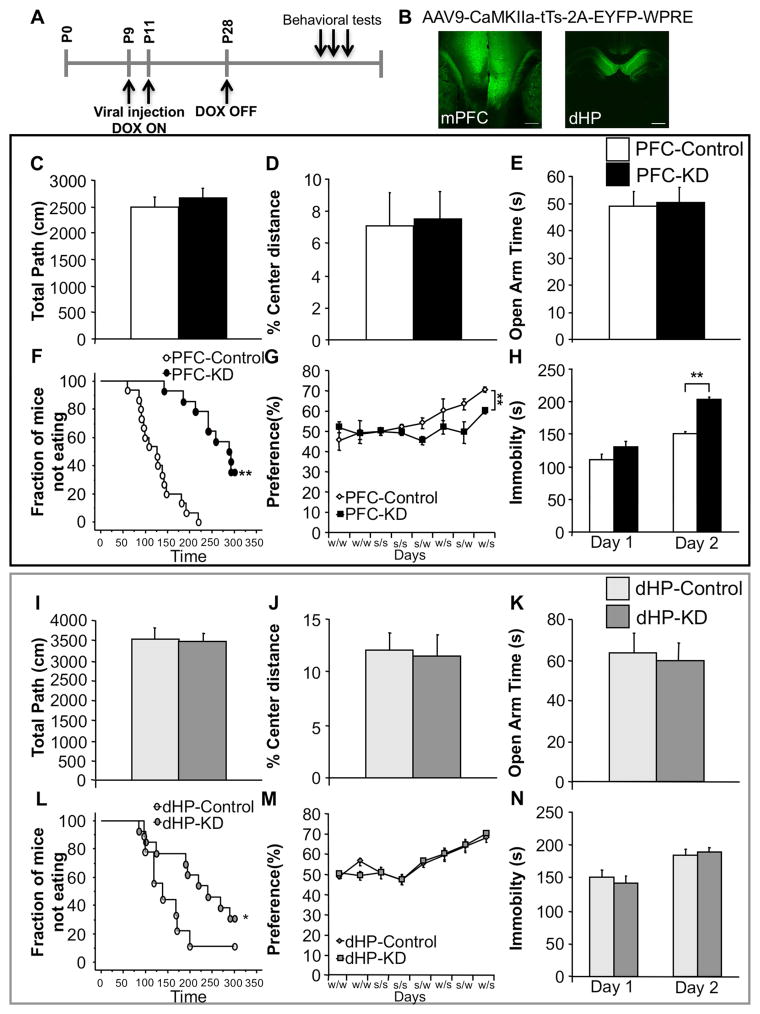
In order to test the function of heteroreceptors in modulating behavior in anxiety and depression-related paradigms, we generated 4 groups of male mice: Control (ON DOX whole-life), Hetero-KO (OFF DOX whole-life), Adolescent knock-down (Adolescent KD) (Removal of DOX at P28) and Rescue (Removal of DOX at P28 and back on DOX at P43) (Figure 4A). In the open-field, no differences were detected between Hetero-KO, Adolescent KD, Rescue mice and their controls in either total path travelled (Figure 4B) or percent center distance (Figure 4C). Likewise, no differences were detected in time spent in the open arms of the elevated-plus maze (Figure 4D).
