Abstract
DNA molecules that encode a small, high-speed self-hydrolyzing deoxyribozyme are used as templates for rolling circle amplification (RCA) to produce single-stranded DNAs (ssDNAs) of single- and multiple-unit lengths. Including self-cleaving deoxyribozymes in RCA products can be exploited to generate large amounts of ssDNAs with defined sequence, length, and with precise termini. We also demonstrate the use of this method to efficiently generate ssDNA size markers by using deoxyribozyme reaction conditions that permit partial processing.
Keywords: deoxyribozymes, self-hydrolysis, rolling-circle amplification, single-stranded DNA ladder
Deoxyribozymes are DNA molecules that form structures capable of catalyzing chemical reactions (1–3). Given the central role of DNA in genetic information storage and its importance in biotechnology, deoxyribozymes might find utility in engineered organisms or as reagents for various molecular applications (4–6). Of particular interest to us are DNAs that catalyze self-processing reactions (7–10). Such deoxyribozymes could be harnessed to create DNA constructs that become modified based on their inherent catalytic activities when exposed to specific reaction conditions. For example, engineered self-cleaving deoxyribozymes that employ oxidation (7), depurination (8), or hydrolysis (9–12) mechanisms have been created previously by using various directed evolution strategies. Versions of these or other self-cleaving deoxyribozymes that operate with appropriate speeds and chemical characteristics might find broad utility for various applications involving DNA cleavage.
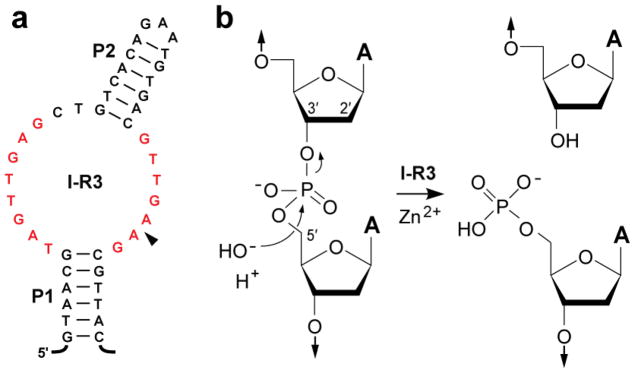
Recently, we identified two classes of engineered self-cleaving deoxyribozymes that hydrolyze DNA with high speed and sequence specificity (13). One such deoxyribozyme, named I-R3 (Figure 1a), carries a small catalytic core composed of 17 nucleotides flanked by either one or two base-paired substructures. Representatives of this deoxyribozyme class exhibit an observed rate constant (kobs) for DNA hydrolysis of ~1 min−1 (half-life of ~40 sec) when incubated near neutral pH and in the presence of millimolar concentrations of Zn2+. This deoxyribozyme cleaves the phosphoester bond between the 3′ oxygen and the phosphorus center of an ApA linkage to yield a 3′ cleavage fragment with a 5′ phosphate group (Figure 1b).
Figure 1. Schematic representation of a class I self-hydrolyzing deoxyribozyme and its chemical reaction.
(a) Sequence and secondary structure of the I-R3 self-cleaving deoxyribozyme. Nucleotides in red are highly-conserved and the arrowhead identifies the site of hydrolysis. (b) Proposed reaction for hydrolysis of the 3′ phosphoester linkage to yield products with 3′ hydroxyl and 5′ phosphate termini.
We speculated that an efficient self-cleaving deoxyribozyme would be useful for cleaving multimeric ssDNA products that are generated by rolling-circle amplification (RCA). RCA generates concatemer DNA products since DNA polymerase is using a circular DNA template (14,15). Whereas most applications involving RCA exploit its ability to amplify weak biochemical signals in various diagnostic systems (16,17), a self-cleaving deoxyribozyme would permit the concatemers to be resolved into unit-length DNA products.
Materials and methods
Rolling circle amplification (RCA)
A 10 μL or 2 μL aliquot of 1 μM circular DNA template (prepared by CircLigase [Epicentre Biotechnologies, Madison, WI, USA] according to the manufacturer’s directions) was combined with an equivalent amount (in moles) of primer in a total of 50 μL containing 40 mM Tris-HCl (pH 7.5 at 23°C, 50 mM KCl, 10 mM MgCl2, 5 mM (NH4)2SO4, and 4 mM DTT. To ensure the binding of the primer to the template, an annealing procedure was performed by stepwise 2-min incubations at 80°C, 60°C, 45°C, and 23°C. After annealing, 1 μL of 10 mM dNTPs, 1 μL of 10 mg/mL BSA, and 2 μL of 10 unit/μL Phi 29 DNA polymerase (New England BioLabs, Ipswich, MA, USA) were added to initiate DNA synthesis. The reaction was incubated at 30°C for 4 h and stopped by inactivating the enzyme at 65°C for 10 min. The products were precipitated by using 2.5 volumes of 100% ethanol, centrifuged to recover the DNA pellet, and the DNA was resuspended in a 50 μL solution containing 50 mM HEPES (pH 7.0 at 23°C) and 100 mM NaCl.
