Highlights
-
•
LPS-induced anorexia depends on COX-2, but not in brain endothelial, myeloid or neural cells.
-
•
LPS-induced anorexia and fever are elicited by prostaglandin synthesis in distinct cell groups.
-
•
Conditioned taste aversion induced by LPS is prostaglandin-independent.
Keywords: Anorexia, Inflammation, Conditioned place aversion, Fever, Lipopolysaccharide, Cyclooxygenase
Abstract
Systemic inflammation evokes an array of brain-mediated responses including fever, anorexia and taste aversion. Both fever and anorexia are prostaglandin dependent but it has been unclear if the cell-type that synthesizes the critical prostaglandins is the same. Here we show that pharmacological inhibition or genetic deletion of cyclooxygenase (COX)-2, but not of COX-1, attenuates inflammation-induced anorexia. Mice with deletions of COX-2 selectively in brain endothelial cells displayed attenuated fever, as demonstrated previously, but intact anorexia in response to peripherally injected lipopolysaccharide (10 μg/kg). Whereas intracerebroventricular injection of a cyclooxygenase inhibitor markedly reduced anorexia, deletion of COX-2 selectively in neural cells, in myeloid cells or in both brain endothelial and neural cells had no effect on LPS-induced anorexia. In addition, COX-2 in myeloid and neural cells was dispensable for the fever response. Inflammation-induced conditioned taste aversion did not involve prostaglandin signaling at all. These findings collectively show that anorexia, fever and taste aversion are triggered by distinct routes of immune-to-brain signaling.
1. Introduction
Anorexia, i.e. loss of appetite, and taste aversion are common and debilitating symptoms that occur as consequence of acute and chronic inflammatory disease (Saper et al., 2012). While it is well established that inflammation-induced anorexia is prostaglandin (PG) dependent, as demonstrated by the amelioration of the food intake by cyclooxygenase (COX) inhibitors (Baile et al., 1981, Langhans et al., 1989, Lugarini et al., 2002, Swiergiel and Dunn, 2002), the cellular source of the prostaglandins that evoke anorexia has not been identified. This is in contrast to fever, another cardinal symptom of the inflammatory response. As demonstrated in genetically modified mice, fever is dependent on PGE2 synthesized in brain endothelial cells (Engstrom et al., 2012, Wilhelms et al., 2014), and its subsequent binding to dedicated receptors on thermosensory neurons in the preoptic hypothalamus (Lazarus et al., 2007). However, prostaglandins are induced in several other cell-types upon inflammation and the induction in different cell-types may mediate distinct symptoms and has even been reported to elicit opposing effects (An et al., 2014, Serrats et al., 2010). In the same vein, neural prostaglandin synthesis is critical for the hyperalgesia induced by a local inflammation but dispensable for fever (Vardeh et al., 2009), whereas brain endothelial prostaglandins synthesis is critical for immune-induced fever but dispensable for the reduction in locomotor activity triggered by the same stimulus (Wilhelms et al., 2014).
We here examined the cellular source for prostaglandins eliciting inflammation-induced anorexia by using mice with cell-type specific deletions of COX-2, the COX isoform that is responsible for eliciting both fever (Cao et al., 1997, Li et al., 1999) and anorexia (Lugarini et al., 2002, Swiergiel and Dunn, 2002). To directly test if anorexia and fever are driven by COX-2 in the same cell-types we also monitored fever induced by LPS of the same type and dose (10 μg/kg) as used in the anorexia experiments. Furthermore, we included analysis of inflammation-induced conditioned taste aversion (CTA) since this symptom is closely related to anorexia but has been shown, at least partly, to involve distinct mechanisms (Bauer et al., 1995, Kopf et al., 2011).
2. Materials and methods
2.1. Animals
All experiments followed international and national guidelines, and were approved by the animal care and use committee in Linköping. Mice were kept on a 12–12 h light-dark cycle. Food and water were provided ad libitum if not stated otherwise. Adult mice (older than 8 weeks), of both sexes were used. During the experimental period, all mice were housed individually. For fever experiments, mice were kept at near-thermoneutral ambient temperature of 28 °C ± 1 °C (humidity 42–49%). All other experiments were performed at room temperature (21 °C ± 1 °C, humidity 40–43%).
C57B/6 wild type (WT) mice used for the pharmacological studies were purchased from Scanbur (Karlslunde, Denmark). COX-1 KO (Langenbach et al., 1995) and COX-2 KO (Morham et al., 1995) were purchased from Taconic Biosciences Inc (Ejby, Denmark), and were on a mixed B6;129P2 background. Mice that had the gene encoding COX-2 flanked by loxP sites (floxed) (Ishikawa and Herschman, 2006) were crossed with mice expressing Cre under the control of the nestin promoter (MGI: J:57315, The Jackson Laboratory, Bar Harbor, ME), the LysM promoter (MGI: J:67924, Jackson Laboratories) or Slco1c1 promoter (Ridder et al., 2011) to obtain mice with deletion of COX-2 in the neural cells (Cox2ΔNes), myeloid cells (Cox2ΔLysM), or brain endothelial cells (Cox2ΔbEnd), respectively. Homozygous floxed (Cox2fl/fl) but Cre negative littermates were used as controls (WT). C57B/6 WT mice implanted with cannulas into the brain ventricular system were purchased from Jackson Laboratories (Bar Harbor, ME). In general, littermates were used as controls except for the experiment with COX-2 knockouts in which mice with the same genetic background but not from the same colony were used. In all experiments the groups were balanced regarding sex and age.
To induce Cre-recombinase activity in mice with a Slco1c1-CreERT2 construct, 100 μL tamoxifen solution (1 mg tamoxifen dissolved in a 1:10 mixture of ethanol and sunflower seed oil) was administered intraperitoneally (i.p.), twice a day for 5 consecutive days.