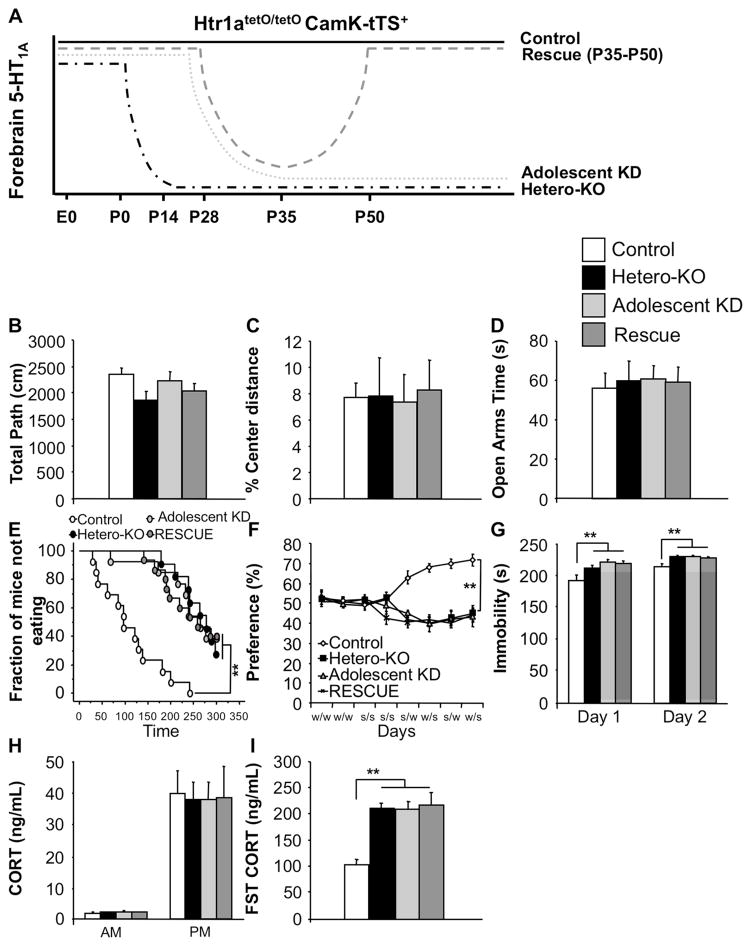
Figure 4. Suppression of 5-HT1A heteroreceptors beginning at or during adolescence results in a depression-like phenotype.
(A) Control and Hetero-KO mice were generated by maintaining Htr1atetO/tetO-CamKII-tTS+ mice in the presence or absence of DOX throughout life. Adolescent knockdown (Adolescent KD) mice were removed from DOX at P28 and Rescue mice were withdrawn from DOX from P28 to P43. (B) No group differences were detected in either total distance (ANOVA for main effect of group: F(3,45)=0.010; p=0.9987) or (C) % center distance in the open-field (ANOVA for main effect of group: F(3,45)=0.029; p=0.9934; n=11–15/group). (D) No group differences were detected in time spent in the open arms of the elevated-plus maze (ANOVA for main effect of group: F(3,45)=0.045; p=0.9851; n=11–15/group). (E) Hetero-KO, Adolescent KD and Rescue mice displayed increased latency in the NSF paradigm (Kaplan-Meier survival analysis with Mantel-Cox and Bonferroni correction: p<0.01; n=11–15/group). (F) Hetero-KO, Adolescent KD and Rescue mice displayed a decreased sucrose preference (ANOVA repeated measures for main effect of group: F(3,21)=6.516; p<0.01; Fisher post hoc: p<0.01 all groups vs. control; n=11–15/group) and (G) decreased mobility compared with the controls in a 2 day FST (ANOVA for main effect of group: Day1: F(3,44)=6.001, p<0.01; Fisher post hoc: p<0.01 all groups vs. control; Day 2: F(3,44)=5.198, p<0.01; Fisher post hoc: p<0.01 all groups vs. control; n=11–15/group). (H) No difference in CORT levels at the onset of both the light and the dark phase was detected among groups (ANOVA for main effect of group: AM: F(3,35)=0.743; p=0.5339; PM: F(3,35)=0.032; p=0.9922; n=9–10/group). (L) Hetero-KO, Adolescent KD and Rescue mice display an enhanced CORT response to a forced-swim stressor compared to controls (ANOVA for main effect of group: F(3,35)=12.974; p<0.01; Fisher post hoc: p<0.01 all groups vs. control; n=9–10/group). Male mice were tested starting at 12–14 weeks of age. Data are represented as mean±SEM. *p≤0.05, **p<0.01. See also Figure S4 and S5.
