Abstract
Using the domestic cat as a non-rodent, larger animal model, the objective of the present study was to determine the impact of a brief incubation in a hypertonic microenvironment on (1) ovarian follicle and oocyte growth in vitro, (2) developmental capacity of the resident oocyte, and (3) expression of aquaporin (AQP) genes in parallel with genes involved in the regulation of folliculogenesis. In Study 1: Secondary or early antral follicles encapsulated in alginate were allocated to one of three treatment groups: 1) culture in standard medium at 290 mOsm for 15 d (Control); 2) incubation in 350 mOsm medium for 1 h followed by culture in standard medium for 15 d (Hypertonic-1h); or 3) incubation in 350 mOsm medium for 24 h followed by incubation in standard medium for additional 14 d (Hypertonic-24h). After measuring follicle and oocyte diameters on Day 15, in vitro-grown oocytes were incubated for 24 h before assessing their nuclear status. In Study 2: secondary or early antral follicles were subjected to one of the three treatments: 1) culture in standard medium at 290 mOsm for 48 h; 2) incubation in 350 mOsm medium for 1 h followed by culture in standard medium for additional 47 h; or 3) incubation in 350 mOsm medium for 24 h followed by culture in standard medium for additional 24 h. At the end of the culture period, all follicles were assessed for mRNA level of Cyp17a1, Cyp19a1, Star (Steroidogenesis genes)AQP1, 3, 5, 7 and 8 as well as Fshr using qPCR. Freshly collected follicles also were subjected to gene expression analysis and served as ‘Non-cultured control’. Hypertonic-24h follicles grew larger (P < 0.05) than the control, whereas those in Hypertonic-1h group exhibited intermediate growth, especially when the culture started at the early antral stage. Oocytes in the Hypertonic-24h group were larger and resumed meiosis at a higher rate than in the other treatments. In vitro culture affected (P < 0.05) mRNA expression of Cyp19a1, Star, AQP1 and AQP7 in both secondary and early antral stage while FSHr was only affected in the former compared to the non-cultured control. Pre-incubating follicles in 350 mOsm medium for 24 h enhanced (P < 0.05) Star and AQP7 while decreasing (P < 0.05) AQP1 expression compared to the control in secondary follicles, but not in the early antral stage. In summary, short-term hypertonic exposure promoted cat follicle development in vitro (including the meiotic competence of the enclosed oocyte) possibly through a mechanism that does not involve water transport genes.
Keywords: in vitro follicle culture, hypertonic exposure, cat, meiotic maturation, gene expression
1. Introduction
In addition to live animal models, much of our contemporary understanding about the mechanisms regulating ovarian folliculogenesis are partly derived from incubating follicles in vitro while manipulating the surrounding biochemical and mechanical environment [1–3]. Beyond improving fundamental knowledge, this research area has practical implications. Because the ovary contains thousands of small, immature follicles, the development of an effective culture system could provide access to enormous numbers of viable oocytes from a given donor that could be matured and fertilized in vitro to produce embryos. This emerging fertility preservation technology would benefit the genetic management of numerous animal genotypes (including those used to study human diseases [4, 5] and diverse wildlife species [5]. Other beneficiaries are young female cancer patients who are at risk for permanent infertility due to their therapeutic treatments [6].
A three dimensional (3D) culture system involving alginate has been shown effective for growing mouse follicles to produce oocytes capable of fertilization and live births after embryo transfer [7]. This system has provided encouraging results for advancing follicle development in the human [8–10], rhesus monkey [11], baboon [12], goat [13], and dog [14, 15], although with limited or no success at producing fertilizable oocytes. The lack of direct application from achievements in the mouse to other species likely is due to physical, anatomical and physiological differences [15, 16]. For example, the size of a mature cow, sheep, or human follicle is five- to 20-fold larger than for the same stage mouse follicle [16]. Furthermore, the mouse oocyte becomes ‘competent’ upon follicular antrum development [16], whereas this capacity in larger species, including the cow [17], dog [18], human [19], and cat [20, 21]) is more closely affiliated with the follicle and oocyte reaching a required size. For example, a human follicle must reach at least 5 mm in diameter for its resident oocyte to be able to complete nuclear maturation [19, 22]. Such observations probably mean that follicles from larger species require an in vitro microenvironment that allows meeting some minimal size requirement, likely in the presence of an antral cavity.
To-date, mechanisms regulating antrum formation and expansion within the ovarian follicle have not been fully elucidated. It has been suggested that transport of water into the follicular antral cavity is facilitated by water channel proteins, or aquaporins (AQPs) [23–26] that are localized in granulosa cells. Thus far, at least 13 AQP subtypes (AQP1-13) have been found in mammalian cells [27]. APQ1 to 5 as well as AQP7 to 9 are present and participate in water transport and development of ovarian follicles of multiple mammalian species [23, 26, 28]. Specifically, mRNA and protein expressions of APQ1 and 5 are highest during the follicular period of cycling pigs, suggesting a role(s) in follicle advancement [28]. In the rat, AQP7, 8, and 9 facilitate water transport in antral stage follicles [23]. Finally, it has been shown that APQ1 and 9 may play roles in preovulatory follicle growth and ovulation in the human [26, 29]. Based on these earlier observations, we speculated that AQPs may be exerting an influence by increasing water transport, that in turn, promoting the formation and expansion of the antral cavity.
Previous investigations explored the relationship between APQ expression and osmolality of the culture medium. Specifically, it has been determined that subjecting pre-implantation stage mouse embryos (zygote-16 cell) to a short-term osmotic, hydrostatic, or oxidative challenge stimulates ‘adaptive’ and positive developmental responses [30]. More precisely, such embryos in a hypertonic environment (350 mOsm) are stimulated to produce more p38 mitogen-activated kinase that, in turn, increases AQP3 and 9 [31]. Furthermore, when pig zygotes are exposed to a slight hypertonicity for 48 h, more blastocysts form after transfer back into isotonic medium [32], and these embryos are more cryo-tolerant [28].
Using the domestic cat as a non-rodent, larger animal model, the objective of the present study was to assess the impact of a brief incubation in a hypertonic microenvironment on (1) follicle and oocyte growth in vitro, (2) developmental capacity of the resident oocyte, and (3) expression of AQP in parallel with genes involved in the regulation of folliculogenesis (follicle stimulating hormone receptor (Fshr) and steroidogenesis regulation, including Cyp17a1, Cyp19a1 and Star). We hypothesized that short-term exposure of follicles to a hyperosmolar medium enhance follicle growth, and developmental competence of the residence oocyte by modulating expression of genes regulating water transport and folliculogenesis.
2. Materials and Methods
2.1. Chemicals
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
2.2. Collection of ovarian follicles
Ovaries from 60 domestic cats (6 mo-3 yr of age) were recovered after routine ovariohysterectomy at local veterinary and spay clinics and transported on ice in Leibovitz’s L-15 medium supplemented with 10 IU/ml penicillin G sodium and 10 µg/ml streptomycin sulfate. Without predetermination, all ovaries used in the present study contained visible antral follicles on the gonadal surface and no evidence of corpora lutea or albicantia. Follicle isolation was performed within 6 h of the surgery. Briefly, the cortical sections (2–3 mm thick) were dissected from each ovary’s surface and individual follicles physically-isolated using 23 g needles under stereomicroscopic viewing. The isolated follicles were placed in ‘collection medium’ comprised of Minimum Essential Medium (MEM) plus 3 mg/ml bovine serum albumin (BSA), 2 mM glutamine, 25 mM Hepes, and 10 IU/ml penicillin G sodium and 10 µg/ml streptomycin sulfate. Follicles were classified as secondary (mean ± standard error of mean [SEM] = 208 ± 7.9 µm diameter) or early antral (329.8 ± 5.4 µm) stage using the following criteria established earlier in our laboratory [21]. Specifically, follicles with multiple layers of granulosa cells and an apparent basement membrane, but lacking a clearly visible antral cavity (under stereomicroscopy), were considered to be in the secondary stage; those containing evidence of a small antral cavity were classified as in the early antral stage.