Self-hydrolyzing deoxyribozyme reactions
The single-stranded DNA concatemers from RCA were allowed to fold by 2-min stepwise incubations at 80°C, 60°C, 45°C, and 37°C. At 37°C, the self-cleavage reaction was initiated by mixing the above 50 μL solution with an additional 50 μL containing 50 mM HEPES (pH 7.0 at 23°C), 100 mM NaCl, and 4 mM ZnCl2. At different time points (2 min, 5 min, 15 min, 30 min, 1h, and 2h), a 10 μL aliquot was removed and mixed with 10 μL of stop buffer containing 95% formamide and 20 mM EDTA. Products were separated by 8% PAGE or 1.5% agarose under denaturing or non-denaturing conditions as indicated for each experiment. Bands were visualized with SYBR Gold nucleic acid gel stain (Invitrogen, Grand Island, NY, USA), and imaged by ultraviolet transillumination.
Purification of ss100 DNA ladder products
The ten shortest ss100 DNA ladder products (ranging from 100 to 1000 nucleotides) were separated by denaturing (8 M urea) 8% polyacrylamide gel electrophoresis (PAGE). Individual bands were visualized by UV shadowing, excised from the gel, and were eluted by crush-soaking overnight in a single tube containing 10 mM Tris-HCl (pH 7.5 at 23°C), 200 mM NaCl, and 1 mM EDTA. DNA was recovered from solution by the addition of 2.5 volumes of 100% ethanol followed by centrifugation. The resulting DNA pellet was resuspended in deionized H2O and samples were used for the electrophoresis mobility assays depicted in Fig. 4.
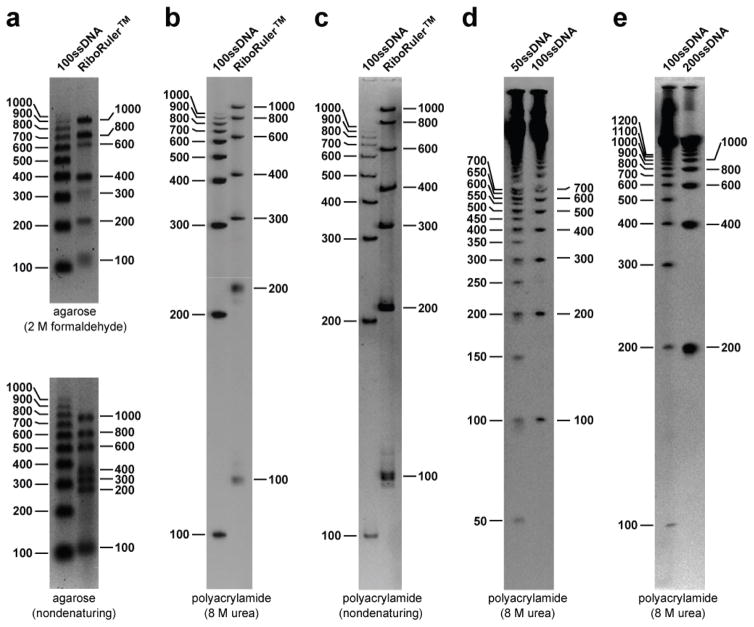
Figure 4. Comparison of ssDNA and ssRNA ladders by gel electrophoresis.
(a) Agarose gel separation of ss100 DNA Ladder and RiboRuler samples using denaturing (2 M formaldehyde) (top) or non-denaturing (bottom) 1.5% agarose gel electrophoresis. For these assays, and for those in b and c, the first ten bands of the ss100 DNA ladder (100–1000 bases long) were purified from the larger RCA products by PAGE before use. (b) Denaturing (8 M urea) and (c) non-denaturing 8% PAGE separation of ss100 DNA Ladder and RiboRuler samples. (d) Comparison of ss50 DNA Ladder and ss100 DNA Ladder by denaturing 8% PAGE. (e) Comparison of ss100 DNA Ladder and ss200 DNA Ladder by denaturing 8% PAGE.
Oligonucleotides
A list of oligonucleotides used in this study is provided below. Underlined sequences in the template strands encode the I-R3 deoxyribozyme. ss50, ss100, ss200 refer to the template DNAs used to generate single-stranded DNA ladders of the increment lengths indicated.