In the NSF paradigm, the latency to eat was greater for the Hetero-KO, Adolescent KD and Rescue mice compared to their controls (Figure 5E). No changes were seen in home cage consumption (Figure S5A). In depression related tests, Hetero-KO, Adolescent KD and Rescue mice failed to develop a preference for sucrose (Figure 4F) and displayed decreased mobility in the FST relative to their controls on both days of the test (Figure 4G). Decreased mobility in the FST was accompanied by increased CORT levels in Hetero-KO, Adolescent KD, and Rescue mice when compared to their controls (Figure 4I), but no changes were seen in baseline CORT levels between groups (Figure 4H).
Figure 5. Suppression of PFC, but not dHP, pyramidal 5-HT1A heteroreceptors results in a depression-like phenotype.
(A) Timeline. (B) Representative images depicting virus infection in the mPFC (Left) and dHP (Right). Scale bar=100μm. (C) No differences were detected between PFC-KD and control (PFC-Control) mice in total path (ANOVA for main effect of group: F(1,27)=0.451; p=0.5076) or (D) % center distance in the open-field (ANOVA for main effect of group: F(1,27)=0.022; p=0.8827; n=14–15/group). (E) Time spent in the open arms of the elevated-plus maze was not different (ANOVA for main effect of group: F(1,27)=0.026; p=0.8741; n=14–15/group). (F) PFC-KD showed increased latency to eat in the NSF compared to PFC-Control (Kaplan-Meier survival analysis with Mantel-Cox: p<0.01; n=14–15/group). (G) PFC-KD displayed decreased sucrose preference compared to PFC-Control mice (ANOVA repeated measures for main effect of group: F(1,23)=10.424; p<0.01; n=11–14/group). (H) In the FST PFC-KD mice showed increased immobility compared to PFC-KD mice on day 2 (ANOVA for main effect of group: Day 1, F(1,27)=3.226; p=0.0836; Day 2, F(1,27)=91.645; p<0.01). (I) No differences were detected between dHP-KD and dHP-Control mice in total path (ANOVA for main effect of group: F(1,20)=0.067; p=0.7990) or (J) % center distance in the open-field (ANOVA for main effect of group: F(1,20)=0.018; p=0.8952; n=9–13/group). (K) No change in time spent in the open arms of the elevated-plus maze (ANOVA for main effect of group: F(1,20)=0.070; p=0.7940; n=9–13/group). (L) In the NSF, dHP-KD mice showed increased latency to feed compared to dHP-Control mice (Kaplan-Meier survival analysis with Mantel-Cox: p=0.05; n=9–13/group). (M) No changes were detected in the sucrose preference test (Two-way ANOVA repeated measures time x group interaction for choice testing days (5–8): F(3,60)=0.059; p=9809; n=9–13/group). (N) In the FST no changes were observed (ANOVA for main effect of group: Day 1, F(1,20)=0.248; p=0.6239; Day 2, F(1,20)=0.178; p=0.6775; n=9–13/group). Male mice were tested starting at 12–14 weeks of age. Data are represented as mean±SEM. *p≤0.05, **p<0.01. See also Figure S6 and S7.
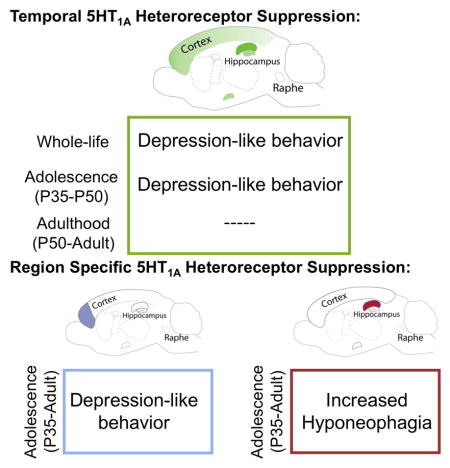
In summary, the Adolescent KD and the Rescue mice behave like Hetero-KO mice, demonstrating that susceptibility to depression-like behaviors is sensitive to alterations in 5-HT1A heteroreceptor levels during adolescence.