2.3. Follicle encapsulation and in vitro culture
Follicles were individually-encapsulated in 0.5% (w/v) alginate (FMC BioPolymers, Philadelphia, PA, USA) using methods adapted from Songsasen et al. [14]. In brief, alginate (50 µl) was pipetted onto a cover of a 65 mm Petri dish. A group of five to seven follicles was transferred into each alginate drop. Then each follicle was aspirated in ~5 µl of the surrounding alginate and transferred into a calcium chloride (5 mM CaCl2/14 mM NaCl) solution. After allowing cross-linking for 2 min, each alginate-encapsulated follicle was washed twice in collection medium before being transferred into a 4-well culture plate (Nunc™, Fisher Scientific, Pittsburgh, PA, USA), each well containing 500 µl of pre-equilibrated growth medium. The latter was comprised of MEM containing 3 mg/ml BSA, 4.2 µg/ml insulin, 3.8 µg/ml transferrin, 5 ng/ml selenium), and 1 µg/ml FSH (Bioniche Animal Health, Belleville, ON, Canada). Individual follicles were cultured for a total of 15 d (or 360 h; Study 1) or 48 h (Study 2) at 38.5°C in humidified 5% CO2. For those incubated for 15 d, half of the culture medium (~250 µl) was aspirated from each culture well and this volume immediately replaced with fresh growth medium at every 72 h time-point.
2.4. Follicle and oocyte assessments
The diameter of each follicle was measured at the onset of in vitro culture (Day 0) as well as 7 and 15 d of in vitro incubation using an inverted microscope (Leitz DMIL, Leica Microsystem, Buffalo Grove, IL, USA) equipped with an ocular micrometer. Each follicle was sized from the outer layer of the somatic cells, with the measurements including the widest diameter and perpendicular width to the initial assessment. The mean of these two metrics then was calculated and reported as ‘follicle diameter’. On each measurement day, a given follicle was also recorded as having ‘survived’ or ‘degenerated’. Follicles were classified as degenerated [14] when there was: 1) a decrease (>10%) in diameter (compared to the previous observation point; 2) evidence of oocyte deterioration (fracture, non-homogeneous discoloration, or cellular extrusion); and/or 3) granulosa cell fragmentation.
At the end of culture (Day 15), follicles from each incubated group were mechanically-removed from alginate beads by gentle repeated aspiration using a P1000 pipette. Oocytes were then recovered from the resident follicles using 23 g needles. The diameter of each enclosed oocyte (excluding the zona pellucida) was then assessed using the same inverted microscope equipped with an ocular micrometer.
2.5. In vitro oocyte maturation
Oocytes were matured in vitro for 24 h using our conventional protocol for the cat [33] in MEM supplemented with 1 µg/ml FSH, 1 µg/ml luteinizing hormone (LH; Bioniche Animal Health), and 1 µg/ml estradiol under a 38.5°C in humidified, 5% CO2 in air conditions. At the end of the IVM period, oocytes were fixed in 4% paraformaldehyde and stored overnight at 4°C, stained with Hoechst 33342, and then evaluated for nuclear status using fluorescent microscopy (BX40, Olympus, Waltham, MA, USA) [33]. The stained oocytes were classified as being in one of the following stages: germinal vesicle (GV); germinal vesicle breakdown (GVB); metaphase I (MI); or metaphase II (MII) [33]. Oocytes with fragmented cytoplasm/chromatin or without chromatin were considered degenerate.
2.6. RNA extraction
Total RNA was extracted from a group of secondary and early antral follicles (n = 6–12/treatment/replication) using absolutely RNA Nanoprep kit (Agilent technologies, Santa Clara, California, USA) following the manufacturer’s protocol. Extracted RNA was treated by RapidOut DNA removal kit (Thermo-Scientific, Carlsbad, California, USA) to remove genomic DNA contamination. The quantity and purity of extracted RNA was assessed using a Spectrophotometer (Nanodrop-One, Thermo- Scientific, Carlsbad, California, USA).
2.7. Quantitative RT-PCR (qRT-PCR)
Complementary DNA was synthesized from mRNA (1.74 ng from each sample) using Superscript III First strand synthesis system (Invitrogen, Thermo-Scientific, Carlsbad, California, USA) according to the manufacturer’s instructions, and stored at −20°C until qRT-PCR analysis. With the exception for Fshrthe gene-specific primers were designed by Beacon designer software package (Premier Biosoft, Palo Alto, California, USA). Fshr primer sequences were those previously reported by Hobbs et al [34]. All primer sequences used in the present study are shown in Table 1. The relative expression of all genes were normalized to an endogenous control gene, Succinate Dehydrogenase Complex Flavoprotein Subunit A (SDHA), which shown to be more stable when compared with Tyrosine 3-Monooxygenase/Tryptophan 5-Monooxygenase Activation Protein Zeta (YWHAZ), Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin in RefFinder software [35]. With the exception of Aqp7, each PCR reaction (total volume 20 µl) consisted of 2 µl DNA and 18 µl of reaction mixture that contained 10 µl of FastStart Essential DNA green master (Roche, Basel, Switzerland), 1 µl each of 10 µM for forward and reverse primers and 6 µl of nuclease free water. For Aqp7 expression, the concentrations of forward and reverse primers were 5 µM. The reaction were performed with the following settings: 95°C for 10 min, followed by 45 cycles of 95°C for 30 sec, specific annealing temperature (Table 1) for 10 sec, and 72°C for 10 sec. All amplifications were performed in triplicate using LightCycler® 96 (Roche, Basel, Switzerland). Reactions without cDNA were performed in parallel as negative control. Primer efficiency was assessed in each gene by serial dilution of DNA. The Ct value of each gene was normalized against the average Ct of SDHA (housekeeping gene) to generate delta Ct (ΔCt). Relative expression was calculated using efficiency correction model to normalize gene expression of follicles in the Fresh, non-culture control as described by [36].
Table 1.
Primers used for qPCR analysis of cat follicles.