2.2. Drugs
To induce anorexia, 100 μL LPS (O55:B5, Sigma Aldrich, St. Louis, MO; 10 μg/kg) was given i.p. The unspecific COX-inhibitor indomethacin (Confortid; Alpharma, Langenfeld, Germany; 5 mg/kg) and the COX-2 specific inhibitor parecoxib (Dynastat; Pfizer, New York, NY; 10 mg/kg) were given i.p. at a volume of 100 μL. These doses were selected from previous studies (Nilsberth et al., 2009, Ruud et al., 2013a) and tested by titration to robustly inhibit anorexia induced by the given dose of LPS. Indomethacin (Confortid) was also administered intracerebroventricularly (i.c.v.; 15 μg in 3 μL). The COX-1 specific inhibitor SC-560 (Cayman chemicals, Ann Arbor, MI; 30 mg/kg in 300 μL) was given by gavage. The selected dose has previously been show to robustly inhibit COX-1 in a food intake paradigm (Ruud et al., 2013a). Indomethacin, parecoxib and LPS were diluted in saline, whereas SC-560 was diluted in a mixture of methylcellulose and tween 80. Due to the problem with tolerance against LPS, each animal was only given LPS once.
2.3. Fever experiments
One week prior to the recordings, temperature transmitters (model TA11TAF10, Data Sciences International, New Brighton, MN) were inserted into the abdominal cavity of the mice under brief gas anesthesia (isofluoran, 1%). Temgesic was given peri-operatively. Basal core temperature was measured 24 h prior to experimental onset. About 2–3 h after lights on, mice were given a single i.p. injection of LPS or saline and core body temperature was measured for 12 h. Experiments were performed during daytime in order to minimize activity-related changes in body temperature. Fever was defined as any prolonged LPS-induced increase in body temperature (i.e. when LPS-treated mice had a body temperature that was statistically significantly higher than that of NaCl-treated mice).
2.4. Food intake experiments
Mice were single housed for a minimum of 5 d prior to food intake measurements. All food intake measurements were performed during the active period of the mice, i.e. during the dark period. On the test day, food was withdrawn and mice were given a single injection of either LPS or saline 1 h before onset of the dark period (7 p.m.). At dark period onset, pre-weighed food was given, and the weight of the food was measured again at 4, 7 and/or 13 h after injection. Visible food spillage in the cage was measured and accounted for. If food spillage was detected after experimental endpoint, the data from that particular animal was excluded. Pretreatment with indomethacin or SC-560 was done once, 1 h before LPS or saline injection. Parecoxib was given 30 min before LPS or saline and an additional dose was given after 4.5 h. The timing for administration of pretreatment was chosen in concordance with studies in which the drugs have been demonstrated to be efficient in inhibiting a LPS response (Fritz et al., 2016, Nilsberth et al., 2009).
For experiments using i.c.v. administration of indomethacin, mice were single housed 5 d before experimental onset. On experimental day, food was withdrawn 2 h prior to dark period onset. Four hours after food withdrawal, 3 μL indomethacin (15 μg) was injected with a Hamilton syringe into the i.c.v. cannula under brief gas anesthesia (isofluoran, 1%), and LPS was administered i.p. simultaneously. Ten minutes after injections, pre-weighed food was reintroduced and food intake was measured after 1 h by weighing the food tray.
2.5. Conditioned taste aversion experiments
All behavioral testing was conducted during the dark phase. Mice were separated and housed one and one a minimum of 5 d prior to experimental onset. Days 1–7, mice were habituated to water deprivation for 4 h a day. Day 8 (conditioning day), mice were water deprived for 4 h, then given access to a saccharin solution (0.15%) for 1 h, and immediately thereafter given an injection of either LPS or saline. Days 9–10 mice were only exposed to water deprivation. At the test day (Day 11) mice were water deprived for 4 h and then given saccharin solution for 1 h after which saccharin intake was measured. Pretreatment with SC-560 and parecoxib was given 1 h before LPS injection on the conditioning day (Day 8), i.e. at the same time mice got access to saccharin. The timing for administration of these inhibitors is in concordance with the timing of administration in the food intake studies.
2.6. Statistics
Results are presented as mean ± SEM. Anorexia and fever were analyzed with a 2-way repeated measures ANOVA, followed by a post hoc analysis using Tukey’s (anorexia) or Holm-Sidak’s (fever) multiple comparisons test. Mean fever and saccharin intake were analyzed with a 2-way ANOVA, followed by a post hoc analysis using Tukey’s multiple comparisons test. P < 0.05 was considered statistically significant.
3. Results
3.1. LPS-induced anorexia is COX-2 dependent
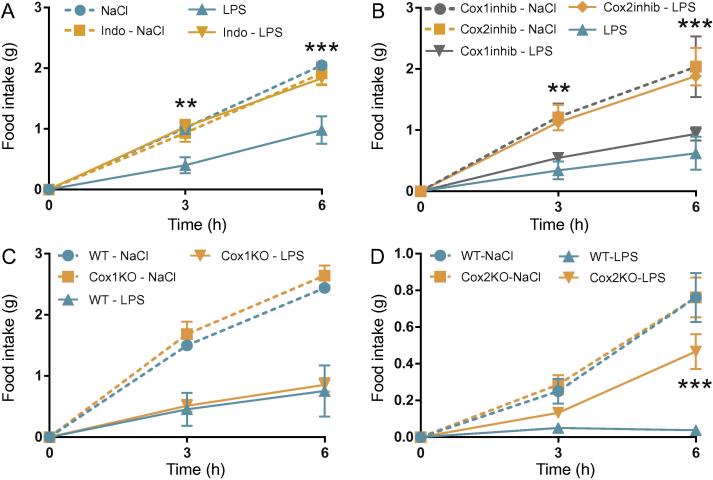
In order to induce anorexia, we used a low dose (10 μg/kg) of LPS. The dose was kept low in order to mimic natural disease conditions. As expected, LPS induced a robust anorexia that was evident both 3 and 6 h after injection (Fig.1A) and to some extent also after 12 h (Supplementary Fig. 1A). For the subsequent analysis of anorexia, we restricted our primary analysis to the first 6 h and show data from 12 h in Supplementary Fig. 1A–G. To confirm that the LPS-induced anorexia was prostaglandin dependent, we pretreated mice with the non-selective COX-inhibitor indomethacin. Whereas LPS induced a robust anorexia in vehicle-treated mice, indomethacin-treated animals displayed normal food intake (Fig.1A). To examine if the anorexia in the present paradigm was driven by COX-1 or COX-2, we next used selective inhibitors of the two enzymes. Mice given the COX-1 selective inhibitor SC-560 showed anorexic responses comparable to those of vehicle treated mice when both groups were injected with LPS (Fig.1B). In contrast, mice given the COX-2 selective inhibitor parecoxib showed a strongly attenuated inflammation-induced anorexia (Fig.1B). We next validated these findings using mice with genetic deletions of COX-1 or COX-2. As expected, both WT mice and mice lacking COX-1 displayed normal anorexic responses to LPS (Fig.1C). Mice without COX-2 displayed a blunted anorexic response (Fig.1D) but the effect of the COX-2 deletion was less strong compared to that obtained by pharmacological COX-2 inhibition. This difference may be explained by the compensatory induction of COX-1, and its potential functional replacement of COX-2, which is seen in mice with genetic deletion of COX-2 (Kirtikara et al., 1998, Zhang et al., 2002).