Adolescent loss of 5-HT1A heteroreceptors in mPFC pyramidal neurons, but not in the dHP, is sufficient to reproduce the Hetero-KO phenotype
As 5-HT1A heteroreceptors are widely expressed in the forebrain, the phenotype observed in the Hetero-KO mice could be due either to the global developmental disruption of 5-HT1A heteroreceptors or to effects of a restricted population of heteroreceptors. To test this, we generated an AAV9-CaMKIIa-tTs-2A-EYFP-WPRE virus that when injected into Htr1Ateto/teto mice results in a DOX dependent, spatially restricted suppression of pyramidal 5-HT1A heteroreceptors. Given our c-fos results and the literature on stress-coping strategies, we hypothesized that the phenotype might be due to effects in the mPFC. We also tested the dHP as an alternative effector region because it is one of the forebrain regions with the highest 5-HT1A receptor expression (Aznavour et al., 2009; Aznavour et al., 2006; Descarries and Riad, 2012).
To test this, Htr1Ateto/teto male mice raised on DOX were injected bilaterally in the mPFC or dHP with AAV9-CaMKIIa-tTs-2A-EYFP-WPRE at P9–P11. On P28 DOX was removed from the chow of the animals to allow for suppression of 5-HT1A transcription starting in adolescence (PFC-KD; dHP-KD) while the other half were maintained on DOX (PFC-Control; dHP-Control) (Figure 5A,B and S6A,B). We validated the viral approach by examining the response of EYFP-expressing mPFC pyramidal neurons to 5-CT using patch-clamp recordings. We found that only 30% of pyramidal mPFC neurons from PFC-KD mice respond to 5-CT while 82% of cells from PFC-Control mice respond with a hyperpolarizing response (Table 2). Both groups of male mice respond robustly to baclofen (100% response), indicating functional Gαi/o coupling to GIRK channels (Table 2).
Table 2. 5-HT1A heteroreceptor response to 5-HT1A agonist in the PFC of PFC-KD mice.
Whole cell recordings from mPFC cells showing percentage of pyramidal cells responding to bath perfused drug. PFC-KD mPFC cells show a decreased proportion of pyramidal cells that respond to 5-CT compared to controls (PFC-Control) (PFC-Control: 9/11;PFC-KD: 3/10)(Fisher’s: p<0.05; n=10–11/group). All cells in both groups responded to Baclofen (PFC-Control: 11/11;PFC-KD: 9/9) (Fisher’s: p=1.0; n=10–11/group).
| Viral Injection Site | Pyramidal 5-CT | Pyramidal Baclofen |
|---|---|---|
| PFC-Control | 82% | 100% |
| PFC-KD | 30%* | 100% |
Having validated the viral approach, we determined whether suppression of mPFC and/or dHP 5-HT1A heteroreceptors had any consequence on conflict based anxiety tasks. No differences were observed in either total distance travelled or in percent center distance between PFC-KD or dHP-KD compared to their respective controls in the open-field test (Figure 5C,I). Likewise, no differences were detected in time spent in the open arms in the elevated-plus maze (Figure 5D,J).
Interestingly, when tested in the NSF, both PFC-KD and dHP-KD mice show an increased latency to eat when compared to their respective controls (Figure 5F,L). Importantly, we did not detect any change in home cage consumption (Figure S7A,D).
Next, having observed that suppression of PFC and dHP 5-HT1A receptors at P28 impacts NSF but not open-field or elevated-plus behavior, we asked whether the PFC or dHP manipulations could further reproduce the Hetero-KO mice phenotype. We found that PFC-KD but not dHP-KD mice show a decreased preference for sucrose when compared to controls (Figure 5G,M). We also observed that PFC-KD but not dHP-KD mice displayed increased immobility in the FST during the second day of testing (Figure 5H,N). This increased immobility in PFC-KD mice was not accompanied by an increase in CORT elicited by FST (Figure S7C). Further, no changes were observed between dHP groups after stress (Figure S7F), and no baseline changes in CORT levels were observed in any of the groups tested (Figure S7B,E).
Taken together these results suggest that while adolescent suppression of dHP 5-HT1A heteroreceptors impacts NSF behavior, suppression in the PFC recapitulates the major behavioral phenotypes of adult Hetero-KO mice.