| Gene | Sense | Sequence 5'-3' | Accession number |
Annealing temperature (°C) |
Product length (bp) |
Primer efficiency |
|---|---|---|---|---|---|---|
| SDHA | Forward | GCCTTGGATCTCCTGATG | DQ402986 | 57 | 75 | 1.067 |
| Reverse | ATGGATGGACCCGTCTTC | |||||
| YWHAZ | Forward | CGTTACTTGGCTGAGGTT | XM_006943328 | 57 | 77 | 1.216 |
| Reverse | GCTTCCTGGTATGCTTGT | |||||
| GAPDH | Forward | CATCACCATCTTCCAGGA | NM_001009307 | 57 | 81 | 1.009 |
| Reverse | CCAGTAGACTCCACAACA | |||||
| β-actin | Forward | ATCCACGAGACCACCTTC | AB051104 | 57 | 75 | 1.077 |
| Reverse | CACCGTGTTAGCGTAGAG | |||||
| Aqp1 | Forward | GGCTCGTCAGTGATTACTC | XM_003982907 | 60 | 103 | 0.924 |
| Reverse | CCAGGATGAAGTCGTAGATG | |||||
| Aqp3 | Forward | ATCTATGCCTTGGCTCAG | XM_011288588 | 59 | 96 | 1.056 |
| Reverse | CTCATTCTTGGCGAAGTC | |||||
| Aqp5 | Forward | TATGGGCTGGCACCAATCA | XM_003988711 | 57 | 77 | 1.092 |
| Reverse | GCTTGACCCTGTGTTGTGTT | |||||
| Aqp7 | Forward | CGTGAATCCTGGTATGAG | XM_006939211 | 57 | 78 | 1.321 |
| Reverse | CCGATGAAGATGAAGAGG | |||||
| Aqp8 | Forward | CGTGAATCCTGGTATGAG | XM_006942229 | 59 | 77 | 0.946 |
| Reverse | CCGATGAAGATGAAGAGG | |||||
| Cyp17a1 | Forward | CCGAGATGAGTTGCTGAG | NM_001009371.2 | 57 | 105 | 0.87 |
| Reverse | GAGTTCATCCTGGCTTGG | |||||
| Cyp19a1 | Forward | CAATCCTGCTGCTCACTG | GU306147.1 | 57 | 84 | 0.917 |
| Reverse | CCATGCAATAGCCAGGAC | |||||
| Star | Forward | ATGGAAGCGATGGGAGAG | NM_001246196.1 | 57 | 90 | 1.066 |
| Reverse | CAACTCGTGGGTGATGAC | |||||
| FSHR | Forward | GCCTCACCTACCCTAGCCACTG | NM_001048014.1 | 64 | 163 | 0.8374 |
| Reverse | GTAACTGGATTCCTCGTCTTCTG | |||||
2.8. Experimental design
2.8.1. Study 1: Influence of short-term exposure to a hypertonic medium on follicle growth and oocyte developmental competence
Secondary (n = 47) and early antral (n = 86) follicles were encapsulated in 0.5% alginate and then randomly allocated (according to follicle size) to one of the three culture conditions: 1) controls (Control), isotonic (290 mOsm) medium only with no exposure at any time to hypertonic medium (secondary, n = 16; early antral, n = 30); 2) ‘Hypertonic-1h’, short incubation in 350 mOsm growth medium (secondary, n = 15; early antral, n = 28) adjusted by supplementing with an appropriate NaCl concentration for 1 h; or 3) ‘Hypertonic-24h, short incubation in 350 mOsm growth medium for 24 h (secondary, n = 16; early antral, n = 28). Follicles in the Control group were exposed throughout the experiment only to our standard growth medium (as described above). Hypertonic exposure groups were cultured in the adjusted (to 350 mOsm) growth medium for 1 or 24 h before transfer to the isotonic medium followed by culture for 15 or 14 days, respectively. 350 mOsm was chosen based on a previous study conducted in the mouse, where this osmolality was shown to impact APQ expression in the mouse embryo [31]. Follicle survival and diameter were assessed as described above. At the end of culture, all oocytes were recovered and assessed for diameter and morphology. Only morphologically-normal oocytes (i.e., those displaying a spherical shape with dark, homogeneous cytoplasm) were subjected to IVM (as described above) and then evaluated for nuclear status (as above). The experiment was comprised of six culture trial replications.
2.8.2. Study 2: Influence of pre-exposure to a hypertonic culture medium on gene expression
Secondary (n = 155) and early antral follicles (n = 148) were isolated and randomly allocated to one of the three treatments: 1) culture in standard medium at 290 mOsm for 48 h; 2) incubation in 350 mOsm medium for 1 h followed by culture in standard medium for additional 47 h; or 3) incubation in 350 mOsm medium for 24 h followed by culture in standard medium for additional 24 h. At the end of the incubation period, all follicles were assessed for mRNA levels of Cyp17a1, Cyp19a1, Star, AQP1, 3, 5, 7 and 8 as well as Fshr, as described above. This experiment consisted of three qPCR replications. Each qPCR replication involved a pool 6 to 12 follicles per treatment group.
2.9. Statistical analysis
All data were expressed as mean ± standard deviation (SD). Follicle and oocyte diameter data were tested for normality using Shapiro-Wilk normality test and variance homogeneity using Bartlett’s test. Follicle diameter data were normally distributed with homogeneity variance; thus comparisons among three culture treatments were performed using a mixed-effect ANOVA model (random effects = ‘culture trial’, fixed effects = follicle stage + culture condition) followed by Tukey’s range test (SysStat 12 Version 12.00.08, Systat Software, Inc.). For oocyte diameter data, comparison among culture treatments were performed using Krukal Wallis Rank Sum Test. Comparison of nuclear maturation among the oocytes from different culture treatments were performed using a Fisher Exact test and RStudio3.2.2 (http://www.r-project.org/). Linear mixed effect models (lmer function in lmerTest package in R) was used to compare gene expression level between fresh, non-cultured follicles to those of in vitro culture groups. Significance was set at P < 0.05.
3. Results
3.1. Study 1: Influence of short-term exposure to a hypertonic medium on follicle growth and oocyte developmental competence
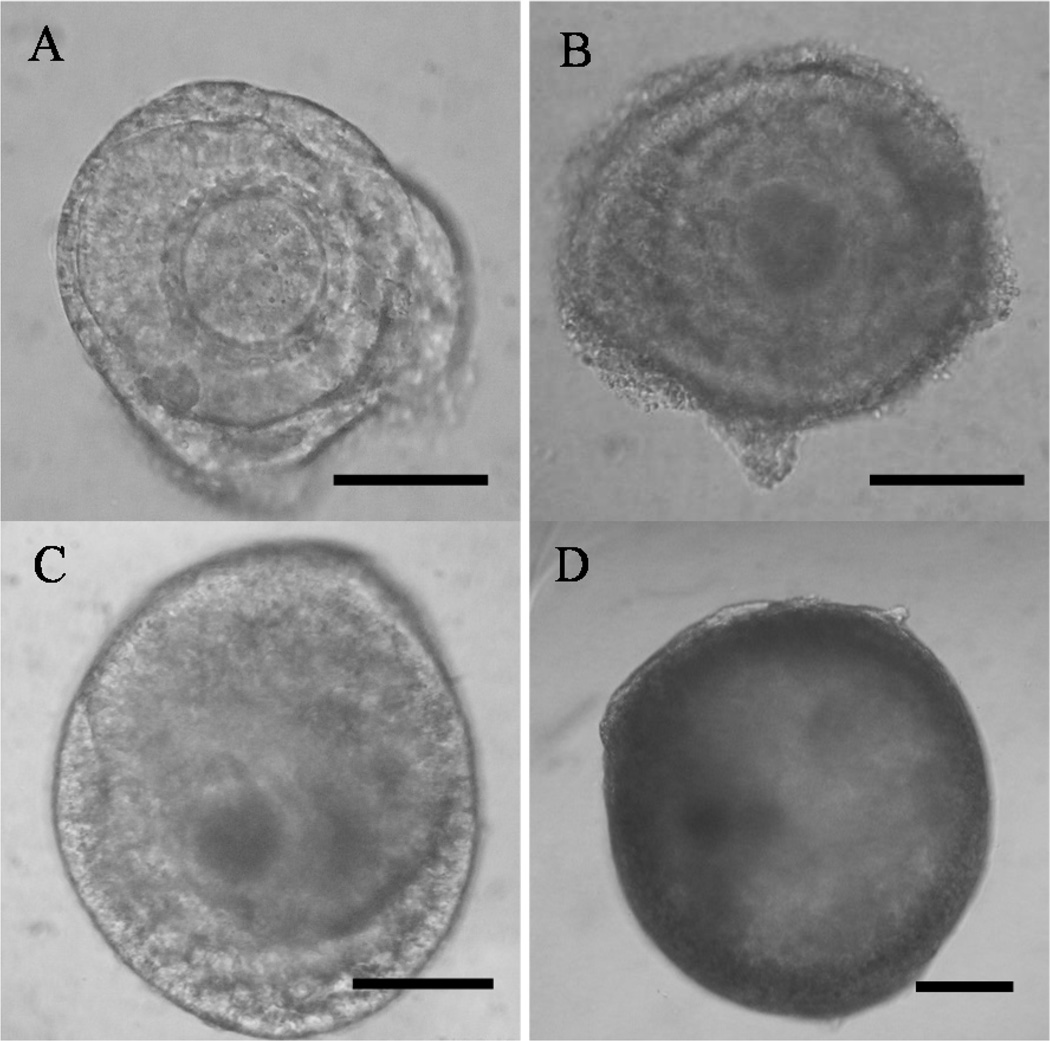
All follicles, regardless of initial developmental stage or culture treatment, retained a spherical shape, 80% of which had normal morphology, including the classical dark, homogeneous oocyte cytoplasm characteristic of the species (Fig. 1). Follicles in all groups also increased in size (P < 0.05) over the 15 d culture compared to Day 0 (Table 2). Follicle extrusion (breakdown of the basement membrane and expulsion of granulosa cells and usually the oocyte) occurred in ~10% of all follicles regardless of culture treatment. All of the extruded follicles were ≥400 µm in diameter.