-
ss50 DNA Template
5′-pTAGGTAACGCTTCAACGTCACATTCTGTGACAGCTCAACTACGTTACTTG
-
ss50 DNA primer
5′-AGCGTTACCTACAAGTAACGTA
-
ss100 DNA Template
5′-pCTTGACTGCTTATGAGCATGGTGTATATGTGCCGAATTAGGTAACGCTTCAACGTCACATTCTGTGACAGCTCAACTACGTTACTTGGTCTGCAATGATA
-
ss100 DNA primer
5′-AGCGTTACCTAATTCG
-
ss200 DNA Template
5′-pCTTGACTGCTTATGAGCATGGTGTATATGTGCCGAATTAGGTAACGCTTCAACGTCACATTCTGTGACAGCTCAACTACGTTACTTGGTCTGCAATGATAGAATGTGGTATTCCTAAATCTCAACTGATGAATCTTTCTACCTGTAATAATGTTGTTCCGTTAGTTCGTATGATTAACGTAGATATCTCTCCTCAGCATA
-
ss200 DNA primer
5′-AGCGTTACCTAATTCG
Results and discussion
To demonstrate the activity of a self-cleaving deoxyribozyme, we sought to prepare a collection of ssDNAs of defined sequence and length, wherein RCA amplification products ranged from a single-unit DNA (100 nucleotides) to greater than 10 unit DNA repeats in the concatemeric sequence (Figure 2). We reasoned that such a range of DNA products might be useful as ssDNA size markers (DNA ladders) for gel electrophoresis applications. Our studies were initiated by preparing a 100-nucleotide circular DNA template for RCA. This was generated from a synthetic DNA template prepared by solid-phase chemical synthesis. The synthetic DNA template includes a 44-nucleotide sequence complementary to deoxyribozyme I-R3 along with 56 randomly-chosen nucleotides. The synthetic DNA was ligated to form a ssDNA circle by using CircLigase, a protein enzyme that efficiently couples a linear DNA carrying both 5′ phosphate and 3′ hydroxyl termini (Figure 2, i) (18).
Figure 2. Scheme for producing an ssDNA ladder using RCA and a self-hydrolyzing deoxyribozyme.
Synthetic linear DNAs carrying a complement of a self-hydrolyzing deoxyribozyme are (i) treated with CircLigase to create circular ssDNAs. The resulting DNA templates are (ii) incubated with DNA primer and Phi 29 DNA polymerase to generate concatemeric ssDNA products. These products are (iii) incubated in the presence of Zn2+ to promote cleavage of the deoxyribozymes. Partial digest of the deoxyribozymes (~single-hit kinetics) yields a variety of ssDNA lengths that differ in size by the unit-length (1X) of the template DNA, which is evident upon (iv) separation of the products by gel electrophoresis.
Upon incubation of this circular DNA template with Phi 29 DNA polymerase and complementary DNA primer, a single-stranded concatemer consisting of multiple linear copies of the sequence complementary to the circular template is produced (Figure 2, ii). At the junction of each DNA repeat resides the sequence corresponding to the I-R3 class I self-hydrolyzing deoxyribozyme. However, the deoxyribozymes do not cleave until they are exposed to the conditions needed for robust self-processing (50 mM HEPES [pH 7.0 at 23°C], 100 mM NaCl, 2 mM ZnCl2) (Figure 2, iii). By halting the reaction before all deoxyribozymes have cleaved, a mixture of products representing a range of unit-length DNAs is generated. This mixture of deoxyribozyme cleavage products can serve as an ssDNA ladder when separated by gel electrophoresis (Figure 2, iv) or by other methods that can separate large ssDNAs.
The distribution of products generated by implementing our RCA/self-cleaving deoxyribozyme scheme was examined by denaturing (8 M urea) 8% polyacrylamide gel electrophoresis (PAGE) (Figure 3). The smallest ten major bands of the sample (spanning from 100 to 1000 nucleotides) are well resolved, whereas larger product bands are less-well resolved (Figure 3a). Initially, we also observed a few unanticipated bands (Figure 3a, asterisks), perhaps caused by the presence of excess circular DNA templates interacting with amplification products, these are eliminated when the circular ssDNA template concentration is reduced from 200 to 40 nM during the RCA amplification (Figure 3b). Importantly, this reduction in template concentration does not compromise the yield of RCA products. Thus, the RCA reaction followed by a short incubation under deoxyribozyme cleavage conditions permit the generation of an ssDNA ladder of 100-nucleotide increments (termed ss100 DNA Ladder) that is free of undesired bands (Figure 3b). It should be noted our system can permit bias toward production of short or long concatemers, respectively, simply by increasing or decreasing catalysis time. In our hands, an incubation time of approximately 30 minutes was sufficient to yield near equal intensities for the ten bands ranging in size from 100 to 1000 nucleotides.
