Abstract
Objective
This narrative review examines six important non-nutritive substances in breastmilk, many of which were thought to have little to no biological significance. The overall objective of this narrative review is to provide background on key bioactive factors in breastmilk believed to have an effect on infant outcomes (growth and body composition).
Methods
The evidence for the effects of the following six bioactive compounds in breastmilk on infant growth outcomes are reviewed: insulin, leptin, adiponectin, ghrelin, interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α).
Results
The existing literature on the effects of breastmilk insulin, ghrelin, IL-6 and TNF-α and their associations with infant growth and adiposity is sparse. Of the bioactive compounds reviewed, leptin and adiponectin are the most researched. Data reveals that breastmilk adiponectin has negative associations with growth in infancy.
Conclusions
There is a need for innovative, well-designed studies to improve causal inference and advance our understanding in the effects of breastmilk and its components on offspring growth and body composition. The recommendations provided, along with careful consideration of both known and unknown factors that affect breastmilk composition, will help improve, standardize and ultimately advance this emergent field.
Keywords: breastfeeding, prevention, body composition, breastmilk
INTRODUCTION
“Breastfeeding is an unequalled way of providing ideal food for the healthy growth and development of infants” (World Health Organization) (1) and “one of the most highly effective preventive measures a mother can make in protecting the health of her infant and herself is to breastfeed” (US Surgeon General, January 2011). Declarative, definitive statements such as these carry much weight in setting both national and world health policy for breastfeeding as a major protectorate against obesity. Unanimous support for breastfeeding comes from the Center for Disease Control and Prevention (2), the World Health Organization (3), the American College of Obstetricians and Gynecologists (4) and the American Academy of Pediatrics (5), with Healthy People 2020 going as far as to declare breastfeeding of national importance (6).
One of the most important purported benefits of breastfeeding is obesity prevention. The case for breastfeeding as a means to reduce obesity risk is built upon seven systematic review-meta analyses from 81 studies spanning four decades (1970 through 2010) (3, 7–12). A recent review reported a reduction of 12% to 24% (odds ratio for becoming overweight/obese 0.76 to 0.96) in the prevalence of overweight/obesity in children who were breastfed compared to those who were not breastfed (12), supporting an earlier report by the American Academy of Pediatrics (5).
The broad support by national organizations for the wide-ranging health benefits of breastfeeding, coupled with specific claims about the benefits of breastfeeding for obesity prevention, can make it appear as though breastfeeding’s effects on offspring overweight/obesity are resolved (13). They are not. Several researchers have recently challenged claims that breastfeeding is an effective tool for reducing obesity (14–16). A common critique of the literature is the frequent failure of studies to control for important confounders, both known (maternal body mass index (BMI), ethnicity, socioeconomic status) and unknown (15). Many studies on the effects of breastfeeding are fundamentally limited in their ability to infer causation primarily because of their associational study design (17, 18); while others have questioned the methods used for the inclusion or exclusion of data that are analyzed (19). Furthermore, even when a a randomized design is used to isolate the effect of a component of breastmillk on infant growth, it is important to remember that this component is just one component among many causally related to infant outcomes, and that there are likely other causal pathways, unrelated to the isolated breastmilk component, leading to infant growth outcomes (20).
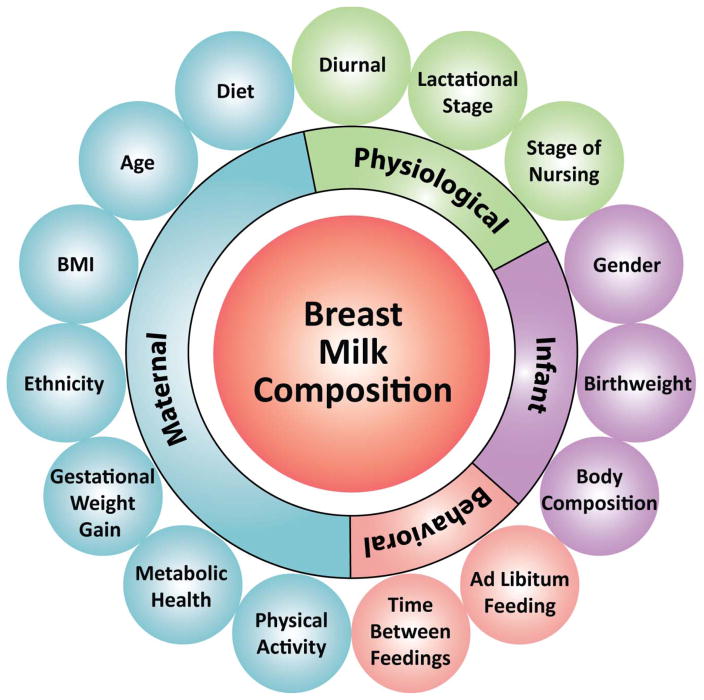
Given these concerns, some have concluded that there is no causal link between breastfeeding and childhood overweight/obesity and/or at best, breastfeeding provides a modest degree of protection (3, 16, 18, 21–23). Criticism of the claim that breastfeeding reduces the risk of offspring obesity often invites contentious debate and ad-hominem vitriol. This may be due, in part, to a failure to fully appreciate the compositional fluidity of breastmilk, its heterogeneity and the vast constellation of factors that influence its composition, making it difficult to isolate any single factor in breastmilk as having a causal effect on infant outcomes (Figure 1).
Figure 1.
Maternal, physiological and behavioral factors that impact breastmilk composition.
Breastmilk is not a uniform unvarying constant factory-made product; rather, it is a biological product produced by women with markedly varying genotypes, phenotypes and diets. To add to the complexity, the composition of breastmilk is influenced by a myriad of maternal, infant and environmental factors. Some of these influences are better understood than others, including diurnal variations (fat concentration greatest mid-morning ~ 10:00 am (24)), stage of nursing (fore vs hind milk (25)), and lactational stage (colostrum, transitional and mature (25, 26)). The influence of other factors, including those factors which are the focus of this review - non-nutritive bioactive components, are just beginning to be understood (27), while many other factors remain unknown (28). The major challenge to causal claims about the effects of breastmilk and its specific components is that there are both known and unknown factors influencing maternal breastmilk composition, and these factors may, in turn, affect infant outcomes independent of their effect on breastmilk composition.
This narrative review delves into an emergent body of literature, examining six important non-nutritive substances in breastmilk. Many of these substances were, until recently, believed to be absent or of little biological significance. The intent of this narrative review is to provide a background on key bioactive factors in breastmilk believed to have an effect on infant outcomes (growth and body composition), review their importance in human health, important evidence of their effects, as well as discuss methodological challenges researchers face in studying the effects of breastmilk and its constituent components on infant outcomes—namely the inability to randomly assign human infants to breastmilk.
As an operational definition of these substances, we will refer to hormones (insulin, leptin, adiponectin, ghrelin) and cytokines (IL-6, TNF-α) as “bioactive compounds” (29). In reviewing each of these components, we first provide a brief synopsis of their role in human health and metabolism as a foundation for understanding why their presence in breastmilk, insofar as each compound is bioavailable to the infant, has the potential to affect developmental and metabolic health outcomes in infants. After discussing the presence and heterogeneity of these substances in breastmilk, we will review evidence, when available, that these bioactive components are associated with offspring growth and adiposity. This review is not intended to be a systematic review of all articles on the six selected bioactive compounds, factors that modulate their presence, or their effects on infant adiposity. Nor do we review other potentially important bioactive compounds including neuropeptides, anti-inflammatory agents, proteins, vitamins, polyphenols, macronutrients, immunoglobulins, chemokines, growth factors, oligosaccharides and glycans, all of which have been shown to be present in breastmilk in varying concentrations (30, 31). We conclude the review with six suggestions to improve and standardize a rapidly growing research area with the potential to make significant contributions to infant and child health.