Fig. 1.
Cat secondary (A) and early antral follicles (B) before and the same follicles after (C, D, respectively) pre-exposure to the 350 mOsm culture medium for 24 h followed by isotonic culture for 14 d. Bars represent 100 µm. All photographs were taken at 200X magnification.
Table 2.
Mean (± SD) diameter of cat secondary or early antral follicles at the onset of culture (Day 0) and after 15 days of in vitro culture (Day 15) in isotonic medium (290 mOsm; Control), pre-exposure to hypertonic medium (350 mOsm) for 1 h followed by in vitro culture, or pre-exposure to hypertonic medium (350 mOsm) for 24 h followed by in vitro culture.
| Culture group | Secondary follicle diameter (n) | Early antral follicle diameter (n) | ||
|---|---|---|---|---|
| (µm) | ||||
| Day 0 | Day 15 | Day 0 | Day 15 | |
| Control | 209.0 ± 61.7 (16) |
327.0 ± 77.0 (13)* |
323.5 ± 51.7 (28) |
460.0 ± 103.6 (23)a* |
| Hypertonic-1 h | 206.1 ± 40.5 (15) |
349.8 ± 89.3 (12)* |
329.0 ± 53.1 (28) |
505.6 ± 98.2 (21)ab* |
| Hypertonic-24 h | 198.0 ± 34.9 (16) |
366.2 ± 103.1 (14)* |
337.0 ± 44.6 (28) |
538.0 ± 103.7 (25)b* |
Within columns, values with different superscripts indicate differences (P < 0.05) among culture treatments.
Within rows, values with an asterisk indicate a difference (P < 0.05) between Days 0 and 15 within the same culture group and initial follicular stage
When culture began at the secondary stage, there were no differences (P > 0.05) in the final mean diameter at 15 d among the three culture groups. However, when culture initiated at the early antral stage, exposure to the hypertonic medium for 24 h was beneficial as the final mean diameter of follicles in this treatment was larger (P < 0.05) than that of the Control group (Table 2). The final size of early antral follicles in the Hypertonic-1h condition was intermediate (P > 0.05) between the counterpart treatments (Table 2).
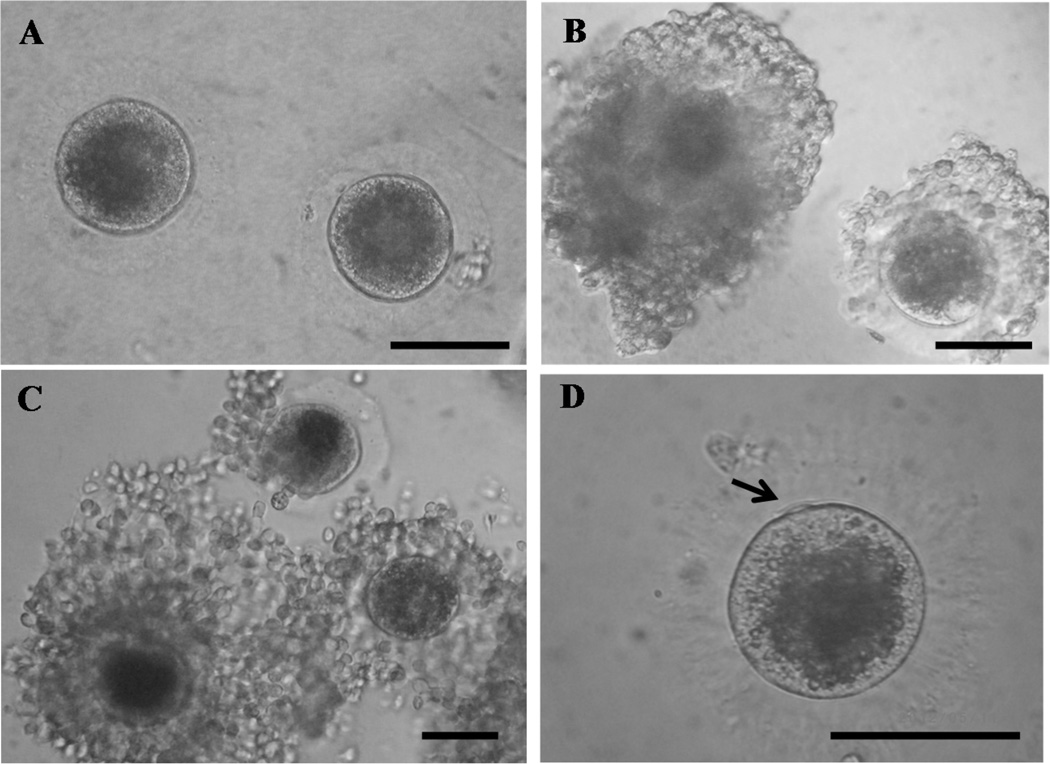
There was no difference (P > 0.05) in the oocyte diameter recovered post-culture, although both secondary and early antral follicles pre-incubated in hypertonic solution for 24 h tended (P < 0.07) to be larger by 3 to 5% compared to the Control or 1 h treatment, respectively at Day 15 (secondary: Control, 92.0 ± 13.7 [n = 12], Hypertonic-1h, 94.5 ± 14.1 [n = 8], Hypertonic-24h, 97.8 ± 6.14 µm [n = 15]; early antral: Control, 100.5 ± 5.0 [n = 23], Hypertonic-1h, 102.8 ± 10.0 [n = 21], Hypertonic-24h, 105.7 ± 12.4 µm [n = 25]). There also were distinctive morphological differences among oocytes, with those recovered from control follicles being mostly void of cumulus cells (Fig. 2A). By contrast, oocytes recovered from hypertonic-exposed follicles were fully or partially surrounded by several layers of compact somatic cells (Fig. 2B) that underwent expansion after IVM (Fig 2C). Culture condition also affected (P < 0.05) the ability of resident oocytes to resume meiosis in vitro. Less than 30% of pooled oocytes recovered from secondary and early antral follicles in the Hypertonic-24h treatment remained at the GV stage after 24 h IVM compared to ~about 60% for both the Control and Hypertonic-1h groups (Table 3). Approximately 3-fold more oocytes from the Hypertonic-24h group completed meiotic maturation compared to the Control or Hypertonic-1h treatments (Table 3), although this difference was not significant (P = 0.14) due to small sample size.
Fig. 2.
In vitro grown oocytes recovered from follicles (A, 200X) cultured in isotonic growth medium for 15 d or (B, 200X) pre-incubated in the same medium at 350 mOsm for 24 h before the same isotonic culture for 15 d. Morphology of oocytes from the latter culture condition (C, 200X) after in vitro maturation (D, 400X). The arrow indicates first polar body. Bars represent 100 µm.
Table 3.
Nuclear status after in vitro maturation of oocytes recovered from cat follicles after 15 days of in vitro culture (Day 15) in isotonic medium (290 mOsm; Control), pre-exposure to hypertonic medium (350 mOsm) for 1 h followed by in vitro culture, or pre-exposure to hypertonic medium (350 mOsm) for 24 h followed by in vitro culture.
| Culture group | Numbers of oocyte | Numbers of oocyte at (%)* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Secondary | Early Antral |
GV | GVBD | MI | MII | Degenerated | |||
| Total | Secondary | Early antral |
||||||||
| Control | 29 | 8 | 20 | 17 (58.6)a |
2 (6.9)a |
3 (10.3) |
2 (6.8) |
0 (0.0) |
2 (6.8) |
5 (17.2) |
| Hypertonic-1 h | 33 | 10 | 23 | 19 (57.6)a |
6 (18.2)ab |
0 (0.0) |
2 (6.1) |
1 (10.0) |
1 (4.3) |
6 (18.2) |
| Hypertonic-24 h | 33 | 8 | 25 | 9 (27.3)b |
11 (33.3)b |
3 (9.1) |
7 (21.2) |
1 (12.5) |
6 (18.2) |
3 (9.1) |
No. in parentheses = percentage of total oocytes in that treatment.