Fig. 1.
COX-2 is critical for LPS-induced anorexia. Food intake in mice given LPS intraperitoneally. LPS induced a robust anorexia (2-way ANOVA, group × time F (6, 38) = 7.081, P < 0.001) that was blocked by unspecific inhibition of COX with indomethacin (A; n = 6, Indo-LPS; n = 6, Indo-NaCl; n = 5, LPS; n = 6, NaCl). Specific inhibition of COX-2 with parecoxib also blocked the anorexia (B; n = 6, LPS; n = 4, NaCl) whereas the COX-1 selective inhibitor SC-560 was without effect (B; n = 6, LPS; n = 3, NaCl; 2-way ANOVA, group × time F (4, 18) = 6.785, P = 0.0016). Similar results were seen using mice lacking COX-1 (C; n = 7, KO-LPS; n = 7, KO-NaCl; n = 7, WT-LPS; n = 5, WT-NaCl; 2-way ANOVA, group × time F (6, 44) = 16.60, P < 0.001) or COX-2 (D; n = 9, KO-LPS; n = 8, KO-NaCl; n = 8, WT-LPS; n = 8, WT-NaCl; 2-way ANOVA, group × time F (6, 58) = 11.17, P < 0.001). *P < 0.05, **P < 0.01, ***P < 0.001; repeated measurement ANOVA followed by Tukey’s post hoc test. Only differences between genotypes are indicated.
3.2. LPS-induced anorexia occurs independently of COX-2 expression in neural-, myeloid- or brain endothelial cells
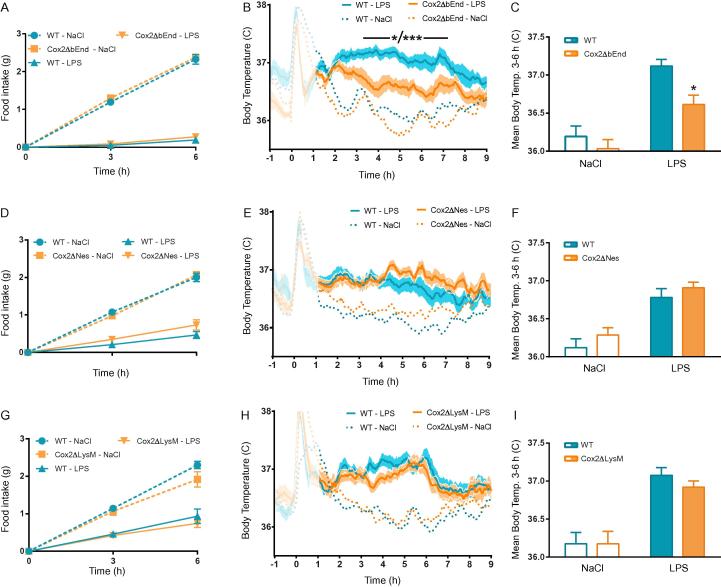
To determine which cell type that generates the prostaglandins responsible for LPS-induced anorexia, we used cell-type specific deletions of COX-2. Since brain endothelial COX-2 has been shown to be involved in immune-induced fever (Engstrom et al., 2012, Wilhelms et al., 2014), we first generated mice lacking COX-2 in brain endothelial cells (Cox2ΔbEnd). This was done by crossing mice in which critical parts of the gene encoding COX-2 was floxed (Ishikawa and Herschman, 2006), with a mouse line expressing an inducible Cre-recombinase under the control of the Slco1c1-promoter. This promoter drives Cre expression selectively in the brain endothelium (Ridder et al., 2011) and has previously been used to dissect the role of prostaglandin synthesis in those cells for the febrile response (Eskilsson et al., 2014, Wilhelms et al., 2014). When these mice were tested for LPS-induced anorexia, we found that Cox2ΔbEnd mice became anorectic to the same extent as littermates with intact COX-2 expression (WT; Fig.2A). We next investigated the febrile response in Cox2ΔbEnd mice to the same low dose of LPS as was used in the food intake experiments and found that it was significantly attenuated (Fig.2B, C). These findings indicate that the prostaglandins that trigger immune-induced fever and anorexia, respectively, are synthetized in distinct cell types. Next, we generated mice lacking COX-2 in neural cells (i.e. neurons, astrocytes and oligodendrocytes; Cox2ΔNes) (Braun et al., 2012). To this end we used a mouse line in which Cre expression was driven by the nestin promoter. Similar to the mice with COX-2 deletion in the brain endothelium, the mice lacking COX-2 in neural cells reduced their food intake in response to LPS to the same degree as did WT mice (Fig.2D). The mutant mice also showed a normal febrile response after injection of the same dose of LPS (Fig.2E, F). Finally, we deleted COX-2 in myeloid cell using the LysM-Cre line. Mice without COX-2 in myeloid cells (Cox2ΔLysM) showed a normal LPS-induced anorexia (Fig.2G) and a febrile response that was almost identical to the one seen in WT mice (Fig.2H, I).
Fig. 2.