1. BIOACTIVE COMPONENTS IN BREASTMILK
Is it biologically plausible that the following bioactive compounds can be absorbed into the infant’s circulation? Breastmilk molecules can get into the infant’s circulation via six potential mechanisms that have been previously identified (32, 33).
First, breastmilk, especially colostrum and early milk, has high concentrations of α1-antitrypsin, a known protease inhibitor (34, 35). Second, the gastrointestinal tract of the infant favors the absorption of bioactive compounds and proteins in breastmilk due to high gut pH (35) (36, 37). Third, immaturity of pancreatic enzymatic activity favors absorption of bioactive factors by resisting proteolysis (35). Fourth, there are known adipokine gastrointestinal tract receptors for specific bioactive compounds (38, 39). Fifth, an important non-digestive pathway for the absorption of peptides and bioactive compounds is paracellular diffusion, which plays a major role in infancy (32). Sixth, breastmilk molecules, such as microRNAs and others, can be transferred into the infant’s circulation via exosomes or live breastmilk cells (33, 40). We believe these six factors collectively demonstrate the biological and physiological plausibility that non-nutritive bioactive compounds can enter an infant’s circulation and exert meaningful outcomes on the health of an infant, thereby providing a working, albeit theoretical framework for future research on bioactive compounds in breastmilk.
Insulin
Insulin plays an essential role in blood glucose homeostasis and liver metabolism and its presence in breastmilk has been known for some time (41, 42). It has been shown that insulin concentrations range between 0 and 11.7 μg/l in breast milk samples collected 3–7 days after delivery and between 0 and 14 μg/l in samples collected 3 months after delivery (43). In another sample of breastmilk collected from non-diabetic healthy mothers at 6-weeks post-partum, insulin concentrations ranged from 4.5 ± 7.6 μIU/mL in normal-weight mothers and 30.1 ± 56.3 μIU/mL in overweight/obese mothers (44). Recent research has sought to examine sources of variation in insulin levels in breastmilk, including the effects of gestational age (45), metabolic abnormalities (46) and maternal body mass (47). Insulin concentrations in breastmilk do not appear to vary by gestational age (45), but may be positively associated with pregravid BMI and hyperglycemia, and negatively associated with insulin sensitivity (46). Regarding maternal BMI, Andreas et al. (2014), in a systematic review of the effect of maternal BMI on hormones in breastmilk, identified four studies that examined its effect on breastmilk insulin (48). Two studies reported a positive correlation between maternal BMI and breastmilk insulin (44, 46), while two studies reported no correlation (45, 47). While there is significant variation in breastmilk both over time and between mothers and some of this variation is associated with metabolic health, the evidence for an association between maternal BMI and breastmilk insulin remains mixed.
Little published data exist examining breastmilk insulin levels and the associations with infant outcomes in healthy non-diseased mothers. A recent study reported negative associations between breastmilk insulin concentrations and body weight, weight-for-length z-score, BMI-for-age z-score and total lean mass in infants from both normal weight and obese mothers at 1-month of age (47).
Leptin
Since its initial discovery, leptin’s role in the long-term regulation of human energy balance obesity has been extensively documented (49). Recent research has indicated that leptin may additionally influence the short-term regulation of food intake, as leptin and leptin receptor proteins are now known to exist in the human stomach (50). Given the influence of leptin on both long- and short-term regulation of energy balance and food intake, its production in the human placenta and mammary epithelial cells and its presence in breastmilk, it has been of interest for its possible effects on offspring energy-intake and adiposity (51).
Immunoreactive leptin has been detected in breastmilk (52, 53), but with significant variation in measured concentrations ranging from 1.4 ± 0.2 μg/l in one early study (52) to 10.1 ± 2.6 μg/l in another early study (53). Subsequent research continues to observe significant variation in leptin concentration, due in part to breastmilk fractions used in the analysis and sample treatments (54). The preponderance of the literature reveals clear evidence that breastmilk leptin is positively correlated with maternal BMI (47, 55). Andreas et al. (2014), in their systematic review of the literature, reported that ten of the fifteen studies found a positive association between maternal BMI and leptin concentration in breastmilk at all measured time points, with no studies finding a negative correlation. Of all the bioactive compounds currently identified in breastmilk, leptin by far provides the clearest indication that maternal BMI is associated (positively) with leptin breastmilk concentrations.
Several studies have reported inverse associations between breastmilk leptin and infant weight gain (ΔBMI, Δweight gain, BMI-for-age z-score) and length (weight-for-length z-score) in the first two years of life (47, 56–58). Others have reported no association between breastmilk leptin and growth (59–61). A recent study has brought clarity and reconciliation to these disparate findings. In a sample of 152-breastfeeding mothers (exclusive and partial) breastmilk leptin was obtained at 6-weeks and 4-months post-partum with infant outcomes obtained at 4-, 12- and 24-months (62). Breastmilk leptin at 6-weeks was not associated with any infant clinical outcomes (growth or body composition). However, breastmilk leptin at 4-months was inversely associated with infant body weight (adjusted β coefficients [95% CI]: -604.96 g [−1166.2; −44.7], P=0.037) and lean mass (−400.95 g [−777.6; −24.3], P=0.039) at 4-months, but not at 12 or 24-months. At this time the data is far from conclusive in determining the exact role leptin plays on early growth and body composition.
Adiponectin
Adiponectin is synthesized predominantly by adipose tissue, with evidence also demonstrating synthesis in other tissues such as salivary gland and mammary epithelial cells, and is one of the most abundant proteins in human circulation (plasma concentration is 5–10 μg/mL) (63–65). Since its initial discovery in 1995, adiponectin has become a major research focus due to its anti-diabetic, anti-inflammatory and anti-atherogenic effects (66). Adiponectin plays an important role in regulating energy metabolism, acting to stimulate glucose uptake and fatty acid oxidation, inhibit liver gluconeogenesis and improve insulin sensitivity (67). Recent research suggests that adiponectin may also influence food intake and energy expenditure, as indicated by evidence that adiponectin is present in human cerebrospinal fluid and that adiponectin receptors are expressed in the human hypothalamus (68).
