Abstract
Monocytes play a vital role in HIV-associated neurocognitive disorder (HAND), postulated to transport HIV into the brain and secrete pro-inflammatory cytokines. We analyzed cytokines released by cultured peripheral blood mononuclear cells enriched with the CD14+ marker isolated from HIV-infected individuals with HAND and normal cognition (NC) in combination antiretroviral therapy (cART)-naïve and after one year on treatment. Interleukin-8 and monocyte chemoattractant protein-1 (MCP-1) levels were higher in HAND compared to NC at baseline (p=0.002 and p<0.0001). These cytokines remained higher in HAND patients one year after cART and was significant when NC patients who were initially HAND were excluded (p=0.012 and p=0.002). Both correlated with baseline CD14+ PBMC HIV DNA levels supporting the role of HIV DNA reservoir size and monocyte cytokines in HAND persistence.
Keywords: HIV, Neurocognitive, Cognition, Cytokines, DNA
INTRODUCTION
Despite access to effective combination antiretroviral therapy (cART), HIV-associated neurocognitive disorder (HAND) continues to affect 35-50% of HIV-infected individuals. HIV-infected monocytes, particularly those with an activated phenotype are theorized to traverse the blood brain barrier, a means of HIV transport to the brain 1, 2. It is thought that, once in the brain, they contribute to an inflammatory milieu that ultimately results in neuronal damage and contributes to HAND 3, 4. These events are likely influenced by autocrine and paracrine responses of cytokines that are secreted by monocytes, resident macrophages and microglia. In turn, the cytokines attract more inflammatory cells including monocytes and activate macrophages and microglia to secrete detrimental cytokines. This cytokine overproduction causes neuronal damage and a loss of function.
The persistence of HIV is due to the establishment of viral reservoirs that may include lymphocytes, monocytes and macrophages. While T-cells may provide a significant contribution to viral reservoirs in general, monocyte and macrophage reservoirs are thought to be the primary contributors to HAND because they are the key cell types that are infected with HIV-1 in the central nervous system1. In previous studies, we quantified HIV DNA in CD14+ enriched PBMCs and have demonstrated a tight link to HAND 5-7. We hypothesize, that the persistence of HAND is in part due to these reservoirs, which provide a constant source of inflammation through cytokine production.
Several cytokines such as IL-8 and IFN-γ have been implicated in the neuropathogenesis of HIV 8. These studies typically analyze cytokines measured from plasma samples. In contrast, the current study investigated cytokines produced in cultures of CD14+ enriched PBMCs to more accurately investigate the role of monocyte secretory products and to provide a tighter link to concurrently measured HIV DNA levels. Since discordant results have been described with plasma cytokines 9, we further hypothesized that CD14+ PBMC supernatants may be a more reliable indicator of monocyte-related pathways. In this study, inflammatory cytokines that were shown to have involvement in HAND 10-16 were measured in supernatants from cultured peripheral monocytes by isolating CD14+ enriched PBMC. We previously reported that CD14+ PBMC subsets were higher in patients with HAND 17 and now demonstrated that supernatants from isolated CD14+ in culture have markers of immune activation in patients with HAND.
METHODS
Patient Cohort
Participants were enrolled in SEARCH011 (NCT00782808) and provided consent approved by the Institutional Review Boards (IRB) of UCSF, Chulalongkorn University, Phramongkutklao Hospital and the University of Hawaii. Selection criteria were previously reported 17. Briefly, community physicians referred participants if they met the Thai Ministry of Public Health criteria for treatment that included CD4<350 cells/mm3 or symptomatic disease. A total of 63 participants were enrolled but two were excluded at entry due to opportunistic CNS infections. Subjects started first-line cART typically with lamivudine (3TC) + nevirapine (NVP) + either stavudine (d4T) or zidovudine (ZDV) or tenofovir (TDF). Participants intolerant to this regimen were changed based on clinical acumen, most commonly to efavirenz (EFV) for nevirapine complications.