LPS-induced anorexia is unaffected in mice with cell-type specific deletions of COX-2, but mice with COX-2 deletion in brain endothelial cells show attenuated febrile response. Mice lacking COX-2 in brain endothelial cells (Cox2ΔbEnd) showed intact anorexia (A; n = 6, Cox2ΔbEnd-LPS; n = 6, Cox2ΔbEnd-NaCl; n = 6, WT-LPS; n = 7, WT-NaCl; 2-way ANOVA, group × time F (6, 42) = 123.7, P < 0.001) but attenuated fever in response to LPS (B, C; n = 11, Cox2ΔbEnd-LPS; n = 10, Cox2ΔbEnd-NaCl; n = 13, WT-LPS, n = 12, WT-NaCl; 2-way ANOVA, group × time F (900, 12,900) = 5.589, P < 0.001). Mice lacking COX-2 in neural cells (Cox2ΔNes) showed intact anorexia (D; n = 6, Cox2ΔNes-LPS; n = 6, Cox2ΔNes-NaCl; n = 6, WT-LPS; n = 6, WT-NaCl; 2-way ANOVA, group × time F (6, 88) = 48.49, P < 0.001) and fever (E, F; n = 16, Cox2ΔNes-LPS; n = 15, Cox2ΔNes-NaCl; n = 14, WT-LPS, n = 14, WT-NaCl; 2-way ANOVA, group × time F (729, 13,122) = 2.891, P < 0.001) after injection of LPS. The same was the case for mice lacking COX-2 in myeloid cells (G; n = 9, Cox2ΔLysM-LPS; n = 6, Cox2ΔLysM-NaCl; n = 9, WT-LPS; n = 9, WT-NaCl; and H, I; n = 12, Cox2ΔLysM-LPS; n = 12, Cox2ΔLysM-NaCl; n = 11, WT-LPS, n = 11, WT-NaCl; Anorexia: 2-way ANOVA, group × time F (6, 58) = 20,89, P < 0.001; Fever: group × time F (849, 11,886) = 4.497, P < 0.001). *P < 0.05, **P < 0.01, ***P < 0.001 repeated measurement ANOVA (A, D, G) or ANOVA (B, C, E, F, H, I) followed by Tukey’s (anorexia) or Holm-Sidak’s (fever curves) post hoc tests. Only differences between genotypes are indicated.
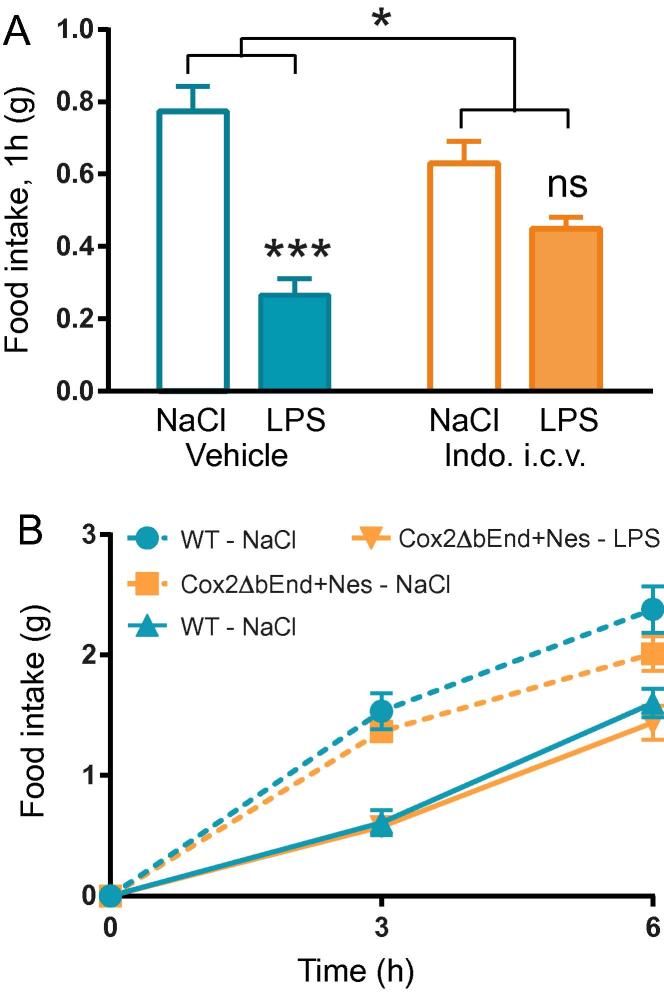
A reason why none of the cell-type specific COX-2 deletions blocked anorexia could be that two mutually redundant sites of COX-2 expression exist that each are sufficient to drive anorexia. If this were the case, it is likely that both sites were cerebral, since COX-2 inhibitors injected into the brain have been shown to block LPS-induced anorexia in the rat (Kopf et al., 2011). We tested if cerebral COX is necessary for inducing LPS-elicited anorexia also in the mouse by injecting indomethacin i.c.v. Indeed, indomethacin blocked the anorexia the first hour after LPS injection (Fig.3A; we restricted the analysis to one hour since drugs given i.c.v. are rapidly eliminated from the brain). The selected indomethacin dose (15 μg/mouse) had no effect when administered systemically (data not shown). Next, we generated mice lacking COX-2 in both brain endothelial cells and neural cells (Cox2ΔbEnd + Nes). This combination of Cre-lines was chosen since both lines affect the brain whereas the deletions induced by LysM mostly affect peripheral sites. Despite the dual deletion, Cox2ΔbEnd + Nes mice displayed normal LPS-induced anorexia (Fig.3B).
Fig. 3.
LPS-induced anorexia is attenuated in mice given indomethacin i.c.v. but remain unaffected in mice lacking COX-2 in both brain endothelial and neural cells. A. Food intake in response to i.p. injection of LPS and i.c.v. injection of indomethacin (n = 3, vehicle + NaCl, vehicle + LPS, indo + NaCl; n = 4, indo + LPS; 2-way ANOVA, pre-treatment × treatment F (1, 9) = 10.28, P = 0.0107). B. Mice lacking COX-2 in brain endothelial and neural cells (Cox2ΔbEnd ± Nes) showed intact anorexia (D; n = 6, Cox2ΔbEnd + Nes-LPS; n = 8, Cox2ΔbEnd + Nes-NaCl; n = 11, WT-LPS; n = 9, WT-NaCl; 2-way ANOVA, treatment F (3, 33) = 13.94, P < 0.001).