GV = germinal vesicle; GVBD = germinal vesicle breakdown; MI = metaphase I; MII = metaphase II.
Within columns, values with different superscripts indicate differences (P < 0.05) among groups within the same nuclear stage.
3.2. Study 2 Influence of pre-exposure to a hypertonic culture medium on gene expression
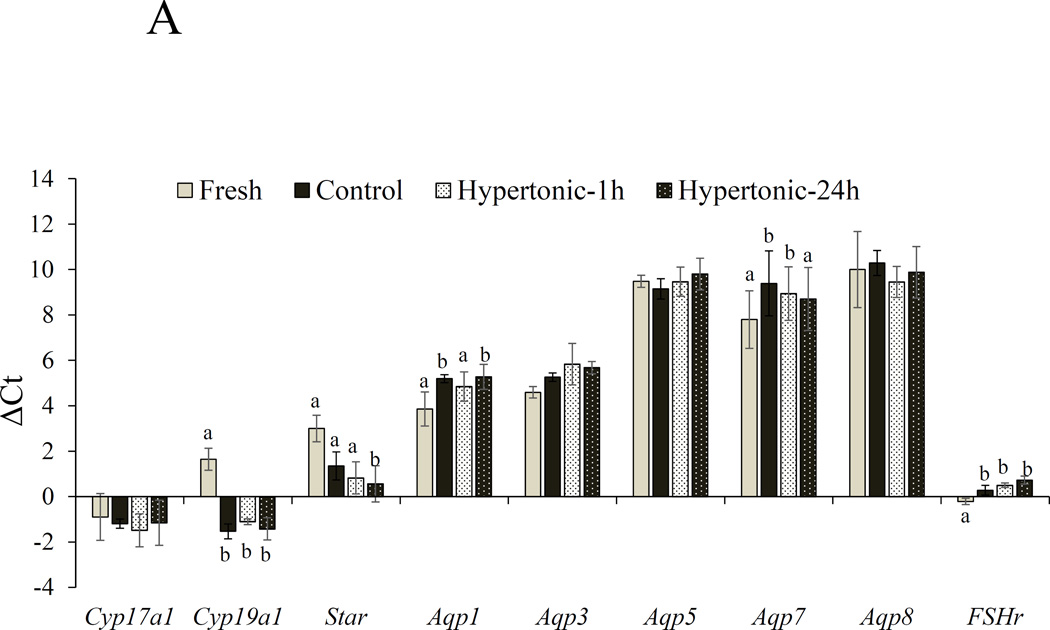
For secondary follicles, in vitro culture significantly altered mRNA expression of Cyp19a1, Star, Aqp1, 7 and Fshr compared with the fresh, non-cultured control (Fig. 3A). Furthermore, expression levels of these genes were also affected by culture treatments. Specifically, Cpy19a1 increased (P < 0.05; Figs. 3A) 11 to 18 fold in cultured follicles compared with the fresh cohorts. However, no differences (P > 0.05) in the transcript level among culture conditions were observed. Star was upregulated (8 fold, P < 0.05) only in Hypertonic-24h treatment compared with the Fresh control. Expression of Aqp1 mRNA decreased (P < 0.05) in Control and Hypertonic-24h compared with fresh, non-culture follicles with no changes in mRNA expression in Hypertonic-1h treatment (P > 0.05). Aqp7 expression was down-regulated (P < 0.05; Figs 3A) in Control and Hypertonic-1h compared with in the fresh counterparts. Fshr mRNA level was also reduced in all cultured follicles (P< 0.05; Figs 3A).
Fig. 3.
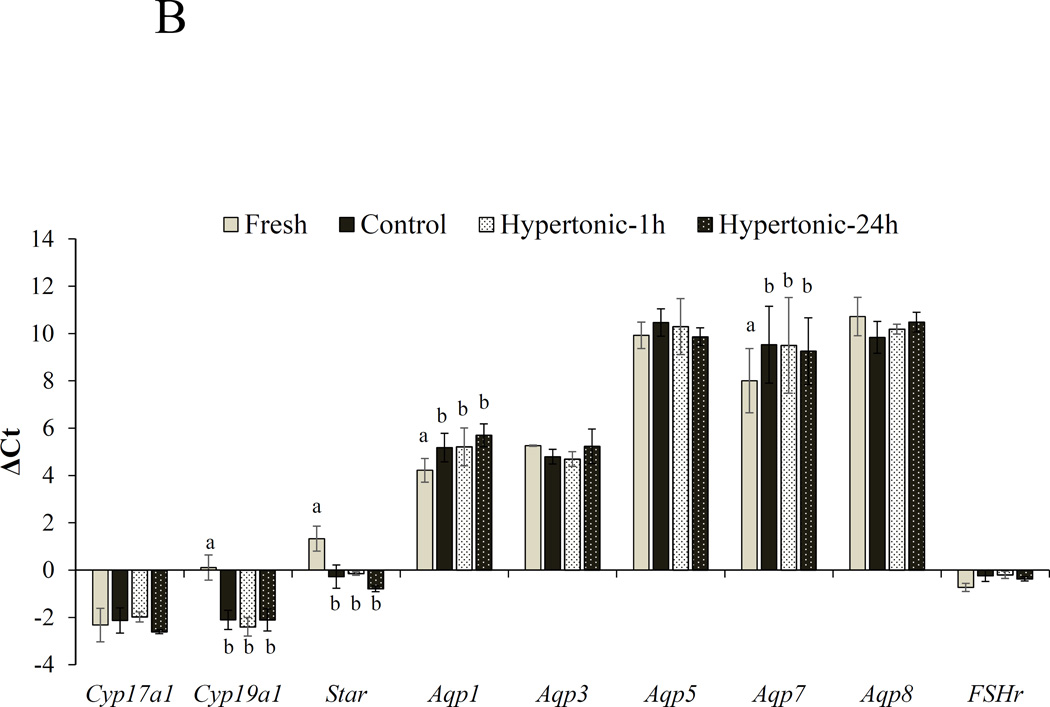
ΔCt values (mean ± SEM) of Cyp17a1, Cyp19a1, Star, Aqp1, Aqp3, Aqp5, Aqp8 and Fshr in freshly collected (A) secondary and (B) early antral follicles (Fresh) or after cultured in isotonic (290 mOsm) for 48 h (Control) or pre-exposed to the same medium at 350 mOsm for 1 h (Hypertonic-1h) or 24 h (Hypertonic-24 h) followed by the same isotonic culture for 48 or 24 h, respectively. Ct is the number of cycles to exceed the analysis threshold (lower Ct values represent higher mRNA levels). ΔCt is the difference in gene expression between the gene of interest and a panel of housekeeping genes (Sdha). a,bIndicates a difference between fresh and a culture group at P < 0.05.
For early antral follicles, in vitro culture influenced expression levels of Cyp19a1, Star, Aqp1 and Aqp7 (Fig. 3B). Cyp19a1 and Star transcripts were upregulated (P < 0.05; 6–8 fold for Cyp19a1, 4–6 fold for Star) whereas Aqp1 and Aqp7 mRNA was downregulated (P < 0.05) in cultured follicles compared with Fresh cohorts (Figs 3B). However, pre-incubating follicles in hypertonic medium either for 1 or 24 h had no impact (P > 0.05) on mRNA expression of these genes.