Figure 3. Gel electrophoresis of the 100mer ssDNA ladder.
(a) PAGE separation of the products of RCA subjected to incomplete deoxyribozyme self-cleavage. M designates a marker lane containing a mixture of linear and circular 100mer template ssDNAs. C-100 designates the circular 100mer DNA template. Asterisks identify bands possibly corresponding to circular template DNAs interacting with RCA products. Bands were visualized by staining with SYBR Gold. (b) PAGE separation of RCA and deoxyribozyme cleavage products generated using 40 nM instead of 200 nM of the template DNA concentration used in a. Annotations are as described in a.
When seeking size markers for ssDNAs, some researchers use denaturing conditions to create ssDNAs from double-stranded DNAs (dsDNAs) of known length. However, incomplete denaturation can cause confusion since ssDNA and dsDNA have different electrophoretic mobilities. We and others have occasionally resorted to using single-stranded RNAs as surrogates for ssDNA size markers, but again, the difference in mobility between DNA and RNA can cause confusion.
To illustrate this latter effect, we compared the electrophoretic mobilities of the ss100 DNA Ladder constituents with ssRNAs present in the commercially available RNA marker preparation called RiboRulerTM (Fermentas Inc., Lafayette, CO, USA). Electrophoretic separation of ss100 DNA Ladder and RiboRuler nucleic acids in adjacent lanes of either denaturing or non-denaturing agarose gels revealed substantial differences in the mobilities of bands (Figure 4a). For example, the DNAs in the ss100 DNA Ladder generated consistent banding patterns, whereas the RNAs in the RiboRuler sample exhibit some differences between denaturing and non-denaturing conditions, likely due to the formation of strong RNA structures formed by particular RNA sequences. Also, all the DNA and RNA molecules of equal size do not co-migrate, which highlights the disadvantages of using RNA markers as surrogates for ssDNA. Likewise, differences in gel mobility between these DNAs and RNAs are also observed when the two samples are separated by denaturing (Figure 4b) and non-denaturing (Figure 4c) PAGE.
The method used to produce ss100 DNA Ladder can be used to produce markers of any unit size increment simply by varying the number of nucleotides in the template DNA. For example, the addition of six nucleotides to the 44 nucleotides of the I-R3 deoxyribozyme complementary sequence yielded 50-nucleotide unit-length ssDNA products (ss50 DNA Ladder) (Figure 4d) whereas the addition of 156 nucleotides yielded ssDNA markers with 200-nucleotide increments (ss200 DNA Ladder) (Figure 4e).
Thus, our combined RCA and deoxyribozyme scheme allows for the production of any ssDNA markers with increments of ~50 nucleotides or larger. Furthermore, ssDNA markers produced by this method can be easily internally- or 5′-radiolabeled using standard methods. For example, radiolabeling by using γ-32P[ATP] and polynucleotide kinase can be carried out after removal of the 5′ phosphate group generated by deoxyribozyme hydrolysis. This makes possible the production of ssDNAs for use as markers that can overcome the problems of structure formation and altered mobility observed with some existing RNA markers. Moreover, since DNA is more stable than RNA, ssDNA markers will have a storage time that is far greater than RNAs. Samples of ss100 DNA Ladder can be obtained for evaluation or application from the Coli Genetics Stock Center at Yale University (http://cgsc.biology.yale.edu/).
In summary, we have developed a simple and effective method to produce ssDNAs of defined sequence and length from engineered circular DNA templates. This approach permits the efficient synthesis of DNAs that can be much longer and carry less chemical damage than those prepared by existing solid-phase DNA synthesis methods. In the current study, we demonstrate the use of such ssDNA products as markers for gel electrophoresis applications. Markers of this type could be useful when conducting experiments on natural ssDNAs (e.g., bacteriophage genomes) or on cDNA products made from natural RNAs. Additional applications involving complete digestion with a deoxyribozyme should permit the production of uniform-length sequence specific ssDNAs for other applications. Moreover, one could envision the incorporation of deoxyribozymes or DNA aptamers with other functions that would yield multifunctional DNA constructs that are produced by RCA.
Acknowledgments
We thank the Breaker laboratory for helpful discussions. This work was supported by grants from DARPA and the NIH (GM022778). Research in the Breaker laboratory is also supported by the Howard Hughes Medical Institute.