3.3. LPS-induced conditioned taste aversion is prostaglandin-independent
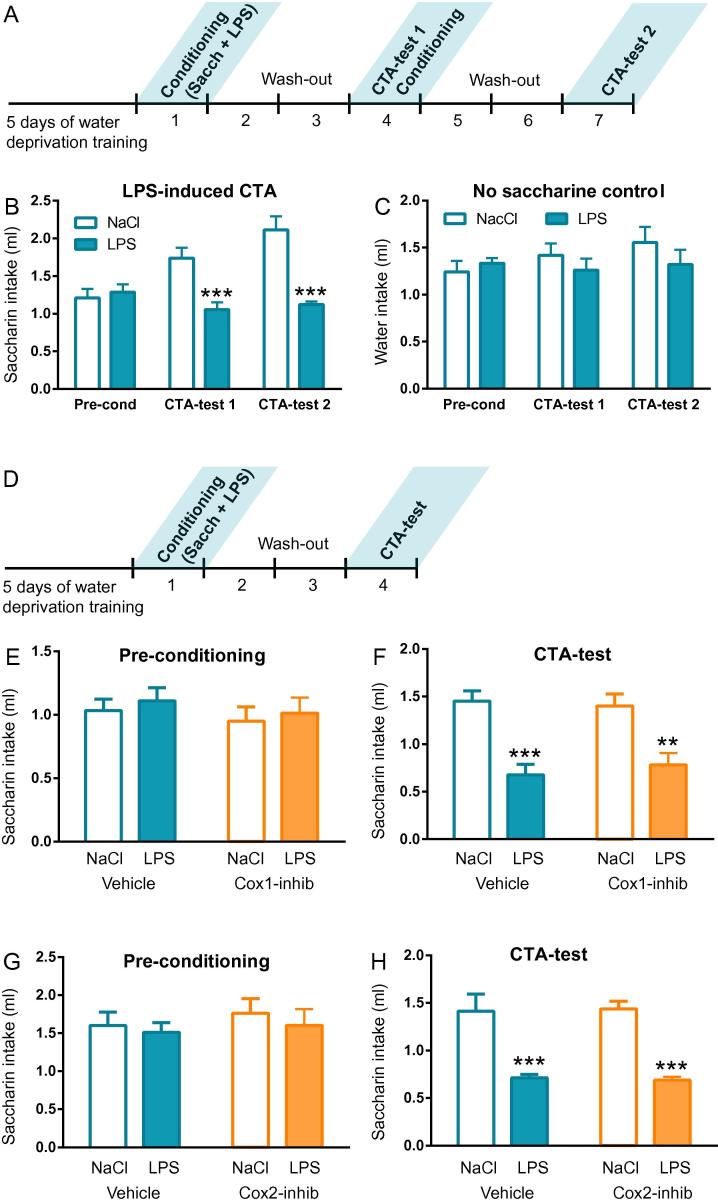
We established a protocol for conditioned taste aversion with the same dose of LPS as was used in the anorexia and fever experiments (Fig.4A). Intraperitoneal injection of 10 μg/kg of LPS given after saccharine drinking robustly reduced saccharin drinking three days later (Fig.4B). This effect was a specific reaction to the taste, since fluid intake was normal in mice subjected to the same protocol but without access to saccharine (Fig.4C). One further conditioning session made the effect more pronounced (Fig.4B), but since the difference was only incremental we continued with a protocol including only one conditioning session (4D). Since unspecific COX inhibition induced taste aversion by itself (Supplementary Fig. 1H–I), we moved directly to inhibition of COX-1 and COX-2. Neither COX-1 inhibition (Fig.4E, F) nor COX-2 inhibition (Fig.4G, H) had any effect on the conditioned taste aversion induced by LPS. These observations indicate that the anorexia and the taste aversion induced by inflammation are triggered by distinct mechanisms and that only the mechanism behind anorexia requires prostaglandins.
Fig. 4.
LPS-induced conditioned taste aversion is neither dependent on COX-1 nor on COX-2. Conditioned taste aversion was initially tested by a protocol with two conditioning sessions (A). Saccharine drinking was reduced already after one conditioning session and further reduced after a second conditioning session (B, CTA-test 1; n = 6, LPS; n = 6, NaCl; 2-way ANOVA, session × treatment F (2, 20) = 12.87, P < 0.001). The same protocol but without saccharine did not result in reduced fluid intake at the test session (C; n = 6, LPS; n = 5, NaCl), showing that the aversion is specifically related to the taste of saccharine. Since one conditioning session was enough to induce taste aversion we used only one conditioning session for the subsequent experiments (D). COX-1 inhibition with SC-560 did not affect saccharine intake before the conditioning (E; n = 22, LPS; n = 21, NaCl), and did not interfere with the reduction in saccharine intake induced by conditioning (F; 2-way ANOVA, treatment × pre-treatment F (1, 82) = 0.4391, P = 0.509; treatment F (1, 82) = 34.62, P < 0.001). The same was the case for COX-2 inhibition with parecoxib (G, H; n = 8, LPS; n = 8, NaCl; 2-way ANOVA, treatment × pre-treatment F (1, 28) = 0.06074, P = 0.807; treatment F (1, 28) = 51.08, P < 0.001). *P < 0.05, **P < 0.01, ***P < 0.001; ANOVA followed by Tukey’s post hoc test.
4. Discussion
Anorexia and fever are hallmarks of systemic inflammation. Both these symptoms are dependent on prostaglandins synthesized by COX-2 (Johnson et al., 2002, Li et al., 1999, Lugarini et al., 2002, Swiergiel and Dunn, 2002), but it has been unclear if the cellular source of the critical prostaglandins is identical. Using cell-type specific gene deletions, we here studied the role of prostaglandin synthesis in anorexia, fever and conditioned taste aversion induced by the same dose of LPS.
We recently demonstrated that mice with selective deletion of COX-2 in the endothelial cells of the blood-brain barrier displayed attenuated LPS-induced fever (Wilhelms et al., 2014). Here we extended this finding by showing that fever in response to an LPS dose ten times lower than the one used in our previous study also is attenuated in these mice, although the attenuation is less pronounced. Possible reasons for the only partial effect by the gene deletion on fever include incomplete recombination by the Cre-line and contribution of COX-2 from other cell-types. In contrast to the attenuated fever, mice lacking COX-2 expression in the endothelial cells of the blood-brain barrier displayed an intact LPS-induced anorexia. Taken together with the finding that deletion of Tak1 in the brain endothelium dampens fever but not anorexia in response to IL-1β (Ridder et al., 2011), the present data indicates that the brain endothelium is not involved in the immune-to-brain signaling underpinning anorexia and, thus, that the prostaglandin-dependent mechanism triggering anorexia is distinct from the one eliciting fever. These findings are also supported by studies showing that LPS-induced fever is dependent on microsomal prostaglandin E synthase-1 (mPGES-1) production (Engblom et al., 2003) but that mPGES-1 knock-out mice still develop anorexia after LPS (Elander et al., 2007), emphasizing the discrepancy between the mechanisms generating anorexia and fever.