Given the role of adiponectin in energy metabolism, the discovery of its presence in breastmilk has led to much interest in its potential influence on offspring growth and adiposity. Breastmilk adiponectin concentrations vary considerably across the lactational stage with high inter-individual variation. The concentration of adiponectin in breastmilk is highest in colostrum, after which adiponectin levels decrease (46, 69). In samples of breastmilk collected at 1–7 days and at 3-months post-partum, median (25th, 75th percentiles) adiponectin concentration levels were 50.0 ng/mL (21.9, 104.6 ng/mL) in samples collected 1–7 days and 12.3 ng/mL (9.9, 17.2 ng/mL) at 3-months (46). In an early study of breastmilk at 6-weeks postpartum, adiponectin concentrations ranged from 0.8 ng/mL to 110.0 ng/mL (70) and in a separate study, adiponectin concentrations in breastmilk samples collected 1–180 days postpartum ranged from 4.0 ng/mL to 105.7 ng/mL (71). Variation in breastmilk adiponectin has been associated with maternal factors including parity, length of gestation, smoking, race/ethnicity and type of delivery. Breastmilk adiponectin levels have been positively associated with length of gestation, and inversely associated with parity (46). Additionally, smoking may impact breastmilk adiponectin given higher concentrations have been reported in mothers who do not smoke compared to those that do smoke (60). Furthermore, maternal ethnicity may also influence breastmilk adiponectin levels. Martin et al. (2006) found median breastmilk adiponectin concentrations to be significantly lower at 1-month postpartum among Mexican mothers compared with non-Hispanic white women (69). With respect to maternal BMI, Andreas et al. (2014) identified nine studies examining the effect of maternal BMI on breastmilk adiponectin, with only two studies reporting a positive correlation between maternal BMI and breastmilk adiponectin, while the remaining seven studies reported no association (48). As such, evidence for an association between maternal BMI and breastmilk adiponectin remains inconclusive.
Adiponectin survives digestion with transporters detected in the gut, favoring the bioavailability of adiponectin (60, 72). In Western developed countries clear and negative associations are reported between higher breastmilk adiponectin concentrations and lower weight-for-age z-scores, weight-for-length z-scores and body weight in the first 4-months of life (72–74). Brunner et al. (2015) have extended these negative associations to show higher adiponectin levels are associated with lower lean mass when using skinfolds (62). Continued follow-up out past one year and up to two years of age show a reversal, where exposure to higher breastmilk adiponectin levels are associated with an increased weight-for-age z-score and weight-for-length z-scores (60, 75). Interestingly, this positive association is extended to increased percent body fat and total fat mass (62). Furthermore, it has been shown that there is an increased risk for being overweight at two years of age with increasing breastmilk adiponectin concentrations (60). In societies where scarcity of food (Ceba, Philippines) exists and mothers exhibit low BMI’s contrary findings have been reported in the first months of life (76). When breastmilk adiponectin is stratified into tertiles across the first two years of life, infants exposed to the greatest levels of adiponectin had the highest weight-for-age z-scores throughout the entire testing period (3-, 6-, 9-, 12-, 15-, 18-, 21- months). When comparing this study to others, it appears there is a threshold for adiponectin (~15 ng/mL), where concentrations not meeting this threshold result in a linear association between breastmilk adiponectin and infant growth in the first few months of life. It has been proposed that breastmilk adiponectin exhibits pleiotropic properties in the first two years of life based upon food availability and exert differentially between early infancy (< 6-months) and later in childhood (2-years) with the associations tied to maternal BMI (76).
Ghrelin
Ghrelin is an orexigenic hormone produced predominantly by the stomach. Its regulatory role in short-term food intake has been well established and, more recently, evidence suggests an important role for ghrelin in long-term regulation of energy homeostasis and body weight (77). Ghrelin acts both centrally and peripherally to influence appetite, food intake, gastric acid secretion and gut motility (77). Research suggests a role for ghrelin in glucose and lipid metabolism, demonstrating that exogenous ghrelin administration has an inhibitory effect on insulin secretion and fat oxidation and promotes fatty acid storage, resulting in elevated blood glucose levels and increased adiposity (77). In general, circulating ghrelin concentrations are inversely associated with adiposity and weight gain, suggesting that ghrelin also plays an important role in long-term regulation of energy balance and body weight (77).
Ghrelin is present in breastmilk and produced by the human mammary gland, mammary epithelial cells and placenta (78, 79). As such, the potential role of breastmilk ghrelin in infant feeding behaviors, growth and adiposity is of interest. Numerous studies have indicated that breastmilk ghrelin levels increase progressively from birth through at least 6-months postpartum. Levels of ghrelin in human colostrum range from 616 ± 78 pg/mL in one study (80) to 4181 ± 456 pg/mL in another study (81). Ilcol et al. (2007) demonstrated a 1.7-fold increase in ghrelin levels in transitional milk (collected 4–10 days after delivery) compared to colostrum and a 3.7-fold increase in mature milk (collected 91–180 days after delivery) compared to colostrum (82), an increase comparable to Aydin et al.’s (2008) finding of a 2-fold increase in ghrelin in breastmilk collected 25 days after delivery compared to colostrum collected 2 days after delivery (80). In addition to progressively increasing with the lactational stage, breastmilk ghrelin concentrations are observed to be significantly higher in fore vs. hind milk (83). Andreas et al. (2014) report of the three studies identified, none found a significant correlation between maternal BMI and breastmilk ghrelin levels (84–86). An additional study also reported no significant correlation between maternal BMI and ghrelin concentration in breastmilk (87). However, a recent study found an inverse association between maternal BMI and ghrelin concentration in breastmilk (88). Overall, the evidence for an association between breastmilk ghrelin and maternal factors, including maternal BMI, remains unclear.
Extant data linking breastmilk ghrelin and infant outcomes are especially sparse. In the four studies looking at breastmilk ghrelin, none specifically looked at infant outcomes.
IL-6
IL-6 is a multifunctional cytokine secreted by adipose tissue. Although IL-6 is secreted by many cell types, including immune cells and myocytes, approximately 30% of total IL-6 production is attributable to adipose tissue in non-acute inflammatory conditions (89). Mostly known as a pro-inflammatory cytokine, IL-6 is also involved in anti-inflammatory activities and plays a vital role in neuronal function and development (90). Several studies have found a positive association between obesity and IL-6 levels in humans, as well as a positive association between IL-6 and insulin resistance and type-2 diabetes (91).
The sheer breadth in effects of IL-6 observed in human development, immune function, and metabolic regulation makes its presence in breastmilk a potentially important contributor to health and weight-related infant outcomes. Several studies have measured IL-6 in breastmilk, with substantial variation between studies (92, 93). In one study, breastmilk IL-6 in thirty healthy mothers, ranged from 0.81 pg/mL to 306 pg/mL (94), while a second study reported the milk IL-6 concentration at 3.8 ± 5.3 pg/mL (47). In a review of immune markers in breastmilk, Agarwal et al. (2011) reported IL-6 concentration levels ranging from 7.3 to 81 pg/mL in colostrum to a mean concentration of 78 pg/mL at 3-months and 105 pg/mL at 6-months (95).
The number of studies in a healthy non-diseased population looking at associations between breastmilk IL-6 and infant growth and body composition, like much of the other bioactive compounds in this review, are limited. We are aware of one study, using dual energy X-ray absorptiometry to measure infant body composition, finding a negative association between breastmilk IL-6 and weight-for-length z-score, BMI-for-age z-score, total fat mass, percentage of fat and total lean mass in the infant at 1-month (47).
TNF-α
TNF-α, so named for its observed necrotizing effect on tumors, is a potent pro-inflammatory cytokine secreted by a variety of cell types including macrophages and adipocytes. In human adipose tissue, TNF-α is associated with reduced insulin-stimulated glucose disposal and decreased lipoprotein lipase activity (96). In humans, obesity is associated with a significant increase in the expression of TNF-α mRNA and increased secretion of the TNF- α protein.