4. Discussion
Imposing a sub-lethal stress, such hydrostatic pressure or an anisotonic solution, to rodent gametes or embryos has been demonstrated to improve cell survival and development in vitro [30]. It is believed that such an effect may be expressed via up-regulation of APQ expression. In the present study, we explored an ‘osmotic challenge’ strategy previously described for mammalian embryos [31], that a hypertonic condition might increase APQ expression [31] that, in turn, would promote antral follicle development. Using the cat model, our hypothesis was that in vitro growth of secondary and/or early antral follicles could be enhanced by pre-incubation in a hypertonic medium, and that this effect might be regulated through one or more of the aquaporin genes. In this study, we made three significant discoveries, the first being that incubating cat follicles in a slightly hypertonic microenvironment (350 mOsm) for 24 h before in vitro culture in isotonic medium enhanced both follicle and oocyte growth. Secondly, this influenced the ability of the oocyte to resume meiosis. Surprisingly, stimulating effect of hypertonic microenvironment did not appear to be caused by upregulation of AQP mRNA, but this treatment stimulated the expression of Star, especially in the secondary follicle.
There have been few investigations directed at understanding the intricacies of folliculogenesis in the domestic cat. Most studies to-date have relied on a 2-dimensional (2D) culture system and conventional culture drops to grow follicles in vitro [37, 38]. Earlier efforts using that approach have demonstrated that cat preantral follicles (<150 µm in diameter) survive for the first 7 d of culture, but then degenerate [38]. Here, we demonstrated that a 3D culture technique utilizing alginate hydrogel (a system previously developed by others) [11, 39] sustained the ability of cat secondary and early antral follicles to grow and survive in vitro for up to 15 d. Furthermore, unlike in earlier cat studies [37, 38], some of the resident oocytes (up to 21%) recovered from donor follicles cultured in the 3D alginate system retained the capacity to resume meiosis and complete nuclear maturation. In that context, this alternative mode of incubation itself imparted advantages for incubating premature cat follicles, as it has for the mouse [7], human [9, 10, 40] and non-human primate [11, 12]. For the human, secondary follicles exposed to such conditions maintain a normal morphological structure, increase in size, and actually form an antral cavity by the 30th day of culture [11]. The major milestone for 3D in vitro follicle culture has been demonstrated in the non-human primate where early stage embryos have been produced from in vitro grown oocytes [41]. The benefit of the 3D/alginate approach is believed to be related to promotion of cell-to-cell contact and cell-to-extracellular matrix interaction within the follicle, both key to normal folliculogenesis [42]. Thus, we speculate that this same incubation system was at least partially overcoming a subpar microenvironment used in earlier cat investigations [37, 38] that likely was disrupting somatic cell-oocyte communication.
The present study proved the first part of our hypothesis that short-term incubation of cat follicles in a slightly hypertonic medium promoted growth and enhanced size and ability of the resident oocyte to resume meiotic maturation. However, the impact was duration-dependent with 1 h being ineffective in generating the significant response achieved with only a 24 h exposure. In a general context, the cat was similar to the pig where developmental responses of the latter’s oocytes to extracellular osmolality also are time dependent. More blastocysts result from incubating pig oocytes in a 327 mOsm culture medium for 2 d compared to culturing in standard medium (273 mOsm) for 5 d. However, there was no advantage to development when pig oocytes were placed in a hypertonic solution for 1 or 3 d [32]. Future studies should examine the effect of other exposure periods (e.g., 6, 12 or 48 hr) and varying medium osmolality on cat ovarian follicle growth in vitro.
While follicles exposed to the hyperosmolar conditions for 24 h were larger than controls, none exceeded 820 µm which was still 20% less than required for fully achieving developmental competence (i.e., complete nuclear maturation and fertilisation [21]). We also observed that, as these incubated follicles reached ~400 to 500 µm with an expanding antral cavity, the basement membrane of 10% of follicles ruptured expelling both granulosa cells and the oocyte itself. This phenomenon has been observed earlier in the goat [43], human [44], and mouse [45], albeit with no known etiology. This phenomenon may be caused by a subtle rupture of the basement membrane at the time of follicle isolation that became apparent as follicle grew larger. Extrusion of oocytes from cultured follicles may be also linked to reduction in numbers of transzonal projections between the oocyte and granulosa cells as reported in the mouse [46]. Alternatively, we speculate that this expulsion event in our system occurred because the cat follicles were unable to expand within the non-degradable alginate, which eventually resulted in disruption of the basement membrane.
In the present study, oocytes from follicles exposed to hyperosmolar treatment for 24 h were 3–5% larger than those recovered from non-hyperosmolar controls. Nevertheless, the size of all the oocytes was smaller (by about ~5–10%) than the minimal diameter of fully grown oocytes from naturally developing Graafian follicles (i.e., circa 110 µm diameter, [21, 47]). Similar to what has been described for the dog [48] and cow [49], developmental competence of the cat oocyte is directly correlated with this cell’s physical diameter [21]. Specifically, the cat oocyte acquires developmental competence (i.e., ability to complete meiotic maturation, fertilization, and develop into an embryo) only when follicle size exceeds 1 mm in diameter and at the onset of antrum formation [21]. Therefore, the overall modest meiotic competence measured across all the treatment groups was likely because these oocytes had not yet completed a full growth pattern. Nonetheless, it was clear that one stimulating factor to follicle size was an increased osmolality of the surrounding culture medium. Importantly, expansive cumulus cell formations were observed around only those oocytes recovered from follicles pre-incubated in hypertonic medium. The latter condition may have afforded better cell-to-cell communication, which already is known to be essential for nutrient transport during intrafollicular oocyte growth and final nuclear/cytoplasmic maturation in the mouse [1, 50, 51], cow [52, 53], and pig [54, 55]). For the cat, we also suspect that the enhanced presence of cumulus cells encompassing oocytes from osmotically-exposed follicles indicating better health which likely contributed to observed improvements in meiotic resumption. Our laboratory has previously demonstrated that cumulus-oocyte communication is important for resumption and completion of meiosis in this species [56]. In this context, the cat differs from the mouse [57], cow [58], and pig [59] where removing the oocyte’s cumulus cells before IVM fails to prevent spontaneous resumption of meiosis in vitro.
There is previous evidence that the influence of osmotic stress on promoting developmental processes is modulated through altered AQP expression. For example, exposure of rat alveolar epithelial cells to a hypertonic solution containing sorbitol increases expression of AQP5 compared to non-hypertonic exposure control [60]. Furthermore, short-term (3 h) exposure to a hyperosmolar medium (via NaCl supplementation) decreases AQP2 expression in a mouse kidney cortical collecting duct cell line [mpkCCD(cl4)] by inhibiting this gene’s transcription [61]. By contrast, extending the exposure time to 24 h enhances expression of AQP2 by stimulating transcription in this cell line [61]. In the mouse, incubating 8-cell stage embryos in hypertonic medium (via an addition of sucrose) for either 6 or 24 h increases AQP3 and 9 mRNA expression compared to the control, with levels of AQP9 also amplified over time [31]. In contrast to the previous studies, our findings rejected the second part of our hypothesis that was, that hypertonic microenvironment stimulates mRNA expression of aquaporins. In fact, we discovered that pre-exposure follicles to hypertonic microenvironment did not affect the expression levels of aquaporin genes in the early antral stage. Modest effect of hypertonic environment on Aqp1 and Aqp7 was observed in the secondary stage follicles, where mRNA expression of the former was sustained in Hypertonic-1h and that of the latter remained constant (compared to fresh follicles) in Hypertonic-24h. This finding is also inconsistent to that of Sales et al. [62] who reported that exposing sheep ovary tissue to a hypertonic concentration of cryoprotectants (ethylene glycol or dimethyl sulfoxide) followed by transferring the cortices back to isotonic medium decreased Aqp3 mRNA expression. It is worth noting that the osmolality of medium used in the present study was much lower (350 mOsm) than that employed in the previous study (>1000 mOsm), and this likely contributes to the discrepancy in the finding between the two reports. Interestingly, in vitro culture appeared to negatively affect mRNA expression of these two genes in both developmental stages. This finding is somewhat similar to that previously reported in the sheep where down regulation of Aqp3, 7 and 9 mRNA has been observed after 12 d in vitro culture, especially in follicles that have formed an antrum [62]. Nevertheless, the same authors have reported the discrepancy between the pattern of mRNA and protein expression of the three aquaporin genes [62]. Thus, future studies should examine the impact of hypertonic microenvironment on protein expression of these water transport genes. While the present study focused on the immediate impact of hypertonic exposure to AQP expression, it may be worthwhile examining long-term effect of hypertonic exposure on gene expression. Our preliminary study revealed that AQP1 expression level increased 2-fold in the Hypertonic-24 h group compared to that of the Control after 15 da in vitro culture (data not shown).