CD14+ PBMC Isolation
Blood was collected at entry and one year after cART in ACD tubes and processed within four hours using a Ficoll Histopaque (Sigma, St Louis, MO) gradient to isolate PBMCs. CD14+ cells (monocytes) were separated from the PBMC using MACS magnetic bead positive selection kit (Miltenyi Biotec, City, ST) and frozen in 10% DMSO/FBS. Purity of CD14+ cells was measured by multi-parameter flow cytometry on every fifth sample for the first 42 cases and the median purity was determined to be 91.9% (min 76.9%; max: 98.7%) 17. Purity data did not show any significant differences between HAND and NC groups, p = 0.48 (two sample Wilcoxon test). HAND: median (IQR) = 94.3 (89.8, 95.4), n = 12 vs. NC: median (IQR) = 94.0 (89.0, 97.2), n = 11.
Cytokine Measurement
CD14+ cells were initially isolated from PBMC and were stored at subzero for HIV DNA analyses at a later time point. Any available cells which were left over after the aliquot was frozen for HIV DNA testing were available for the cytokine tests. Some patients did not have enough cells to establish cell cultures for the cytokine analyses. Thus the number of tests performed varied depending on the available cells from patients. Isolated CD14+ PBMC were cultured in a 96-well plate overnight in RPMI1640 with 2% FBS and 1% pen/strep at 37°C. The plates were then centrifuged and the cell culture supernatants collected and stored at −80°C. The cells were resuspended and frozen in 10% DMSO/FBS. The chemokine and cytokines in the cell culture supernatants were analyzed using a custom 10-plex Milliplex MAP kit (EMD Millipore, Billerica, MA) that detected Fractalkine, IFN-γ, IL-2, IL-4, IL-6, IL-8, IL-10, IP-10, MCP-1, and TNF-α. Cell Culture supernatants and quality controls were prepared and run as described in the manufacturer’s manual with one hour incubation with beads at room temperature and 30 minute incubation with detection antibodies at room temperature. A Luminex 100 system (Luminex, Austin, TX) was used to analyze the samples and cytokine concentrations in the samples were determined using a spline curve-fitting method.
HIV DNA Quantification
Frozen CD14+ PBMCs were thawed in 20%FBS/DMEM and DNA was extracted using the QIAamp DNA Micro Kit (Qiagen, Valencia, CA) as per guidelines by the manufacturer and eluted in 20 uL TE buffer. HIV DNA copies per million were determined through the amplification of regions in the gag and b-globin genes as previously described with a limit of detection of 10 copies per million cells 5.
Statistics
Comparisons between cytokines produced from HAND and NC groups were examined by Wilcoxon rank-sum tests and the comparisons among multiple groups were evaluated by Kruskal-Wallis tests. CD14+ HIV DNA and cytokine association analyses were examined through nonparametric Spearman correlation. All statistical analyses were conducted in SAS version 9.3 (SAS Institute, Cary, NC). A two-sided p<0.05 was regarded as statistically significant. Benjamini–Hochberg correction (q<0.05) was conducted to account for multiple testing.
RESULTS
Participants
The study group consisted of 61 HIV-infected Thais; 28 with an average age of 34.0 years were diagnosed with HAND and 33 with an average age of 35.3 years with NC at baseline (Table S1). After beginning cART, the cognitive status of 18 individuals improved from HAND to NC whereas none of the individuals with baseline NC developed HAND after cART. Three cases were not seen at follow-up resulting in a total of 58; 10 of which met HAND criteria and 48 who had NC 17.