Activation of myeloid cells is critical for LPS-induced anorexia. Thus, deletion in myeloid cells of myeloid differentiation primary response gene 88 (MyD88), which is an adapter protein for Toll like and interleukin-1 receptor signaling, largely inhibits LPS-induced anorexia (Ruud et al., 2013b). Our present findings strongly indicate that the critical MyD88 dependent event in these cells is not the induction of COX-2. Further, our present results show that COX-2 production in neural cells, such as neurons, astrocytes and oligodendrocytes, is not critical for LPS-induce anorexia. Collectively, these data suggests that prostaglandin synthesis in brain endothelial cells, neural cells and myeloid cells is not critical for anorexia. However it should be noted that the role of myeloid cells could vary with the dose of LPS and the time point studied (Chakravarty and Herkenham, 2005).
There are several possible explanations for the somewhat surprising finding that none of the cell type-specific deletions of COX-2 attenuated the inflammation-induced anorexia. One possibility is that COX-2 expressed in a cell-type not targeted in this study is critical for anorexia. Since indomethacin injected i.c.v. (this study) and a COX-2 inhibitor injected in the dorsal raphe nucleus (Kopf et al., 2011) have been shown to attenuate LPS-induced anorexia, it is likely that a cell-type residing in the brain is responsible for the critical prostaglandin synthesis. Since microglia and perivascular cells are not targeted by the Cre-lines used here, they are possible candidates; however, they express no or little COX-2 after immune challenge (Engstrom et al., 2012, Schiltz and Sawchenko, 2002). Another possibility is that, in contrast to what is case for fever, there are redundant COX-2 dependent signaling pathways for anorexia and that COX-2 in one or more of the cell-types investigated is sufficient but not necessary for eliciting anorexia. We found no attenuation of the anorexic response in mice lacking COX-2 in both neural cells and brain endothelial cells, but there are of course several additional combinations that could be tested. Finally, it is possible that COX-2 in one of the cell-types investigated is indeed critical for anorexia but that the Cre-line used to target the deletion to those cells is not efficient enough. However, this is unlikely since the endothelium specific deletion affected fever, and COX-2 deletion in neural cells, using the same Cre-line as used here, has been shown to affect mechanical hyperalgesia (Vardeh et al., 2009). Furthermore, deletion of MyD88 in myeloid cells with LysM-Cre, inhibited LPS-induced anorexia in a previous study from this laboratory (Ruud et al., 2013b).
Based on a previous study (Mormede et al., 2004), we developed a protocol for inflammation-induced conditioned taste aversion. In this protocol, mice learn to associate the taste of saccharine with the effect of LPS. We found that one injection of a low-dose of LPS after saccharine drinking was sufficient to reduce saccharine drinking three days later, and we demonstrate that conditioned taste aversion to a dose of LPS that elicits a prostaglandin-dependent anorexia is independent of prostaglandins. This finding is in line with the results from a previous study using the non-selective COX inhibitor indomethacin and a protocol for conditioned taste aversion in which rats were given LPS prior to exposure to the taste but in which a sweetened diet was used instead of water with saccharin (Weingarten et al., 1993). We recently demonstrated a pathway mediating inflammation-induced conditioned place aversion, using LPS at the dose used in this study. We found that conditioned place aversion induced by LPS is dependent on PGE2 production by COX-1 and subsequent binding of PGE2 to EP1 receptors on dopamine D1 receptor expressing neurons in the striatum (Fritz et al., 2016). Thus, LPS-induced place aversion and taste aversion are elicited by very different mechanisms. On a more general level, most brain-mediated responses to systemic LPS injection are completely or partially prostaglandin dependent. In addition to fever, anorexia and conditioned place aversion, also cortisol release (Elander et al., 2009, Gadek-Michalska and Bugajski, 2004), hyperalgesia (Schmelzer et al., 2006) and lethargy (Harden et al., 2011) in response to LPS are dependent on prostaglandin synthesis. Thus, the immune-to-brain signaling mechanism behind conditioned taste aversion differs in a fundamental way from those behind most other brain-mediated illness symptoms by not being prostaglandin dependent.
5. Conclusion
In conclusion, we show that the anorexia and fever induced by a low dose of LPS are triggered by prostaglandin synthesis in distinct cell-types. Fever is dependent on COX-2 in brain endothelial cells, and whereas anorexia also is COX-2 dependent, it is not inhibited by deletion of COX-2 neither in the brain endothelium, nor in neural cells or myeloid cells. Furthermore, inflammation-induced taste aversion is independent of prostaglandins. These findings illustrate the complexity of the inflammatory induced sickness syndrome, and open up avenues for the selective amelioration of each distinct symptom.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Acknowledgments
This study was supported by the Swedish Medical Research Council (#20725 to DE and #07879 to AB), the European Research Council (ERC-starting grant to DE), the Knut and Alice Wallenberg foundation (DE), the Swedish Brain Foundation (DE and AB), the Swedish Cancer Foundation (#213/692 to AB), and the County Council of Östergötland (DE and AB). We thank Harvey R. Herschman for providing the COX-2 floxed line and Markus Schwaninger for the Slco1c1-CreERT2-line. Further, we thank Maria Kristersson and Michaela Westerdahl for assisting during experiment with COX-2 inhibition in CTA.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbi.2016.12.007.