TNF-α has been detected in breastmilk at sufficient levels to have effects on the development and functioning of the infant immune system (97). TNF-α is secreted by macrophages in the milk and the mammary endothelium, and its presence is thought to improve the immune defense components in the neonate lacking in TNF-α production (98, 99). Breastmilk accomplishes its immuno-protective effects without inflammation due the infrequency of inflammatory mediators and presence of many anti-inflammatory agents in the milk (100). Rudloff et al. (1992) was the first to measure TNF-α in breastmilk, using milk samples collected 24–48 hours post-delivery from healthy women between 18 and 35 years of age. The mean concentration of TNF-α was 620 ± 183 pg/mL and, as suggested by the authors’ calculations, an average newborn could be expected to consume 60 ng of TNF-α during the first two days of lactation. Meki et al. (2003) also measured TNF-α levels in colostrum and mature milk in 55 healthy, vaginally delivered infants, finding mean TNF-α levels in the colostrum to be 402.8 ± 29.7 and 178.3 ± 14.4 pg/mL in mature milk. In Agarwal’s et al.’s (2011) systematic review of immune markers in breastmilk, substantial variation in levels of TNF-α over time was observed, with levels decreasing from a high of a mean 620 pg/mL in colostrum (Rudloff, 1992) to less than a median level of 4.4 pg/mL in milk at 2–3 months (101)
Extant data linking breastmilk TNF-α and infant outcomes are limited. One of the only studies we are aware of (in healthy non-diseased infants) reported a negative association between breastmilk TNF-α and infant total lean mass (by dual energy X-ray absorptiometry) at 1-month of age (47).
2. ANIMAL VS. HUMAN EVIDENCE FOR BIOACTIVE COMPOUNDS AFFECTING OFFSPRING BODYWEIGHT AND ADIPOSITY
The Animal Evidence
The extant animal literature shows a clear and convincing role for the effects of bioactive compounds in breastmilk on growth and body composition in offspring. Elegantly designed cross-fostering animal experiments have demonstrated the effects of lactational programming as well as the effects of breastmilk leptin and insulin. Three murine studies using a cross-fostering approach reported that pups of lean/obesity resistant dams suckled by obese dams had increased body weight and adiposity (102–104). All three studies show clear increases in body weight, ranging from 2.3% to 34.6%, when non-obese pups were cross-fostered onto obese dams and, in the only study looking at offspring adiposity, a 16.8% increase in total fat pads (39.7±1.5 vs. 46.4±2.3; P≤0.05) was reported. All three studies evaluated the effects of bioactive compounds in maternal breastmilk (leptin and insulin). Interestingly, one study reported a 128% greater concentration in breastmilk insulin between lean and obese dams while finding no difference in breastmilk leptin (103). In contrast, Oben et al. (2010) reported a markedly large increase (600%) in breastmilk leptin between obese vs lean dams (~ 35,000 vs. ~ 5,000 pg/ml), as did the Reifsnyder et al. (2000) study, which reported a 330% increase in breastmilk leptin (4,300 vs. 1,000 pg/mL) between obese and lean dams. Collectively, these studies provide a mechanistic framework whereby maternal obesity engenders a metabolically poor breastmilk milieu that in turn creates an offspring with an obesogenic phenotype; in particular, via the positive association between maternal BMI and breastmilk leptin and insulin. The next step is to determine how elevated breastmilk leptin and insulin creates an obesogenic phenotype in the offspring.
In murine models, clear and convincing evidence demonstrate oral leptin administration to be biologically active and absorbed by the gut (105–107). This point is important to keep in mind when trying to understand how specific bioactive compounds in breastmilk significantly influence body weight and adiposity outcomes in offspring The observed effects of oral leptin administration led to the following hypothesis: “persistent and sustained exposure to leptin via breastmilk results in permanent hypothalamic alternations in the offspring, ultimately predisposing offspring to leptin resistance and an obesogenic phenotype” (102). Far from conclusive and currently speculative, animal work such as this shows a viable biological pathway by which leptin (via breastmilk) programs the offspring to a phenotype that is metabolically unhealthy and predisposes the offspring to an increased risk of obesity.
The Human Evidence
Our current understanding of the role for specific bioactive compounds in breastmilk and their effects on offspring adiposity in breastmilk is limited predominately by inadequate study design. Isolating the effects of specific bioactive compounds on offspring adiposity requires randomization; however, the use of randomized controlled trials in humans to study the effects of bioactive breastmilk components is unethical, improbable and impractical. The limited ability to randomize human subjects to breastmilk treatments makes causal inferences especially difficult, given the numerous maternal factors, both genetic and environmental, likely to influence the composition of breastmilk, many of which may also affect infant outcomes independent of their effects on breastmilk composition. As such, many infant outcomes co-vary with maternal outcomes, making it difficult to isolate the effect of these genetic, physiological, and sociological maternal factors from the specific effect of breastmilk, and particular breastmilk components, on infant outcomes. To date, a number of maternal factors have been purported to influence levels of bioactive components in breastmilk, one of the most notable being maternal BMI, which appears to be positively associated with levels of insulin and leptin in breastmilk. In addition, evidence suggests length of gestation, maternal smoking status, parity, race/ethnicity, and type of delivery (vaginal birth or via Cesarean section) all contribute to the variation of specific components in breastmilk. Despite being less well-studied, maternal age, metabolic health during pregnancy, stress, amount of sleep and postpartum weight change could also influence levels of bioactive components in a mother’s breastmilk. As such, the observed association between these breastmilk components and infant outcomes may be, at least in part, explained by the relationship between these maternal factors and infant outcomes. Due to the complex number of maternal factors potentially influencing both breastmilk composition and infant outcomes, it is impossible to match subjects based on all of these factors. Collectively, these issues make testing for causation difficult and onerous for investigators in the field and only highlight the need for innovative study designs.
Researchers from Germany used an ingenious study design that goes beyond (too common) associational studies that, despite the design falling short of true randomization, advances our understanding of breastmilk and infant outcomes (i.e. body weight) (108, 109). One-hundred and twelve diabetic mothers (type 1 diabetes, n=83; gestational diabetes, n=29) were studied prospectively to evaluate the influence of ingestion of either diabetic breastmilk or non-diabetic banked milk on their offspring’s body weight at 2-years of age. All mothers were encouraged to breastfeed for one month, but in the event they could not continue to nurse their child or chose to stop, they were provided non-diabetic banked milk. In the first study, all mothers were divided into tertiles based upon the volume of ingested diabetic breastmilk (1st <56, 2nd 57–124, 3rd ≥125 g/d) during the first week of life (109). This study revealed two interesting results. First, the volume of ingested non-diabetic banked milk was inversely (P=0.001) correlated with body weight at 2-years of age. Second, the number of children classified as overweight at 2-years of age increased as the consumption of diabetic breastmilk increased (1st n=5, 2nd n=11, 3rd n=14, P=0.05), with an odds ratio of [OR] 2.47, 95% CI 1.25–4.87. In the same population, the investigators examined the consumption of diabetic breastmilk starting at the 2nd week through the 4th week of life (108). After adjusting for the volume of diabetic breastmilk consumed in the first week of life, no associations between the consumption of diabetic breastmilk and either body weight or the incidence of being overweight at 2-years old were observed. Neither study looked at the compositional make-up of the breastmilk in either the diabetic breastmilk or the banked breastmilk. Though far from resolved, these studies show tantalizing evidence that the composition of the breastmilk is an important player in infant outcomes with the first week of life being especially “critical” for long-term programming.