Cytokine Analyses
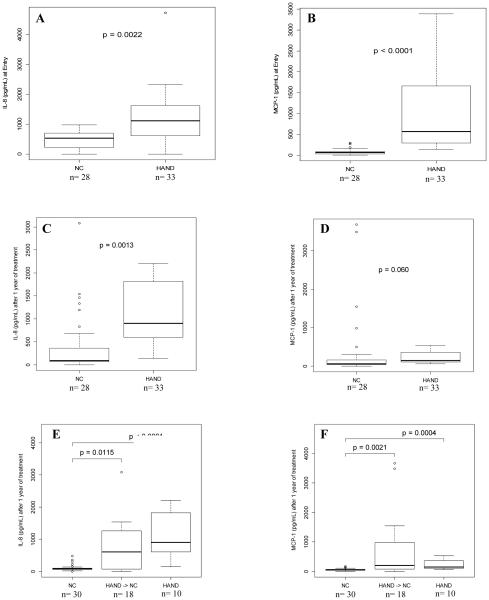
Three of the measured chemokines and cytokines (IFN-γ, IL-2, IL-4) were excluded from analyses because greater than 50% of the samples were below the limit of detection. Of the seven chemokines and cytokines measured in the supernatants, only IL-8 and MCP-1 levels were significantly higher in HAND individuals compared to those with NC at baseline (p=0.002 and p<0.0001, respectively), Figure 1a-1b, Supplement Table S2. The levels of both chemokines remained higher in the supernatants in HAND individuals after one year of treatment; but, this only met our level of statistical significance for IL-8 Figure 1c-1d. Statistical significance was met for both IL-8 and MCP-1 when analyses exclude participants with NC at 12 months who initially were diagnosed with HAND, Figure 1e-1f, Supplement Table S3. Although treatment was associated with improved cognitive status for 18 HAND individuals who became NC, the levels of IL-8 and MCP-1 secreted by their monocytes were still higher than the individuals with NC (p=0.012 and p=0.002, respectively), Figures 1e-1f. IL-8 and MCP-1 supernatant cytokine levels did not correlate with plasma or CSF IL-8 (r=0.039, p=0.775 and r=−0.226, p=0.178 respectively) and MCP-1 cytokine levels (r=0.115, p=0.402 and r=−0.005, p=0.976 respectively), Supplement Table S4.
Figure 1.
A) Monocyte IL-8 secretions between HAND and NC at entry; B) Monocyte MCP-1 secretions between HAND and NC at entry; C) Monocyte IL-8 secretions between HAND and NC after one year of cART; D)Monocyte MCP-1 secretions between HAND and NC after one year of cART; E) Monocyte IL-8 secretions between HAND, NC, and HAND that become NC after one year of cART; F) Monocyte MCP-1 secretions between HAND, NC, and HAND that became NC.
CD14+ enriched PBMC HIV DNA
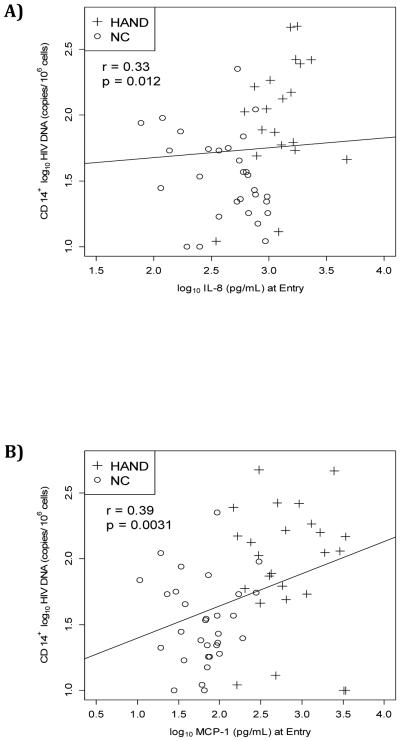
At entry, HIV DNA levels positively correlated with MCP-1 in supernatants (r=0.39, p=0.003) and with IL-8 (r=0.22, p=0.012; correlation was still significant when corrected for multiple testing (Benjamini–Hochberg)), Figures 2a-2b, Supplement Table S5.
Figure 2.
A) Non-parametric Spearman correlation between HIV DNA and IL-8; B) Non-parametric Spearman correlation between HIV DNA and MCP-1.