Appendix A. Supplementary data
Accumulated food intake 12 h after LPS administration (A–G). Twelve hours after LPS administration, a treatment effect from LPS was seen in all experimental groups (A: 2-way ANOVA, treatment F(1, 19) = 7.084, P = 0.0154; B: treatment F(4, 20) = 12.33, P < 0.0001; C: treatment F(1, 22) = 34.89, P = <0.0001; D: treatment F(1, 29) = 35.49, P < 0.0001; E: treatment F(1, 44) = 71.01, P = <0.0001, F: treatment F(1, 29) = 12.53, P = 0.0014, G: treatment F(1, 33) = 6.069, P = 0.0191), treatment × pre-treatment F (1, 33) = 5.567, P = 0.0244. Saccharin intake before and after conditioning with LPS (H-I). **P < 0.01, ***P < 0.001; ANOVA followed by Tukey’s post hoc test (B) or two-way ANOVA followed by Holm-Sidak’s post hoc test (A, C, D, E, F, G).
References
- An Y., Belevych N., Wang Y., Zhang H., Herschman H., Chen Q., Quan N. Neuronal and nonneuronal COX-2 expression confers neurotoxic and neuroprotective phenotypes in response to excitotoxin challenge. J. Neurosci. Res. 2014;92:486–495. doi: 10.1002/jnr.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile C.A., Naylor J., McLaughlin C.L., Catanzaro C.A. Endotoxin-elicited fever and anorexia and elfazepam-stimulated feeding in sheep. Physiol. Behav. 1981;27:271–277. doi: 10.1016/0031-9384(81)90269-9. [DOI] [PubMed] [Google Scholar]
- Bauer C., Weingarten S., Senn M., Langhans W. Limited importance of a learned aversion in the hypophagic effect of interleukin-1 beta. Physiol. Behav. 1995;57:1145–1153. doi: 10.1016/0031-9384(94)00379-j. [DOI] [PubMed] [Google Scholar]
- Braun T.P., Grossberg A.J., Veleva-Rotse B.O., Maxson J.E., Szumowski M., Barnes A.P., Marks D.L. Expression of myeloid differentiation factor 88 in neurons is not requisite for the induction of sickness behavior by interleukin-1beta. J. Neuroinflammation. 2012;9:229. doi: 10.1186/1742-2094-9-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C., Matsumura K., Yamagata K., Watanabe Y. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am. J. Physiol. 1997;272:R1712–R1725. doi: 10.1152/ajpregu.1997.272.6.R1712. [DOI] [PubMed] [Google Scholar]
- Chakravarty S., Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elander L., Engstrom L., Hallbeck M., Blomqvist A. IL-1beta and LPS induce anorexia by distinct mechanisms differentially dependent on microsomal prostaglandin E synthase-1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R258–R267. doi: 10.1152/ajpregu.00511.2006. [DOI] [PubMed] [Google Scholar]
- Elander L., Engstrom L., Ruud J., Mackerlova L., Jakobsson P.J., Engblom D., Nilsberth C., Blomqvist A. Inducible prostaglandin E2 synthesis interacts in a temporally supplementary sequence with constitutive prostaglandin-synthesizing enzymes in creating the hypothalamic-pituitary-adrenal axis response to immune challenge. J. Neurosci. 2009;29:1404–1413. doi: 10.1523/JNEUROSCI.5247-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom D., Saha S., Engstrom L., Westman M., Audoly L.P., Jakobsson P.J., Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat. Neurosci. 2003;6:1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
- Engstrom L., Ruud J., Eskilsson A., Larsson A., Mackerlova L., Kugelberg U., Qian H., Vasilache A.M., Larsson P., Engblom D., Sigvardsson M., Jonsson J.I., Blomqvist A. Lipopolysaccharide-induced fever depends on prostaglandin E2 production specifically in brain endothelial cells. Endocrinology. 2012;153:4849–4861. doi: 10.1210/en.2012-1375. [DOI] [PubMed] [Google Scholar]
- Eskilsson A., Mirrasekhian E., Dufour S., Schwaninger M., Engblom D., Blomqvist A. Immune-induced fever is mediated by IL-6 receptors on brain endothelial cells coupled to STAT3-dependent induction of brain endothelial prostaglandin synthesis. J. Neurosci. 2014;34:15957–15961. doi: 10.1523/JNEUROSCI.3520-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M., Klawonn A.M., Nilsson A., Singh A.K., Zajdel J., Wilhelms D.B., Lazarus M., Lofberg A., Jaarola M., Kugelberg U.O., Billiar T.R., Hackam D.J., Sodhi C.P., Breyer M.D., Jakobsson J., Schwaninger M., Schutz G., Parkitna J.R., Saper C.B., Blomqvist A., Engblom D. Prostaglandin-dependent modulation of dopaminergic neurotransmission elicits inflammation-induced aversion in mice. J. Clin. Invest. 2016;126:695–705. doi: 10.1172/JCI83844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek-Michalska A., Bugajski J. Role of prostaglandins and nitric oxide in the lipopolysaccharide-induced ACTH and corticosterone response. J. Physiol. Pharmacol. 2004;55:663–675. [PubMed] [Google Scholar]
- Harden L.M., du Plessis I., Roth J., Loram L.C., Poole S., Laburn H.P. Differences in the relative involvement of peripherally released interleukin (IL)-6, brain IL-1beta and prostanoids in mediating lipopolysaccharide-induced fever and sickness behavior. Psychoneuroendocrinology. 2011;36:608–622. doi: 10.1016/j.psyneuen.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Ishikawa T.O., Herschman H.R. Conditional knockout mouse for tissue-specific disruption of the cyclooxygenase-2 (Cox-2) gene. Genesis. 2006;44:143–149. doi: 10.1002/gene.20192. [DOI] [PubMed] [Google Scholar]
- Johnson P.M., Vogt S.K., Burney M.W., Muglia L.J. COX-2 inhibition attenuates anorexia during systemic inflammation without impairing cytokine production. Am. J. Physiol. Endocrinol. Metab. 2002;282:E650–E656. doi: 10.1152/ajpendo.00388.2001. [DOI] [PubMed] [Google Scholar]