The beneficial effect of hypertonic microenvironment on follicle and oocyte growth observed in the present study was also reflected by enhanced mRNA expression of genes regulating steroidogenesis. Follicles in Hypertonic-24h group expressed a higher levels of Star mRNA than Fresh control. It also should be noted that mRNA expression of Cyp19a1 and Star were highly upregulated (6 to 18 folds) after in vitro culture regardless of treatment. Cyp19a1 is predominantly expressed in granulosa cells, and have been shown to be upregulated as folliculogenesis progresses both in vivo [62] and in vitro [64]. Upregulation of this steroidogenic genes in cultured follicles confirmed that our in vitro culture system supports follicular cell proliferation, hence promotes growth.
In summary, we demonstrated that exposure of secondary and early antral cat follicles to a slightly hypertonic in vitro environment for 24 h enhanced in vitro growth, promoted cumulus cell-oocyte communication and improved the ability of resident oocytes to resume meiosis. However, parallel assessments of aquaporin genes did not find the beneficial effect to short-term exposure to hyperosmolality on AQP mRNA expression.
Highlights.
Short-term hypertonic incubation enhanced in vitro secondary and early antral follicles
Short-term hypertonic incubation stimulated development of in vitro grown oocytes
Short-term hypertonic exposure did not impact mRNA expression of aquaporin genes
In vitro culture affected aquaporin, steroidogenesis and FSH receptor gene expression
Acknowledgments
The authors thank veterinary clinics in Front Royal, Stephen’s City, and Harrisonburg, VA for donation of domestic cat ovaries. This study was funded by the National Center for Research Resources (R01 R026064), a component of the National Institutes of Health (NIH) and is currently supported by the Office of Research Infrastructure Programs/Office of the Director (R01 OD 010948).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 2.Eppig JJ, O'Brien MO, Wigglesworth K. Mammalian oocyte growth and development in vitro. Mol Reprod Dev. 1996;44:260–273. doi: 10.1002/(SICI)1098-2795(199606)44:2<260::AID-MRD17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Woodruff TK, Shea LD. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3–6. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comizzoli P, Songsasen N, Wildt DE. Protecting and extending fertility options for females of wild and endangered species. Cancer Treat Res. 2010;156:87–100. doi: 10.1007/978-1-4419-6518-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comizzoli P, Wildt DE. On the horizon for fertility preservation in domestic and wild carnivores. Reprod Domest Anim. 2012;6:261–265. doi: 10.1111/rda.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodruff TK. Preserving fertility during cancer treatment. Nat Med. 2009;15:1124–1125. doi: 10.1038/nm1009-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue engineered follicles produce live fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA. Alginate beads as a tool to handle, cryopreserve, and culture isolated human primordial/primary follicles. Cryobiology. 2013;67:64–69. doi: 10.1016/j.cryobiol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Laronda MM, Duncan FE, Hornick JE, Xu M, Pahnke JE, Whelan KA, Shea LD, Woodruff TK. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J Assist Reprod Genet. 2014;31:1013–1028. doi: 10.1007/s10815-014-0252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 20009;24:2531–2540. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, West-Farrell ER, Stouffer RL, Shea LD, Woodruff TK, Zelinski MB. Encapsulated three-dimensional culture supports development of non-human primate secondary follicles. Biol Reprod. 2009;81:587–594. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, Kiesewetter SE, Shea LD, Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84:689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva GM, Rossetto R, Chaves RN, Duarte AB, Araujo VR, Feltrin C, Bernuci MP, Anselmo-Franci JA, Xu M, Woodruff TK, Campello CC, Figueiredo JR. In vitro development of secondary follicles from pre-pubertal and adult goats cultured in two-dimensional or three-dimensional systems. Zygote. 2014;26:1–10. doi: 10.1017/S0967199414000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Songsasen N, Woodruff TK, Wildt DE. In vitro growth and steroidogenesis of dog follicles as influenced by the physical and hormonal microenvironment. Reproduction. 2011;142:113–122. doi: 10.1530/REP-10-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagashima J, Wildt DE, Travis AJ, Songsasen N. Follicular size and stage and gonadotropin concentration affect alginate-encapsulated in vitro growth and survival of pre- and early antral dog follicles. Reprod Fertil Dev. 2015 doi: 10.1071/RD15004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Smitz J, Cortvrindt RG. The earliest stages of folliculogenesis in vitro. Reproduction. 2002;123:185–202. doi: 10.1530/rep.0.1230185. [DOI] [PubMed] [Google Scholar]
- 17.Lonergan P, Monaghan P, Rizos D, Boland MP. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilisation, and culture in vitro. Mol Reprod Fertil. 1994;37:48–53. doi: 10.1002/mrd.1080370107. [DOI] [PubMed] [Google Scholar]
- 18.Songsasen N, Wildt DE. (Size of the donor follicle, but not stage of reproductive cycle or seasonality, influences meiotic competency of selected domestic dog oocytes. Mol Reprod Dev. 2005;72:113–119. doi: 10.1002/mrd.20330. [DOI] [PubMed] [Google Scholar]
- 19.Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- 20.Uchikura K, Nagano M, Hishinuma M. The effect of ovarian status and follicular diameter on maturational ability of domestic cat oocytes. J Vet Med Sci. 2011;73:561–566. doi: 10.1292/jvms.10-0381. [DOI] [PubMed] [Google Scholar]
- 21.Comizzoli P, Pukazhenthi BS, Wildt DE. The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Hum Reprod. 2011;26:2165–2177. doi: 10.1093/humrep/der176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzman L, Ortega-Hrepich C, Albuz FK, Verheyen G, Devroey P, Smitz J, De Vos M. Developmental capacity of in vitro-matured human oocytes retrieved from polycystic ovary syndrome ovaries containing no follicles larger than 6 mm. Fertil Steril. 2012;98:503–507. doi: 10.1016/j.fertnstert.2012.01.114. [DOI] [PubMed] [Google Scholar]
- 23.McConnell NA, Yunus RS, Gross SA, Bost KL, Clemens MG, Hughes FM., Jr Water permeability of an ovarian antral follicle is predominantly transcellular and mediated by aquaporins. Endocrinology. 2002;143:2905–2912. doi: 10.1210/endo.143.8.8953. [DOI] [PubMed] [Google Scholar]
- 24.Rodgers RJ, Irving-Rodgers H-F. Formation of the ovarian follicular antrum and follicular fluid. Biol Reprod. 2010;82:1021–1029. doi: 10.1095/biolreprod.109.082941. [DOI] [PubMed] [Google Scholar]
- 25.Skowronski MT, Kwon T-H, Nielsen S. Immunolocalization of aquaporin 1, 5, and 9 in pig reproductive system. J Histochem Cytochem. 2009;57:61–67. doi: 10.1369/jhc.2008.952499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thoroddsen A, Daham-Kahler P, Lind AK, Weijdegard B, Lindenthal B, Brannstrom MT. The water permeability channels aquaporins 1–4 are differentially expressed in granulosa and theca cells of the preovulatory follicle during precise stages of human ovulation. J Clin Endocrinol Metab. 2011;96:1021–1028. doi: 10.1210/jc.2010-2545. [DOI] [PubMed] [Google Scholar]
- 27.Verkman AS. Aquaporins at a glance. J Cell Sci. 2011;124:2107–2112. doi: 10.1242/jcs.079467. [DOI] [PubMed] [Google Scholar]