Footnotes
Competing Financial Interests
The authors have filed for intellectual property protection on aspects of this work.
References
- 1.Breaker RR. DNA enzymes. Nat Biotechnol. 1997;15:427–431. doi: 10.1038/nbt0597-427. [DOI] [PubMed] [Google Scholar]
- 2.Schlosser K, Li Y. Biologically inspired synthetic enzymes made from DNA. Chem Biol. 2009;16:311–322. doi: 10.1016/j.chembiol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Silverman SK. Deoxyribozymes: selection design and serendipity in the development of DNA catalysts. Acc Chem Res. 2009;42:1521–1531. doi: 10.1021/ar900052y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd AV, Fuery CJ, Impey HL, Applegate TL, Haughton MA. DzyNA-PCR: use of DNAzymes to detect and quantify nucleic acid sequences in a real-time fluorescent format. Clin Chem. 2000;46:625–630. [PubMed] [Google Scholar]
- 5.Liu J, Lu Y. Fluorescent DNAzyme biosensors for metal ions based on catalytic molecular beacons. Methods Mol Biol. 2006;335:275–288. doi: 10.1385/1-59745-069-3:275. [DOI] [PubMed] [Google Scholar]
- 6.Silverman SK. Catalytic DNA (deoxyribozymes) for synthetic applications – current abilities and future prospects. Chem Commun. 2008;14:3467–3485. doi: 10.1039/b807292m. [DOI] [PubMed] [Google Scholar]
- 7.Carmi N, Shultz LA, Breaker RR. In vitro selection of self-cleaving DNAs. Chem Biol. 1996;3:1039–1046. doi: 10.1016/s1074-5521(96)90170-2. [DOI] [PubMed] [Google Scholar]
- 8.Sheppard TL, Ordoukhanian P, Joyce GF. A DNA enzyme with N-glycosylase activity. Proc Natl Acad Sci USA. 2000;97:7802–7807. doi: 10.1073/pnas.97.14.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra M, Sachdeva A, Silverman SK. DNA-catalyzed sequence-specific hydrolysis of DNA. Nat Chem Biol. 2009;5:718–720. doi: 10.1038/nchembio.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Y, Wehrmann RJ, Ibrahim NA, Silverman SK. Establishing broad generality of DNA catalysts for site-specific hydrolysis of single-stranded DNA. Nucleic Acids Res. 2012;40:1778–1786. doi: 10.1093/nar/gkr860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao Y, Chandra M, Silverman SK. Functional compromises among pH tolerance, site specificity, and sequence tolerance for a DNA-hydrolyzing deoxyribozyme. Biochemistry. 2010;49:9630–9637. doi: 10.1021/bi1013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dokukin V, Silverman SK. Lanthanide ions as required cofactors for DNA catalysts. Chem Sci. 2012;3:1707–1714. doi: 10.1039/C2SC01067D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu H, Furukawa K, Weinberg Z, Berenson DF, Breaker RR. Small, high-speed DNAs that hydrolyze DNA. doi: 10.1021/ja403585e. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, Daubendiek SL, Zillman MA, Ryan K, Kool ET. Rolling circle DNA synthesis: small circular oligonucleotides as efficient templates for DNA polymerases. J Am Chem Soc. 1996;118:1587–1594. doi: 10.1021/ja952786k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lizardi PM, Huang X, Zhu Z, Bray-Ward P, Thomas DC, Ward DC. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat Genet. 1998;19:225–232. doi: 10.1038/898. [DOI] [PubMed] [Google Scholar]
- 16.Stougard M, Juul S, Andersen FF, Knudsen BR. Strategies for highly sensitive biomarker detection by rolling circle amplification of signals from nucleic acid composed sensors. Integr Biol. 2011;3:982–992. doi: 10.1039/c1ib00049g. [DOI] [PubMed] [Google Scholar]
- 17.Asiello PJ, Baeumner A. Miniaturized isothermal nucleic acid amplification, a review. Lab Chip. 2011;11:1420–1430. doi: 10.1039/c0lc00666a. [DOI] [PubMed] [Google Scholar]
- 18.Blondal T, Thorisdottir A, Unnsteinsdottir U, Hjorleifsdottir S, Ævarsson A, Ernstsson S, Fridjonsson OH, Skirnisdottir S, et al. Isolation and characterization of a thermostable RNA ligase 1 from a Thermus scotoductus bacteriophage TS2126 with good single-stranded DNA ligation properties. Nucleic Acids Res. 2005;33:135–142. doi: 10.1093/nar/gki149. [DOI] [PMC free article] [PubMed] [Google Scholar]