- Kirtikara K., Morham S.G., Raghow R., Laulederkind S.J., Kanekura T., Goorha S., Ballou L.R. Compensatory prostaglandin E2 biosynthesis in cyclooxygenase 1 or 2 null cells. J. Exp. Med. 1998;187:517–523. doi: 10.1084/jem.187.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf B.S., Langhans W., Geary N., Hrupka B., Asarian L. Evidence that PGE2 in the dorsal and median raphe nuclei is involved in LPS-induced anorexia in rats. Pharmacol. Biochem. Behav. 2011;99:437–443. doi: 10.1016/j.pbb.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Langenbach R., Morham S.G., Tiano H.F., Loftin C.D., Ghanayem B.I., Chulada P.C., Mahler J.F., Lee C.A., Goulding E.H., Kluckman K.D., Kim H.S., Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Langhans W., Harlacher R., Scharrer E. Verapamil and indomethacin attenuate endotoxin-induced anorexia. Physiol. Behav. 1989;46:535–539. doi: 10.1016/0031-9384(89)90032-2. [DOI] [PubMed] [Google Scholar]
- Lazarus M., Yoshida K., Coppari R., Bass C.E., Mochizuki T., Lowell B.B., Saper C.B. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Li S., Wang Y., Matsumura K., Ballou L.R., Morham S.G., Blatteis C.M. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2(−/−), but not in cyclooxygenase-1(−/−) mice. Brain Res. 1999;825:86–94. doi: 10.1016/s0006-8993(99)01225-1. [DOI] [PubMed] [Google Scholar]
- Lugarini F., Hrupka B.J., Schwartz G.J., Plata-Salaman C.R., Langhans W. A role for cyclooxygenase-2 in lipopolysaccharide-induced anorexia in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R862–R868. doi: 10.1152/ajpregu.00200.2002. [DOI] [PubMed] [Google Scholar]
- Morham S.G., Langenbach R., Loftin C.D., Tiano H.F., Vouloumanos N., Jennette J.C., Mahler J.F., Kluckman K.D., Ledford A., Lee C.A., Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Mormede C., Palin K., Kelley K.W., Castanon N., Dantzer R. Conditioned taste aversion with lipopolysaccharide and peptidoglycan does not activate cytokine gene expression in the spleen and hypothalamus of mice. Brain Behav. Immun. 2004;18:186–200. doi: 10.1016/S0889-1591(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Nilsberth C., Elander L., Hamzic N., Norell M., Lonn J., Engstrom L., Blomqvist A. The role of interleukin-6 in lipopolysaccharide-induced fever by mechanisms independent of prostaglandin E2. Endocrinology. 2009;150:1850–1860. doi: 10.1210/en.2008-0806. [DOI] [PubMed] [Google Scholar]
- Ridder D.A., Lang M.F., Salinin S., Roderer J.P., Struss M., Maser-Gluth C., Schwaninger M. TAK1 in brain endothelial cells mediates fever and lethargy. J. Exp. Med. 2011;208:2615–2623. doi: 10.1084/jem.20110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruud J., Nilsson A., Engstrom Ruud L., Wang W., Nilsberth C., Iresjo B.M., Lundholm K., Engblom D., Blomqvist A. Cancer-induced anorexia in tumor-bearing mice is dependent on cyclooxygenase-1. Brain Behav. Immun. 2013;29:124–135. doi: 10.1016/j.bbi.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Ruud J., Wilhelms D.B., Nilsson A., Eskilsson A., Tang Y.J., Strohle P., Caesar R., Schwaninger M., Wunderlich T., Backhed F., Engblom D., Blomqvist A. Inflammation- and tumor-induced anorexia and weight loss require MyD88 in hematopoietic/myeloid cells but not in brain endothelial or neural cells. FASEB J. 2013;27:1973–1980. doi: 10.1096/fj.12-225433. [DOI] [PubMed] [Google Scholar]
- Saper C.B., Romanovsky A.A., Scammell T.E. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat. Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz J.C., Sawchenko P.E. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J. Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer K.R., Inceoglu B., Kubala L., Kim I.H., Jinks S.L., Eiserich J.P., Hammock B.D. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrats J., Schiltz J.C., Garcia-Bueno B., van Rooijen N., Reyes T.M., Sawchenko P.E. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel A.H., Dunn A.J. Distinct roles for cyclooxygenases 1 and 2 in interleukin-1-induced behavioral changes. J. Pharmacol. Exp. Ther. 2002;302:1031–1036. doi: 10.1124/jpet.102.036640. [DOI] [PubMed] [Google Scholar]
- Vardeh D., Wang D., Costigan M., Lazarus M., Saper C.B., Woolf C.J., Fitzgerald G.A., Samad T.A. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J. Clin. Invest. 2009;119:287–294. doi: 10.1172/JCI37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingarten S., Senn M., Langhans W. Does a learned taste aversion contribute to the anorectic effect of bacterial lipopolysaccharide? Physiol. Behav. 1993;54:961–966. doi: 10.1016/0031-9384(93)90309-4. [DOI] [PubMed] [Google Scholar]
- Wilhelms D.B., Kirilov M., Mirrasekhian E., Eskilsson A., Kugelberg U.O., Klar C., Ridder D.A., Herschman H.R., Schwaninger M., Blomqvist A., Engblom D. Deletion of prostaglandin E2 synthesizing enzymes in brain endothelial cells attenuates inflammatory fever. J. Neurosci. 2014;34:11684–11690. doi: 10.1523/JNEUROSCI.1838-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Goorha S., Raghow R., Ballou L.R. The tissue-specific, compensatory expression of cyclooxygenase-1 and -2 in transgenic mice. Prostaglandins Other Lipid Mediat. 2002;67:121–135. doi: 10.1016/s0090-6980(01)00177-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Accumulated food intake 12 h after LPS administration (A–G). Twelve hours after LPS administration, a treatment effect from LPS was seen in all experimental groups (A: 2-way ANOVA, treatment F(1, 19) = 7.084, P = 0.0154; B: treatment F(4, 20) = 12.33, P < 0.0001; C: treatment F(1, 22) = 34.89, P = <0.0001; D: treatment F(1, 29) = 35.49, P < 0.0001; E: treatment F(1, 44) = 71.01, P = <0.0001, F: treatment F(1, 29) = 12.53, P = 0.0014, G: treatment F(1, 33) = 6.069, P = 0.0191), treatment × pre-treatment F (1, 33) = 5.567, P = 0.0244. Saccharin intake before and after conditioning with LPS (H-I). **P < 0.01, ***P < 0.001; ANOVA followed by Tukey’s post hoc test (B) or two-way ANOVA followed by Holm-Sidak’s post hoc test (A, C, D, E, F, G).