Discussion
While the relationship between depression and anxiety disorders is complex, 5-HT signaling appears to be involved in the pathophysiology and treatment of both disorders (Albert et al., 2014; Jans et al., 2007). Our data, together with our previous work demonstrate that changes in 5-HT signaling through 5-HT1A receptors in the brain can result in either a primary anxiety-like phenotype (all receptors and/or autoreceptors (Ramboz et al., 1998; Richardson-Jones et al., 2011)), or a more depression-related phenotype (heteroreceptors in pyramidal cells). Specifically, Hetero-KO mice show no increased anxiety in the open-field or elevated-plus maze, but do show hyponeophagia. They also show a broad depression-like phenotype characterized by anhedonia, increased behavioral despair, and increased stress reactivity. Interestingly, NSF alone is affected in both the 5-HT1A receptor KO (Santarelli et al., 2003) and Hetero-KO mice. Although usually interpreted as an anxiety test, the NSF has an appetitive component. Increases in latency may thus reflect either increased anxiety or less hedonic drive (Dulawa and Hen, 2005). Results from the cookie test and the decreased sucrose preference in Hetero-KO mice suggest that their altered NSF behavior is likely due to anhedonia, rather than anxiety. Taken together, these results suggest that suppression of forebrain 5-HT1A heteroreceptors results in a depression-related phenotype.
The cellular correlates of these behavioral changes were investigated by measuring c-fos induction in response to swim-stress. We detected fewer c-fos positive neurons in DRN projecting mPFC neurons while more 5-HT neurons in the LW of the DRN and in the DRN itself express c-fos relative to controls. These results correspond nicely to a previously described 5-HT1A heteroreceptor-dependent mPFC-DRN polysynaptic negative feedback pathway. In this pathway, cortical glutamatergic descending projections from the mPFC synapse onto GABAergic interneurons in the raphe, which in turn inhibit DRN 5-HT neurons (Challis et al., 2013; Sharp et al., 2007). Importantly, activation of DRN projecting mPFC cells decreases immobility in the FST (Warden et al., 2012). Thus, the decreased c-fos in DRN projecting mPFC cells after a swim-stress is congruent with the observed increased immobility in the FST. These findings are also in line with studies that implicate the mPFC in stress-coping strategies (Amat et al., 2005; Warden et al., 2012) and implicate 5-HT1A heteroreceptors in the development of top-down control in response to stress.
Further, our physiology studies demonstrate mPFC-DRN disruptions at baseline, as Hetero-KO mice show decreased firing rates of their 5-HT neurons. Interestingly, similar changes have been previously observed in other chronic 5-HT models. For example, SERT-KO mice, which show increased behavioral despair, also have decreased DRN firing rates (Lira et al., 2003) and attenuated c-fos activation in the mPFC in response to swim-stress (Soiza-Reilly et al., 2015). Further, in Hetero-KO mice decreased DRN 5-HT and increased mPFC 5-HT levels were detected, suggesting the existence of a local mechanism in the mPFC that regulates 5-HT release in a 5-HT1A heteroreceptor dependent-manner. These results reflect the complex interplay between the mPFC and DRN that can influence each other bi-directionally (Celada et al., 2013; Celada et al., 2001) and the existence of various negative feedback loops that co-exist within the DRN (Bang et al., 2012). One possible explanation for the differences in 5-HT levels is that loss of heteroreceptors specifically disrupts long loop feedback to DRN 5-HT neurons important in maintaining basal mPFC 5-HT levels (Casanovas et al., 1999; Ceci et al., 1994), while maintaining local 5-HT1A autoreceptor mediated feedback important for other target areas and local 5-HT release (Sperling and Commons, 2011).