DISCUSSION
This study measured the differences in cytokine expression from isolated CD14+ enriched PBMC in HIV-infected HAND and NC individuals naïve to cART and after one year on treatment. Among cytokines in our panel, both IL-8 and MCP-1 were significantly associated with HAND at both pre- and post-cART time points. Although previous studies demonstrated the importance of MCP-1 in HAND 18, 19, emerging studies in recent years reveal IL-8 to be equally important 10, 15, 20. They both have been reported to be higher in HAND and remain high despite antiretroviral therapy 15, 20-22. The uniqueness of our findings is that the cytokines were measured from the supernatants of CD14+ enriched isolated monocytes from patients, providing a link to monocyte-associated neuropathogenesis. Other studies reported cytokines in plasma or CSF, which likely represent total cytokines secreted from a broader array of cells as demonstrated by the fact that no correlations were found between the levels of IL-8 and MCP-1 in plasma or CSF. However, a limitation of this study is the monocyte purity which varied between 76.9-98.7%; therefore, contaminating cells such as lymphocytes could have contributed to some of the secreted cytokines that were measured.
Our work further enhances our understanding of neuropathogenesis in HIV because we also linked the cytokine production to the burden of HIV DNA in these cells. This finding buttresses existing reports linking monocytes to HAND and the hypothesis that intracellular CD14+ cellular reservoirs are important in HIV neuropathogenesis 1. These results are consistent with data demonstrating MCP-1 and IL-8 linked to increased monocyte tethering to endothelial cells through binding of E-selectin with IL-8 triggering firm adhesion 23. Such a mechanism would permit an excessive influx of monocytes disrupting the integrity of the blood brain barrier and making it more permeable.
Although cART improves cognition in most of our subjects with HAND, the IL-8 and MCP-1 levels secreted by monocytes remained higher than those who were initially diagnosed and remained NC. This supports the hypothesis that these individuals continue to experience inflammation.
In summary, these data demonstrate a link between CD14+ HIV DNA and cytokines tightly linked to monocytes and of importance to HIV neuropathogenesis. These cytokines are elevated in HAND even after one year on cART that was initiated during chronic infection with CD4<350 cells/mm3. The fact that inflammatory cytokine levels do not return to baseline NC levels regardless of improved cognition; suggests that cART does not completely prevent inflammation and the presence of these monocyte viral reservoirs contribute to the persistence of inflammation.
Supplementary Material
ACKNOWLEDGMENTS
The SEARCH 011 study group includes Nittaya Phanuphak MD, Nitiya Chomchey, Somprartthana Rattanamanee, James LK Fletcher, Duanghathai Sutthichom, and Pairoa Praihirunkit from SEARCH/TRCARC; Nijasri Charnnarong MD and Sukalaya Lerdlum MD from Chulalongkorn University; Yotin Chinvarun MD from Phramongkutklao Medical Center; Mark de Souza PhD MPH, Weerawan Chuenarom and Rapee Trichavaroj from the Armed Forces Research Institute of Medical Sciences; Supunee Jirajariyavej MD from Taksin Hospital; Elijah Mun, Stephanie Chiao, Akash Desai, Edgar Busovaca, Nicholas Hutchings, Collin Adams, Katherine Clifford, and Lauren Wendelken from UCSF
Sources of Funding: This work was supported by R01-NS061696 (VV), R01-NS053345 (BS) and U54 MD007584 and G12 MD007601 (BS, GZ). Its contents are solely the responsibility of the authors and should not be construed to represent the positions of the U.S. NIH, the U.S. Army or the Department of Defense.
Footnotes
Conflicts of Interest: Dr. Valcour has served as a consultant to ViiV Healthcare and Merck in the past year. The authors declare no conflict of interest.