3. RECOMMENDATIONS TO ADVANCE THE FIELD
Recommendations for advancing the field forward
Currently, the state of the literature is sparse, with study findings difficult to interpret and hard to compare. Many lack clarity and specifically defined study endpoints, have poorly described study designs and lack basic study controls (i.e. fasting or non-fasting, complete breast expression or partial). In an attempt to advance the field forward, we have identified six methodological limitations and offer recommendations for fixing them.
Lack in reporting of maternal BMI: Recording maternal BMI is crucial and important if the field is to understand a basic question: does maternal BMI influence the composition of breastmilk and what role, if any does it play on a specific bioactive compound.
Deficiency in standardized study protocols: The standardization of study protocols (i.e. time of day and the feeding of the day i.e. 1st, 2nd, 3rd), paying particular attention to the interval between the last feeding and the time to milk collection. Also, a priori stating the definition used for inclusion into the breastfeeding group (i.e. no formula for the first month of life, up to 6 ounces of formula a week for the first three-months etc). Other aspects related to standardization are documentation of how often the infant was fed at the breast vs. breastmilk given from the bottle and if fore or hind milk was collected. Many factors impact the compositional make up of breastmilk (Figure 1) with many now only being appreciated. Most studies vaguely describe milk collection as in the morning or give no description at all. Greater care and appreciation needs to be made when designing studies with a clear description given when writing the methods and in doing so allowing for replication studies, a bedrock for the scientific process.
Collection of breastmilk in different lactational stages: As reported in this review and the field in general the compositional make-up of milk is dependent upon the lactational stage the sample was taken (i.e. colostrum, early and mature milk). It is fairly established that colostrum is quite different from mature milk, thus study designs should ensure collection between subjects’s falls squarely within the desired stage and report the number of days post-delivery the sample was taken.
Inadequate description in how milk was collected, handled, stored and prepared for analyses: Investigators must provide a thorough description of how the breastmilk was collected, handled and stored (i.e. skimmed milk vs whole, frozen vs refrigerated). This is thought to play a significant role, albeit unknown in how this affects the particular assay used for determining specific bioactive compounds in the milk.
No control for maternal fasted-ness: Standardized maternal fasted-ness at the time of milk collection is important, just as it would be for example, in determining blood lipid levels (cholesterol, LDL, HDL) or diabetes risk (glucose and insulin). It is believed that the maternal diet plays a significant role in breastmilk composition, though little is known of the appearance, peak and disappearance of specific bioactive compounds in the milk.
Limited data on infant outcomes: The literature to date is practically void of infant outcomes, let alone growth and body composition outcomes and their associations with bioactive compounds in breastmilk. At minimum, length and body weight should be collected with each breastmilk collection and when possible, body composition (preferably by air-displacement plethysmography and/or DXA).
Increased implementation of these recommendations, along with a careful consideration of both known and unknown factors identified in Figure 1, will significantly advance an exciting area of research important to health and well-being of future generations.
What is already known about the subject?
Early feeding practices during infancy influence future disease risk.
Breastmilk contains a multitude of bioactive non-nutritive compounds.
The literature on the effects of bioactive compounds in breastmilk and their associations with infant growth and adiposity is sparse.
What does this study add?
This review provided an in-depth analysis of the available evidence looking at the associations between breastmilk insulin, leptin, adiponectin, ghrelin, IL-6 and TNF-α and infant growth and body composition.
In Western societies, adiponectin has negative associations with growth early in infancy.
Further, adiponectin may exhibit pleiotropic properties based upon maternal BMI and the gender of the infant with food scarcity (developed vs. under-developed societies) also playing a potential role.
Acknowledgments
The authors would like to extend appreciation and gratitude to Mikako Kawai PhD from the University of Alabama at Birmingham for designing figures 1 and 2 and for Katy Hammond in helping perform aspects of the literature review.
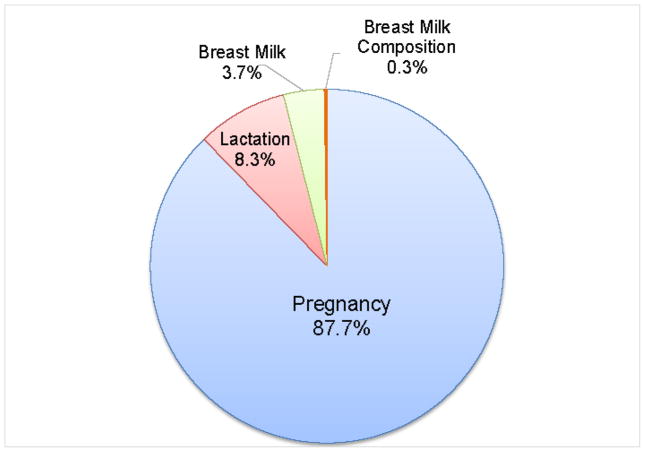
Figure 2.
Percentage of the total number of articles reported in PubMed when keywords of pregnancy, lactation, breastmilk and breastmilk composition are used. This figure was adapted from Hinde 2015 (110).
FUNDING
This work was supported in part by the CMRI Metabolic Research Program and the CMRI Chickasaw Nation Endowed Research Chair in Pediatric Diabetes. Camille R Schneider is supported by a NORC T32 grant funded by NHLBI grant number T32HL105349.
References
- 1.Organization WH. Global strategy on infant and young child feeding. Geneva: 2002. [Google Scholar]
- 2.Breastfeeding report card United States/2014. Atlanta, GA: 2014. p. 8. [Google Scholar]
- 3.Horta BL, Victora CG. Long-term effects of breastfeeding: A systematic review. 2013. [Google Scholar]
- 4.ACOG Committee Opinion No. 361: Breastfeeding: maternal and infant aspects. Obstetrics and gynecology. 2007;109(2 Pt 1):479–80. doi: 10.1097/00006250-200702000-00064. [DOI] [PubMed] [Google Scholar]
- 5.Section on B. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 6.Services USDoHaH, editor. Healthy people 2020. http://healthypeople.gov/2020/2010.