The Hetero-KO phenotype is not the result of an acute loss of 5-HT1A heteroreceptor, rather our results demonstrate that transient disruption during adolescence (roughly after P28 and before P50 (Richardson-Jones et al., 2011)) is sufficient to elicit the behavioral phenotype. Consistent with the importance of normal 5-HT signaling during adolescence, SSRI’s administered during adolescence result in a later mixed phenotype of increased resilience to social defeat and increased anxiety (Iniguez et al., 2014). Furthermore, 5-HT1A receptors (likely autoreceptors) during adolescence, but not early adulthood, are critical in regulating anxiety setpoints (Donaldson et al., 2014; Garcia-Garcia et al., 2015). Adolescence is a time of ongoing brain development in 5-HT1A heteroreceptor expressing areas, particularly in frontal cortical areas (Andersen and Teicher, 2008), whose function is disrupted in depressed patients (Ressler and Mayberg, 2007). Our results further demonstrate that changes in 5-HT signaling through pyramidal 5-HT1A heteroreceptors in the PFC during adolescence is sufficient to drive the major behavioral phenotypes of Hetero-KO mice. Interestingly, changes in 5-HT1A heteroreceptor mediated signaling in the dHP results in only a NSF phenotype suggesting that both the dHP and PFC are part of the circuit controlling this behavior. Overall, our results are consistent with the idea that 5-HT signaling through 5-HT1A heteroreceptors plays an important role in shaping the final development of the mPFC structure during adolescence (Pattwell et al., 2016). Further, our data indicate that plasticity in response to changes in 5-HT1A levels remains in other brain regions in adolescence, but point to the mPFC as being the major node in the circuit that drives these behaviors. Indeed, as animals mature, the circuitry that was initially established to interface with the environment, areas like the amygdala and HP, comes under increasing influence of the PFC (Miller and Cohen, 2001).
In sum, our results identify a sensitive period lasting into adolescence during which the mPFC, the dHP and possibly other structures are sensitive to the amount of 5-HT signaling through 5-HT1A heteroreceptors. In the present study, we have focused on the effects of suppressing 5-HT1A heteroreceptor function resulting in enduring changes that suggest loss of hedonic drive and less active coping to stressors. Whether enhanced signaling through this pathway during this sensitive period might result in the opposite, with enhanced active coping to stressors and improved hedonic drive is an intriguing possibility.
Experimental Procedures
For details, please see Supplemental Information.
Animal husbandry
Male mice were housed in groups of three to five and had ad libitum access to food and water. Animals were maintained on a 12:12 light/dark schedule. Protocols were approved by the Institutional Animal Care and Use Committee and were conducted in accordance to the NIH Guide for the Care and Use of Laboratory Animals.
Generation of the conditional 5-HT1A heteroreceptor knock-out mice
As previously described (Richardson-Jones et al., 2011). Briefly, male Htr1atetO/tetO/CamK–tTS+ mice were bred to Htr1atetO/tetO females. Htr1atetO/tetO-CamK–tTS+ male mice were maintained in the presence or absence of chow containing DOX (40 mg/kg: product #F5545; Bioserv) (Figure 1A).
Behavioral and physiological studies
Male mice were tested starting at 12–14 weeks of age and the battery of behavioral tests took 4–5 weeks to complete. Anxiety tests were completed before other behavioral tests. All behavioral and physiological tests are described in supplemental information.
c-fos Immunohistochemistry
C-fos was induced as previously described by a swim-stressor (10 min) and mice were perfused 2 hours later (Garcia-Garcia et al., 2013). For staining, sections were blocked in 10% NDS (Jackson Immunoresearch) and 1% Triton in PBS for 1 hr. and then incubated with rabbit c-fos antibody (1:5000, Calbiochem PC38) and mouse CamKII (1:200, Millipore), or mouse GAD67 (1:1000, Millipore), or sheep TPH (1:200, Millipore) overnight at 4 °C. After washing with PBS, sections were incubated for 1 hr with secondary antibody (1:200 biotin donkey anti-rabbit, Jackson Immunoresearch) followed by amplification with avidin (1:200, Cy3, Jackson Immunoresearch) complex and either donkey anti-mouse or anti-sheep cy2 (1:200; Jackson Immunoresearch) and NeuroTrace Nissl stain (Invitrogen). Sections were after washed and mounted on glass slides with Prolong Gold Anti-fade Reagent (Invitrogen).
In vivo anesthetized recordings of 5-HT neurons
Single glass micropipettes were positioned 0.2–0.5 mm posterior to the interaural line on the midline and lowered to a depth of 2.5–3.5 mm from brain surface. The DRN 5-HT neurons were identified according to the following criteria: a slow (0.5–2.5Hz) and regular firing rate and a long duration, positive action potential.