REFERENCES
- 1.González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nature Reviews Immunology. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 2.Gartner S, Liu Y. Insights into the role of immune activation in HIV neuropathogenesis. J Neurovirol. 2002 Apr;8(2):69–75. doi: 10.1080/13550280290049525. [DOI] [PubMed] [Google Scholar]
- 3.Verani A, Gras G, Pancino G. Macrophages and HIV-1: dangerous liaisons. Mol Immunol. 2005 Feb;42(2):195–212. doi: 10.1016/j.molimm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 4.Fischer-Smith T, Bell C, Croul S, et al. Monocyte/macrophage trafficking in acquired immunodeficiency syndrome encephalitis: lessons from human and nonhuman primate studies. J Neurovirol. 2008 Aug;14(4):318–26. doi: 10.1080/13550280802132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusao I, Shiramizu B, Liang CY, et al. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012 Winter;24(1):71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agsalda-Garcia M, Shiramizu B, Melendez L, et al. Different levels of HIV DNA copy numbers in cerebrospinal fluid cellular subsets. J Health Care Poor Underserved. 2013 Nov;24(4 Suppl):8–16. doi: 10.1353/hpu.2014.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valcour VG, Shiramizu BT, Shikuma CM. HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J Leukoc Biol. 2010 Apr;87(4):621–6. doi: 10.1189/jlb.0809571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correia S, Cohen R, Gongvatana A, et al. Relationship of plasma cytokines and clinical biomarkers to memory performance in HIV. J Neuroimmunol. 2013 Dec 15;265(1-2):117–23. doi: 10.1016/j.jneuroim.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteiro de Almeida S, Letendre S, Zimmerman J, et al. Dynamics of monocyte chemoattractant protein type one (MCP-1) and HIV viral load in human cerebrospinal fluid and plasma. J Neuroimmunol. 2005 Dec;169(1-2):144–52. doi: 10.1016/j.jneuroim.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Letendre SL, Zheng JC, Kaul M, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011 Feb;17(1):63–9. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moidunny S, Matos M, Wesseling E, et al. Oncostatin M promotes excitotoxicity by inhibiting glutamate uptake in astrocytes: implications in HIV-associated neurotoxicity. J Neuroinflammation. 2016;13(1):144. doi: 10.1186/s12974-016-0613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan L, Liu A, Qiao L, et al. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. Biomed Res Int. 2015;2015:506872. doi: 10.1155/2015/506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrier RD, Hong S, Crescini M, et al. Cerebrospinal fluid (CSF) CD8+ T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND) PLoS One. 2015;10(2):e0116526. doi: 10.1371/journal.pone.0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill AJ, Kolson DL. Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in HAND persistence. Curr HIV/AIDS Rep. 2014 Sep;11(3):325–35. doi: 10.1007/s11904-014-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan L, Qiao L, Wei F, et al. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol. 2013 Apr;19(2):144–9. doi: 10.1007/s13365-013-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porcheray F, Samah B, Leone C, et al. Macrophage activation and human immunodeficiency virus infection: HIV replication directs macrophages towards a pro-inflammatory phenotype while previous activation modulates macrophage susceptibility to infection and viral production. Virology. 2006 May 25;349(1):112–20. doi: 10.1016/j.virol.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS One. 2013;8(7):e70164. doi: 10.1371/journal.pone.0070164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams DW, Calderon TM, Lopez L, et al. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One. 2013;8(7):e69270. doi: 10.1371/journal.pone.0069270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancuta P, Weiss L, Haeffner-Cavaillon N. CD14+CD16++ cells derived in vitro from peripheral blood monocytes exhibit phenotypic and functional dendritic cell-like characteristics. Eur J Immunol. 2000 Jul;30(7):1872–83. doi: 10.1002/1521-4141(200007)30:7<1872::AID-IMMU1872>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Tatro ET, Soontornniyomkij B, Letendre SL, et al. Cytokine secretion from brain macrophages infected with human immunodeficiency virus in vitro and treated with raltegravir. BMC Infect Dis. 2014;14(1):386. doi: 10.1186/1471-2334-14-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamat A, Lyons JL, Misra V, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012 Jul 1;60(3):234–43. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerszten RE, Garcia-Zepeda EA, Lim YC, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999 Apr 22;398(6729):718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.