- 7.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162(5):397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 8.Horta B, Bahl R, Martines J, Victora C. Evidence of the long-term effects of breastfeeding: Systematic reviews and meta-analysis. Geneva, Switzerland: 2007. [Google Scholar]
- 9.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97(12):1019–26. doi: 10.1136/archdischild-2012-302263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 11.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity--a systematic review. Int J Obes Relat Metab Disord. 2004;28(10):1247–56. doi: 10.1038/sj.ijo.0802758. [DOI] [PubMed] [Google Scholar]
- 12.Woo JG, Martin LJ. Does Breastfeeding Protect Against Childhood Obesity? Moving Beyond Observational Evidence. Curr Obes Rep. 2015 doi: 10.1007/s13679-015-0148-9.. [DOI] [PubMed] [Google Scholar]
- 13.Obesity WHTFoC. Solving the challenge of childhood obesity within a generation. Washington DC: 2010. [Google Scholar]
- 14.Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86(6):1717–21. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- 15.Colen CG, Ramey DM. Is breast truly best? Estimating the effects of breastfeeding on long-term child health and wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109C:55–65. doi: 10.1016/j.socscimed.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casazza K, Fernandez JR, Allison DB. Modest Protective Effects of Breast-Feeding on Obesity. Nutrition Today. 2012;47(1):33–8. [Google Scholar]
- 17.Nickel NC. Look before you leap: can we draw causal conclusions from breastfeeding research? J Hum Lact. 2015;31(2):209–12. doi: 10.1177/0890334415572346. [DOI] [PubMed] [Google Scholar]
- 18.Gillman MW. Commentary: breastfeeding and obesity--the 2011 Scorecard. Int J Epidemiol. 2011;40(3):681–4. doi: 10.1093/ije/dyr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cope MB, Allison DB. Critical review of the World Health Organization’s (WHO) 2007 report on ‘evidence of the long-term effects of breastfeeding: systematic reviews and meta-analysis’ with respect to obesity. Obes Rev. 2008;9(6):594–605. doi: 10.1111/j.1467-789X.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95(Suppl 1):S144–50. doi: 10.2105/AJPH.2004.059204. [DOI] [PubMed] [Google Scholar]
- 21.Smithers LG, Kramer MS, Lynch JW. Effects of Breastfeeding on Obesity and Intelligence: Causal Insights From Different Study Designs. JAMA Pediatr. 2015 doi: 10.1001/jamapediatrics.2015.0175. [DOI] [PubMed] [Google Scholar]
- 22.Jiang M, Foster EM. Duration of breastfeeding and childhood obesity: a generalized propensity score approach. Health Serv Res. 2013;48(2 Pt 1):628–51. doi: 10.1111/j.1475-6773.2012.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casazza K, Brown A, Astrup A, Bertz F, Baum C, Brown MB, et al. Weighing the Evidence of Common Beliefs in Obesity Research. Crit Rev Food Sci Nutr. 2015;55(14):2014–53. doi: 10.1080/10408398.2014.922044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hytten FE. Clinical and chemical studies in human lactation. Br Med J. 1954;1(4855):175–82. doi: 10.1136/bmj.1.4855.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitoulas LR, Kent JC, Cox DB, Owens RA, Sherriff JL, Hartmann PE. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br J Nutr. 2002;88(1):29–37. doi: 10.1079/BJNBJN2002579. [DOI] [PubMed] [Google Scholar]
- 26.Jenness R. The composition of human milk. Seminars in perinatology. 1979;3(3):225–39. [PubMed] [Google Scholar]
- 27.Hinde K. Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. Am J Hum Biol. 2009;21(4):512–9. doi: 10.1002/ajhb.20917. [DOI] [PubMed] [Google Scholar]
- 28.Andreas NJ, Kampmann B, Mehring Le-Doare K. Human breast milk: A review on its composition and bioactivity. Early human development. 2015 doi: 10.1016/j.earlhumdev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence RA, Lawrence RM, editors. Breastfeeding: A guide for the medical professional. 7. Maryland Heights, Missouri: Elsevier Mosby; 2011. [Google Scholar]
- 30.Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60(1):49–74. doi: 10.1016/j.pcl.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biesalski HK, Dragsted LO, Elmadfa I, Grossklaus R, Muller M, Schrenk D, et al. Bioactive compounds: definition and assessment of activity. Nutrition. 2009;25(11–12):1202–5. doi: 10.1016/j.nut.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Wada Y, Lonnerdal B. Bioactive peptides derived from human milk proteins--mechanisms of action. J Nutr Biochem. 2014;25(5):503–14. doi: 10.1016/j.jnutbio.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Alsaweed M, Hartmann PE, Geddes DT, Kakulas F. MicroRNAs in Breastmilk and the Lactating Breast: Potential Immunoprotectors and Developmental Regulators for the Infant and the Mother. International journal of environmental research and public health. 2015;12(11):13981–4020. doi: 10.3390/ijerph121113981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowanadisai W, Lonnerdal B. Alpha(1)-antitrypsin and antichymotrypsin in human milk: origin, concentrations, and stability. Am J Clin Nutr. 2002;76(4):828–33. doi: 10.1093/ajcn/76.4.828. [DOI] [PubMed] [Google Scholar]
- 35.Lonnerdal B. Bioactive proteins in human milk: mechanisms of action. J Pediatr. 2010;156(2 Suppl):S26–30. doi: 10.1016/j.jpeds.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Maffei HV, Nobrega FJ. Gastric pH and microflora of normal and diarrhoeic infants. Gut. 1975;16(9):719–26. doi: 10.1136/gut.16.9.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davidson LA, Lonnerdal B. Fecal alpha 1-antitrypsin in breast-fed infants is derived from human milk and is not indicative of enteric protein loss. Acta Paediatr Scand. 1990;79(2):137–41. doi: 10.1111/j.1651-2227.1990.tb11429.x. [DOI] [PubMed] [Google Scholar]
- 38.Savino F, Fissore MF, Liguori SA, Oggero R. Can hormones contained in mothers’ milk account for the beneficial effect of breast-feeding on obesity in children? Clinical endocrinology. 2009;71(6):757–65. doi: 10.1111/j.1365-2265.2009.03585.x. [DOI] [PubMed] [Google Scholar]
- 39.Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. Int J Pediatr Endocrinol. 2009;2009:327505. doi: 10.1155/2009/327505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassiotou F, Geddes DT. Immune cell-mediated protection of the mammary gland and the infant during breastfeeding. Adv Nutr. 2015;6(3):267–75. doi: 10.3945/an.114.007377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulski JK, Hartmann PE. Milk insulin, GH and TSH: relationship to changes in milk lactose, glucose and protein during lactogenesis in women. Endocrinol Exp. 1983;17(3–4):317–26. [PubMed] [Google Scholar]
- 42.Sonksen P, Sonksen J. Insulin: understanding its action in health and disease. British Journal of Anaesthesia. 2000;85(1):69–79. doi: 10.1093/bja/85.1.69. [DOI] [PubMed] [Google Scholar]
- 43.Tiittanen M, Paronen J, Savilahti E, Virtanen SM, Ilonen J, Knip M, et al. Dietary insulin as an immunogen and tolerogen. Pediatric Allergy and Immunology. 2006;17(7):538–43. doi: 10.1111/j.1399-3038.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 44.Ahuja S, Boylan M, Hart S, Román-Shriver C, Spallholz J, Pence B, et al. Glucose and Insulin Levels are Increased in Obese and Overweight Mothers’ Breast-Milk. Food and Nutrition Sciences. 2011;2(3):201–6. [Google Scholar]