Intracerebral in vivo microdialysis
Briefly, two concentric dialysis probes were implanted in the mPFC and DRN. Following recovery, probes were continuously perfused with aCSF, and dialysates collected in the mPFC and in the DRN for analysis by HPLC-amperometry. Freely moving mice were treated with FLX (18 mg/kg; s.c.) and dialysate were collected.
Viral injections and temporal regulation of virally expressed tTS
Briefly, P9-P10 Htr1atetO/tetO male mice were bilaterally infused in the mPFC or dHP with 0.1 microL of the AAV9-CaMKIIa-tTs-2A-EYFP-WPRE (2.20e+13vg/mL) virus at 0.025 microL/min. After surgery, mice were maintained on DOX. At P28, half of the mice were withdrawn from DOX and maintained on normal chow (Figure 5A).
Statistical analysis
Results from data analyses were expressed as mean ± SEM. p≤0.05 was used as the threshold for significance. Group differences were analyzed using a one-way analysis of variance (ANOVA) unless otherwise stated. Repeated measures ANOVA was used for the cookie and sucrose preference tests.
Supplementary Material
Acknowledgments
This work was supported by NIMH R01MH091844 (AD) and NIMH RO1 MH105675 and MH081968 (EDL). AGG was supported by a Spain Science Department and a Sackler Foundation Fellowship, SEC by a BBRF Young Investigator Award, Sackler Foundation Fellowship and a K01 (NIMH 1K01MH107760), AG by Agence Nationale pour la Recherche (ANR-12-SAMENTA-0007), BP by “Fondation pour la Recherche Médicale” (DPP20151033959) and Lundbeck, Phodé, Theranexu.
Footnotes
Authors report no conflicts of interest.
Author Contributions
Conceptualization, A.G.G. and E.D.L.; Methodology, A.G.G., E.D.L.; Investigation, A.G.G., Q.M., S.E.C, B.P.G; Original Draft, A.G.G.; Review & Editing, A.G.G. and E.D.L.; Funding Acquisition, A.G.G., A.D., E.D.L.; Resources, A.M.G., C.K., A.D., E.D.L.; Supervision, A.D., E.D.L.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert PR, Vahid-Ansari F, Luckhart C. Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Frontiers in behavioral neuroscience. 2014;8:199. doi: 10.3389/fnbeh.2014.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nature neuroscience. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55:469–480. [PubMed] [Google Scholar]
- Aznavour N, Benkelfat C, Gravel P, Aliaga A, Rosa-Neto P, Bedell B, Zimmer L, Descarries L. MicroPET imaging of 5-HT 1A receptors in rat brain: a test-retest [18F]MPPF study. Eur J Nucl Med Mol Imaging. 2009;36:53–62. doi: 10.1007/s00259-008-0891-1. [DOI] [PubMed] [Google Scholar]
- Aznavour N, Rbah L, Leger L, Buda C, Sastre JP, Imhof A, Charnay Y, Zimmer L. A comparison of in vivo and in vitro neuroimaging of 5-HT 1A receptor binding sites in the cat brain. J Chem Neuroanat. 2006;31:226–232. doi: 10.1016/j.jchemneu.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur J Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Hervas I, Artigas F. Postsynaptic 5-HT1A receptors control 5-HT release in the rat medial prefrontal cortex. Neuroreport. 1999;10:1441–1445. doi: 10.1097/00001756-199905140-00010. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci A, Baschirotto A, Borsini F. The inhibitory effect of 8-OHDPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–713. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Artigas F. Serotonin modulation of cortical neurons and networks. Frontiers in integrative neuroscience. 2013;7:25. doi: 10.3389/fnint.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13978–13988. 13988a. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, Lu YS, Zhu XH, Li SJ, Wu CY, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Riad M. Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Philos Trans R Soc Lond B Biol Sci. 2012;367:2416–2425. doi: 10.1098/rstb.2011.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Piel DA, Santos TL, Richardson-Jones J, Leonardo ED, Beck SG, Champagne FA, Hen R. Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:291–302. doi: 10.1038/npp.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neuroscience and biobehavioral reviews. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 2012;35:1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Meng Q, Richardson-Jones J, Dranovsky A, Leonardo ED. Disruption of 5-HT function in adolescence but not early adulthood leads to sustained increases of anxiety. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Newman-Tancredi A, Leonardo ED. 5-HT(1A) [corrected] receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacology. 2014;231:623–636. doi: 10.1007/s00213-013-3389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia AL, Venzala E, Elizalde N, Ramirez MJ, Urbiola A, Del Rio J, Lanfumey L, Tordera RM. Regulation of serotonin (5-HT) function by a VGLUT1 dependent glutamate pathway. Neuropharmacology. 2013;70:190–199. doi: 10.1016/j.neuropharm.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos M, Richards CD, Szekely AD, Sharp T. An electrophysiological and neuroanatomical study of the medial prefrontal cortical projection to the midbrain raphe nuclei in the rat. Neuroscience. 1998;87:95–108. doi: 10.1016/s0306-4522(98)00157-2. [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Alcantara LF, Warren BL, Riggs LM, Parise EM, Vialou V, Wright KN, Dayrit G, Nieto SJ, Wilkinson MB, et al. Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:1007–1021. doi: 10.1523/JNEUROSCI.5725-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Molecular psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Kaufman J, DeLorenzo C, Choudhury S, Parsey RV. The 5-HT1A receptor in Major Depressive Disorder. Eur Neuropsychopharmacol. 2016;26:397–410. doi: 10.1016/j.euroneuro.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Sestan N, Weinberger DR, Casey BJ. Mental health. Adolescent mental health--opportunity and obligation. Science. 2014;346:547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon JA, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, et al. Altered depressionrelated behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biological psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Lo Iacono L, Gross C. Alpha-Ca2+/calmodulin-dependent protein kinase II contributes to the developmental programming of anxiety in serotonin receptor 1A knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6250–6257. doi: 10.1523/JNEUROSCI.5219-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ruiz R, Ugedo L. Electrophysiological evidence for postsynaptic 5-HT(1A) receptor control of dorsal raphe 5-HT neurones. Neuropharmacology. 2001;41:72–78. doi: 10.1016/s0028-3908(01)00050-8. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Olivier B. Serotonin: a never-ending story. European journal of pharmacology. 2015;753:2–18. doi: 10.1016/j.ejphar.2014.10.031. [DOI] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, et al. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun. 2016;7:11475. doi: 10.1038/ncomms11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello TJ, Yu Q, Goodfellow NM, Caffrey Cagliostro MK, Teissier A, Morelli E, Demireva EY, Chemiakine A, Rosoklija GB, Dwork AJ, et al. Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:12379–12393. doi: 10.1523/JNEUROSCI.1020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, Leonardo ED. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels BAaHR. Novelty-suppressed feeding in the mouse. In: Gould TD, editor. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Spinger; 2011. pp. 107–121. Neuromethods. [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sharp T, Boothman L, Raley J, Queree P. Important messages in the 'post': recent discoveries in 5-HT neurone feedback control. Trends in pharmacological sciences. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M, Goodfellow NM, Lambe EK, Commons KG. Enhanced 5-HT1A receptor-dependent feedback control over dorsal raphe serotonin neurons in the SERT knockout mouse. Neuropharmacology. 2015;89:185–192. doi: 10.1016/j.neuropharm.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Commons KG. Shifting topographic activation and 5-HT1A receptor-mediated inhibition of dorsal raphe serotonin neurons produced by nicotine exposure and withdrawal. Eur J Neurosci. 2011;33:1866–1875. doi: 10.1111/j.1460-9568.2011.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Molecular psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkers CH, Oosting RS, van Bogaert MJ, Olivier B, Groenink L. Early-life blockade of 5-HT(1A) receptors alters adult anxiety behavior and benzodiazepine sensitivity. Biological psychiatry. 2010;67:309–316. doi: 10.1016/j.biopsych.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortexbrainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, Reichardt LF. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 2000;26:233–245. doi: 10.1016/s0896-6273(00)81153-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.