- 45.Shehadeh N, Khaesh-Goldberg E, Shamir R, Perlman R, Sujov P, Tamir A, et al. Insulin in human milk: postpartum changes and effect of gestational age. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2003;88(3):F214–F6. doi: 10.1136/fn.88.3.F214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ley SH, Hanley AJ, Sermer M, Zinman B, O’Connor DL. Associations of prenatal metabolic abnormalities with insulin and adiponectin concentrations in human milk. Am J Clin Nutr. 2012;95(4):867–74. doi: 10.3945/ajcn.111.028431. [DOI] [PubMed] [Google Scholar]
- 47.Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-alpha in human breast milk with infant growth and body composition. Pediatr Obes. 2012;7(4):304–12. doi: 10.1111/j.2047-6310.2012.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andreas NJ, Hyde MJ, Gale C, Parkinson JR, Jeffries S, Holmes E, et al. Effect of maternal body mass index on hormones in breast milk: a systematic review. PLoS One. 2014;9(12):e115043. doi: 10.1371/journal.pone.0115043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007;8(1):21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 50.Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau J, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47(2):178–83. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith-Kirwin SM, O’Connor DM, Johnston J, de Lancy E, Hassink SG, Funanage VL. Leptin Expression in Human Mammary Epithelial Cells and Breast Milk. The Journal of Clinical Endocrinology & Metabolism. 1998;83(5):1810. doi: 10.1210/jcem.83.5.4952. [DOI] [PubMed] [Google Scholar]
- 52.Casabiell X, Piñeiro V, Tomé MA, Peinó R, Dieguez C, Casanueva FF. Presence of Leptin in Colostrum and/or Breast Milk from Lactating Mothers: A Potential Role in the Regulation of Neonatal Food Intake. The Journal of Clinical Endocrinology & Metabolism. 1997;82(12):4270–3. doi: 10.1210/jcem.82.12.4590. [DOI] [PubMed] [Google Scholar]
- 53.Houseknecht KL, McGuire MK, Portocarrero CP, McGuire MA, Beerman K. Leptin Is Present in Human Milk and Is Related to Maternal Plasma Leptin Concentration and Adiposity. Biochemical and Biophysical Research Communications. 1997;240(3):742–7. doi: 10.1006/bbrc.1997.7736. [DOI] [PubMed] [Google Scholar]
- 54.Savino F, Liguori SA, Petrucci E, Lupica MM, Fissore MF, Oggero R, et al. Evaluation of leptin in breast milk, lactating mothers and their infants. Eur J Clin Nutr. 2010;64(9):972–7. doi: 10.1038/ejcn.2010.105. [DOI] [PubMed] [Google Scholar]
- 55.Schueler J, Alexander B, Hart AM, Austin K, Larson-Meyer DE. Presence and dynamics of leptin, GLP-1, and PYY in human breast milk at early postpartum. Obesity (Silver Spring, Md. 2013;21(7):1451–8. doi: 10.1002/oby.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doneray H, Orbak Z, Yildiz L. The relationship between breast milk leptin and neonatal weight gain. Acta Paediatr. 2009;98(4):643–7. doi: 10.1111/j.1651-2227.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 57.Miralles O, Sanchez J, Palou A, Pico C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring, Md. 2006;14(8):1371–7. doi: 10.1038/oby.2006.155. [DOI] [PubMed] [Google Scholar]
- 58.Schuster S, Hechler C, Gebauer C, Kiess W, Kratzsch J. Leptin in maternal serum and breast milk: association with infants’ body weight gain in a longitudinal study over 6 months of lactation. Pediatr Res. 2011;70(6):633–7. doi: 10.1203/PDR.0b013e31823214ea. [DOI] [PubMed] [Google Scholar]
- 59.Uysal FK, Onal EE, Aral YZ, Adam B, Dilmen U, Ardicolu Y. Breast milk leptin: its relationship to maternal and infant adiposity. Clin Nutr. 2002;21(2):157–60. doi: 10.1054/clnu.2001.0525. [DOI] [PubMed] [Google Scholar]
- 60.Weyermann M, Brenner H, Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: a prospective cohort study. Epidemiology. 2007;18(6):722–9. doi: 10.1097/ede.0b013e3181567ed4. [DOI] [PubMed] [Google Scholar]
- 61.Ucar B, Kirel B, Bor O, Kilic FS, Dogruel N, Aydogdu SD, et al. Breast milk leptin concentrations in initial and terminal milk samples: relationships to maternal and infant plasma leptin concentrations, adiposity, serum glucose, insulin, lipid and lipoprotein levels. J Pediatr Endocrinol Metab. 2000;13(2):149–56. doi: 10.1515/jpem.2000.13.2.149. [DOI] [PubMed] [Google Scholar]
- 62.Brunner S, Schmid D, Zang K, Much D, Knoeferl B, Kratzsch J, et al. Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatr Obes. 2015;10(1):67–73. doi: 10.1111/j.2047-6310.2014.222.x. [DOI] [PubMed] [Google Scholar]
- 63.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 64.Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006;54(7):2295–9. doi: 10.1002/art.21944. [DOI] [PubMed] [Google Scholar]
- 65.Hassiotou F, Savigni D, Hartmann P, Geddes D. Mammary cells synthesize appetite hormones that may contribute to breastmilk. FASEB Journal. 2014;28(1 Supp):38.8. [Google Scholar]
- 66.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 67.Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kos K, Harte AL, da Silva NF, Tonchev A, Chaldakov G, James S, et al. Adiponectin and resistin in human cerebrospinal fluid and expression of adiponectin receptors in the human hypothalamus. J Clin Endocrinol Metab. 2007;92(3):1129–36. doi: 10.1210/jc.2006-1841. [DOI] [PubMed] [Google Scholar]
- 69.Martin LJ, Woo JG, Geraghty SR, Altaye M, Davidson BS, Banach W, et al. Adiponectin is present in human milk and is associated with maternal factors. Am J Clin Nutr. 2006;83(5):1106–11. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- 70.Weyermann M, Beermann C, Brenner H, Rothenbacher D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin Chem. 2006;52(11):2095–102. doi: 10.1373/clinchem.2006.071019. [DOI] [PubMed] [Google Scholar]
- 71.Ozarda Y, Gunes Y, Tuncer GO. The concentration of adiponectin in breast milk is related to maternal hormonal and inflammatory status during 6 months of lactation. Clin Chem Lab Med. 2012;50(5):911–7. doi: 10.1515/cclm-2011-0724. [DOI] [PubMed] [Google Scholar]
- 72.Newburg DS, Woo JG, Morrow AL. Characteristics and potential functions of human milk adiponectin. J Pediatr. 2010;156(2 Suppl):S41–6. doi: 10.1016/j.jpeds.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woo JG, Guerrero ML, Altaye M, Ruiz-Palacios GM, Martin LJ, Dubert-Ferrandon A, et al. Human milk adiponectin is associated with infant growth in two independent cohorts. Breastfeed Med. 2009;4(2):101–9. doi: 10.1089/bfm.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cesur G, Ozguner F, Yilmaz N, Dundar B. The relationship between ghrelin and adiponectin levels in breast milk and infant serum and growth of infants during early postnatal life. The journal of physiological sciences : JPS. 2012;62(3):185–90. doi: 10.1007/s12576-012-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woo JG, Guerrero ML, Guo F, Martin LJ, Davidson BS, Ortega H, et al. Human milk adiponectin affects infant weight trajectory during the second year of life. J Pediatr Gastroenterol Nutr. 2012;54(4):532–9. doi: 10.1097/MPG.0b013e31823fde04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anderson J, McKinley K, Onugha J, Duazo P, Chernoff M, Quinn EA. Lower levels of human milk adiponectin predict offspring weight for age: a study in a lean population of Filipinos. Matern Child Nutr. 2015 doi: 10.1111/mcn.12216. [DOI] [PMC free article] [PubMed]
- 77.Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, et al. Ghrelin. Molecular metabolism. 2015;4(6):437–60. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gronberg M, Tsolakis AV, Magnusson L, Janson ET, Saras J. Distribution of obestatin and ghrelin in human tissues: immunoreactive cells in the gastrointestinal tract, pancreas, and mammary glands. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2008;56(9):793–801. doi: 10.1369/jhc.2008.951145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kierson JA, Dimatteo DM, Locke RG, Mackley AB, Spear ML. Ghrelin and cholecystokinin in term and preterm human breast milk. Acta Paediatr. 2006;95(8):991–5. doi: 10.1080/08035250600669769. [DOI] [PubMed] [Google Scholar]
- 80.Aydin S, Ozkan Y, Erman F, Gurates B, Kilic N, Colak R, et al. Presence of obestatin in breast milk: relationship among obestatin, ghrelin, and leptin in lactating women. Nutrition. 2008;24(7–8):689–93. doi: 10.1016/j.nut.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 81.Dundar NO, Dundar B, Cesur G, Yilmaz N, Sutcu R, Ozguner F. Ghrelin and adiponectin levels in colostrum, cord blood and maternal serum. Pediatr Int. 2010;52(4):622–5. doi: 10.1111/j.1442-200X.2010.03100.x. [DOI] [PubMed] [Google Scholar]
- 82.Ilcol YO, Hizli B. Active and total ghrelin concentrations increase in breast milk during lactation. Acta Paediatr. 2007;96(11):1632–9. doi: 10.1111/j.1651-2227.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 83.Karatas Z, Durmus Aydogdu S, Dinleyici EC, Colak O, Dogruel N. Breastmilk ghrelin, leptin, and fat levels changing foremilk to hindmilk: is that important for self-control of feeding? Eur J Pediatr. 2011;170(10):1273–80. doi: 10.1007/s00431-011-1438-1. [DOI] [PubMed] [Google Scholar]
- 84.Aydin S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides. 2010;31(12):2236–40. doi: 10.1016/j.peptides.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 85.Aydin S, Geckil H, Karatas F, Donder E, Kumru S, Kavak EC, et al. Milk and blood ghrelin level in diabetics. Nutrition. 2007;23(11–12):807–11. doi: 10.1016/j.nut.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 86.Aydin S, Ozkan Y, Kumru S. Ghrelin is present in human colostrum, transitional and mature milk. Peptides. 2006;27(4):878–82. doi: 10.1016/j.peptides.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 87.Kon IY, Shilina NM, Gmoshinskaya MV, Ivanushkina TA. The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Ann Nutr Metab. 2014;65(4):317–23. doi: 10.1159/000367998. [DOI] [PubMed] [Google Scholar]
- 88.Khodabakhshi A, Ghayour-Mobarhan M, Rooki H, Vakili R, Hashemy SI, Mirhafez SR, et al. Comparative measurement of ghrelin, leptin, adiponectin, EGF and IGF-1 in breast milk of mothers with overweight/obese and normal-weight infants. Eur J Clin Nutr. 2015;69(5):614–8. doi: 10.1038/ejcn.2014.205. [DOI] [PubMed] [Google Scholar]
- 89.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 90.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 91.Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87(5):2084–9. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- 92.Meki A, Saleem T, Al-Ghazali M, Sayed A. Interleukins-6, -8 and -10 and tumor necrosis factor-alpha and it soluble receptor I in human milk at different periods of lactation. Nutrition Research. 2003;23:845–55. [Google Scholar]
- 93.Ustundag B, Yilmaz E, Dogan Y, Akarsu S, Canatan H, Halifeoglu I, et al. Levels of cytokines (IL-1beta, IL-2, IL-6, IL-8, TNF-alpha) and trace elements (Zn, Cu) in breast milk from mothers of preterm and term infants. Mediators Inflamm. 2005;2005(6):331–6. doi: 10.1155/MI.2005.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wallace JM, Ferguson SJ, Loane P, Kell M, Millar S, Gillmore WS. Cytokines in human breast milk. British journal of biomedical science. 1997;54(2):85–7. [PubMed] [Google Scholar]
- 95.Agarwal S, Karmaus W, Davis S, Gangur V. Immune markers in breast milk and fetal and maternal body fluids: a systematic review of perinatal concentrations. J Hum Lact. 2011;27(2):171–86. doi: 10.1177/0890334410395761. [DOI] [PubMed] [Google Scholar]
- 96.Zahorska-Markiewicz B, Janowska J, Olszanecka-Glinianowicz M, Zurakowski A. Serum concentrations of TNF-alpha and soluble TNF-alpha receptors in obesity. Int J Obes Relat Metab Disord. 2000;24(11):1392–5. doi: 10.1038/sj.ijo.0801398. [DOI] [PubMed] [Google Scholar]
- 97.Rudloff HE, Schmalstieg FC, Jr, Mushtaha AA, Palkowetz KH, Liu SK, Goldman AS. Tumor necrosis factor-alpha in human milk. Pediatr Res. 1992;31(1):29–33. doi: 10.1203/00006450-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 98.Buescher ES, Malinowska I. Soluble receptors and cytokine antagonists in human milk. Pediatr Res. 1996;40(6):839–44. doi: 10.1203/00006450-199612000-00011. [DOI] [PubMed] [Google Scholar]
- 99.English BK, Burchett SK, English JD, Ammann AJ, Wara DW, Wilson CB. Production of lymphotoxin and tumor necrosis factor by human neonatal mononuclear cells. Pediatr Res. 1988;24(6):717–22. doi: 10.1203/00006450-198812000-00014. [DOI] [PubMed] [Google Scholar]
- 100.Goldman AS, Chheda S, Garofalo R. Evolution of immunologic functions of the mammary gland and the postnatal development of immunity. Pediatr Res. 1998;43(2):155–62. doi: 10.1203/00006450-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Laiho K, Lampi AM, Hamalainen M, Moilanen E, Piironen V, Arvola T, et al. Breast milk fatty acids, eicosanoids, and cytokines in mothers with and without allergic disease. Pediatr Res. 2003;53(4):642–7. doi: 10.1203/01.PDR.0000055778.58807.C8. [DOI] [PubMed] [Google Scholar]
- 102.Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C, et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol. 2010;52(6):913–20. doi: 10.1016/j.jhep.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 103.Gorski JN, Dunn-Meynell AA, Hartman TG, Levin BE. Postnatal environment overrides genetic and prenatal factors influencing offspring obesity and insulin resistance. American journal of physiology. 2006;291(3):R768–78. doi: 10.1152/ajpregu.00138.2006. [DOI] [PubMed] [Google Scholar]
- 104.Reifsnyder PC, Churchill G, Leiter EH. Maternal environment and genotype interact to establish diabesity in mice. Genome Res. 2000;10(10):1568–78. doi: 10.1101/gr.147000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pico C, Oliver P, Sanchez J, Miralles O, Caimari A, Priego T, et al. The intake of physiological doses of leptin during lactation in rats prevents obesity in later life. Int J Obes (Lond) 2007;31(8):1199–209. doi: 10.1038/sj.ijo.0803585. [DOI] [PubMed] [Google Scholar]
- 106.Sanchez J, Oliver P, Miralles O, Ceresi E, Pico C, Palou A. Leptin orally supplied to neonate rats is directly uptaken by the immature stomach and may regulate short-term feeding. Endocrinology. 2005;146(6):2575–82. doi: 10.1210/en.2005-0112. [DOI] [PubMed] [Google Scholar]
- 107.Palou A, Pico C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite. 2009;52(1):249–52. doi: 10.1016/j.appet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 108.Rodekamp E, Harder T, Kohlhoff R, Franke K, Dudenhausen JW, Plagemann A. Long-term impact of breast-feeding on body weight and glucose tolerance in children of diabetic mothers: role of the late neonatal period and early infancy. Diabetes Care. 2005;28(6):1457–62. doi: 10.2337/diacare.28.6.1457. [DOI] [PubMed] [Google Scholar]
- 109.Plagemann A, Harder T, Franke K, Kohlhoff R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care. 2002;25(1):16–22. doi: 10.2337/diacare.25.1.16. [DOI] [PubMed] [Google Scholar]
- 110.Hinde K, editor. Motherhood. Hoboken, NJ: John Wiley and Sons; 2015. [Google Scholar]