- 28.Skowronska A, Mlotkowska P, Eliszewski M, Nielsen S, Skowronski MT. Expression of aquaporin 1, 5, and 9 in the ovarian follicles of cycling and early pregnant pigs. Physiol Res. 2015;64:237–245. doi: 10.33549/physiolres.932825. [DOI] [PubMed] [Google Scholar]
- 29.Qu F, Wang F-F, Lu X-E, Dong M-Y, Sheng J-Z, Lv P-P, Ding G-L, Shi B-W, Zhang D, Huang H-F. Altered aquaporin expression in women with polycystic ovary syndrome: hyperandrogenism in follicular fluid inhibits aquaporin-9 in granulosa cells through the phosphotidylinositol 3-kinase pathway. Hum Reprod. 2010;25:1441–1450. doi: 10.1093/humrep/deq078. [DOI] [PubMed] [Google Scholar]
- 30.Pribenszky C, Vajta G, Molnar M, Du Y, Lin L, Bolund L, Yovich J. Stress for stress tolerance? A fundametally new approach in mammalian embryology. Biol Reprod. 2010;83:690–697. doi: 10.1095/biolreprod.110.083386. [DOI] [PubMed] [Google Scholar]
- 31.Bell CE, Lariviere NMK, Watson PH, Watson AJ. Mitogen-activated protein kinase (MAPK) pathways mediate embryonic responses to culture medium osmolarity by regulating aquaporin 3 and 9 expression and localization as well as embryonic apoptosis. Hum Reprod. 2009;24:1373–1386. doi: 10.1093/humrep/dep010. [DOI] [PubMed] [Google Scholar]
- 32.Mizobe Y, Kurino S, Sata Y, Mori H, Yoshida M, Miyoshi K. Stage-specific effects of osmolarity of a culture medium on development of pig oocytes and minature pig somatic cell nuclear transfer embryos activated by ultrasound treatment. Anim Sci J. 2010;81:453–460. doi: 10.1111/j.1740-0929.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 33.Comizzoli P, Wildt DE, Pukazhenthi BS. Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non-breeding season. Reproduction. 2003;126:809–816. [PubMed] [Google Scholar]
- 34.Hobbs RJ, Howard J, Wildt D, Comizzoli P. Absence of seasonal changes in FSHR gene expression in the cat cumulus oocyte complex in vivo and in vitro. Reproduction. 2012;144:111–122. doi: 10.1530/REP-12-0098. [DOI] [PubMed] [Google Scholar]
- 35.Xie F, Xiao P, Chen D, Xu L, Zhang B. miRDeepFinder: a miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol Biol. 2012;80:75–84. doi: 10.1007/s11103-012-9885-2. [DOI] [PubMed] [Google Scholar]
- 36.Pfaffl MW. Relative quantification. In: Dorak TM, editor. Real-time PCR, International University Line, 0-4153-7734-X. New York, US: 2006. pp. 63–87. [Google Scholar]
- 37.Jewgenow K. Role of media, protein, and energy supplements on maintenance of morphology and DNA-synthesis of small preantral domestic cat follicles during short term culture. Theriogenology. 1998;49:1567–1577. doi: 10.1016/s0093-691x(98)00102-2. [DOI] [PubMed] [Google Scholar]
- 38.Wongbandue G, Jewgenow K, Chatdarong K. Effects of thyroxin (T4) and activin A on in vitro growth of preantral follicles in domestic cats. Theriogenology. 2013;79:824–832. doi: 10.1016/j.theriogenology.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang TR, Yan LY, Yan J, Lu CL, Xia X, Yin TL, Zhu XH, Gao JM, Ding T, Hu WH, Guo HY, Li R, Qiao J. Basic fibroblast growth factor promotes the development of human ovarian early follicles during growth in vitro. Hum Reprod. 2014;29:568–576. doi: 10.1093/humrep/det465. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Lawson MS, Yeoman RR, Molskness TA, Ting AY, Stouffer RL, Zelinski MB. Fibrin promotes development and function of macaque primary follicles during encapsulated three-dimensional culture. Hum Reprod. 2013;28:2187–200. doi: 10.1093/humrep/det093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West ER, Xu M, Woodruff TK, Shea LD. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials. 2007;28:4439–4448. doi: 10.1016/j.biomaterials.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saraiva MV, Celestino JJ, Araujo VR, Chaves RN, Almeida AP, Lima-Verde IB, Duarte AB, Silva GM, Martins FS, Bruno JB, Matos MH, Campello CC, Silva JR, Figueiredo JR. Expression of follicle-stimulating hormone receptor (FSHR) in goat ovarian follicles and the impact of sequential culture medium on in vitro development of caprine preantral follicles. Zygote. 2011;19:205–214. doi: 10.1017/S0967199410000511. [DOI] [PubMed] [Google Scholar]
- 44.Hovatta O, Wright C, Krausz T, Hardy K, Winston RM. Human primordial, primary, and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum. Reprod. 1999;14:2519–2524. doi: 10.1093/humrep/14.10.2519. [DOI] [PubMed] [Google Scholar]
- 45.Mousset-Simeon N, Jouannet P, Le Cointre L, Coussieu C, Poirot C. Comparison of three in vitro culture systems for maturation of early preantral mouse ovarian follicles. Zygote. 2005;13:167–175. doi: 10.1017/s0967199405003151. [DOI] [PubMed] [Google Scholar]
- 46.Hornick JE, Duncan FE, Shea LD, Woodruff TK. Multiple follicle culture supports primary follicle growth through paracrine-acting signals. Reproduction. 2013;145:19–32. doi: 10.1530/REP-12-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Comizzoli P, Wildt DE, Pukazhenthi BS. In vitro compaction of germinal vesicle chromatin is beneficial to survival of vitrified cat oocytes. Reprod Domest Anim. 2009;2:269–274. doi: 10.1111/j.1439-0531.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 48.Otoi T, Ooka A, Murakami M, Kurniani Karja NW, Suzuki T. Size distribution and meiotic competence of oocytes obtained from bitch ovaries at various stages of the oestrous cycle. Reprod Fertil Dev. 2001;13:151–155. doi: 10.1071/rd00098. [DOI] [PubMed] [Google Scholar]
- 49.Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Bovine oocyte diameter in relation to developmental competence. Theriogenology. 1997;48:769–774. doi: 10.1016/s0093-691x(97)00300-2. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez F, Smitz J. Molecular control of oogenesis. Biochim. Biophys. Acta. 2012;1822:1896–1912. doi: 10.1016/j.bbadis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Thomas FH, Vanderhyden BC. Oocyte-granulosa cell interactions during mouse follicular development: regulation of kit ligand expression and its role in oocyte growth. Reprod Biol Endocrinol. 2006;4:19. doi: 10.1186/1477-7827-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luciano AM, Modina S, Vassena R, Milanesi E, Lauria A, Gandolfi F. Role of intracellular cyclic adenosine 3',5'-monophosphate concentration and oocyte-cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte. Biol Reprod. 2004;70:465–472. doi: 10.1095/biolreprod.103.020644. [DOI] [PubMed] [Google Scholar]
- 53.Uzbekova S, Sanchez-Lazo L, Desmachais A, Maillard V, Elis S. Lipolysis in cumulus cells accompanies oocyte maturation in bovine. Reprod Fertil Dev. 2014;27:226. http://dx.doi.org/10.1071/RDv27n1Ab274. [Google Scholar]
- 54.Amano T, Mori T, Matsumoto K, Iritani A, Watanabe T. Role of cumulus cells during maturation of porcine oocytes in the rise in intracellular Ca2+ induced by inositol 1,4,5-trisphosphate. Theriogenology. 2005;64:261–274. doi: 10.1016/j.theriogenology.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Maedomari N, Kikuchi K, Ozawa M, Noguchi J, Kaneko H, Ohnuma K, Nakai M, Shino M, Nagai T, Kashiwazaki N. Cytoplasmic glutathione regulated by cumulus cells during porcine oocyte maturation affects fertilisation and embryonic development in vitro. Theriogenology. 2007;67:983–993. doi: 10.1016/j.theriogenology.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Godard NM, Pukazhenthi BS, Wildt DE, Comizzoli P. Paracrine factors from cumulus-enclosed oocytes ensure the successful maturation and fertilisation in vitro of denuded oocytes in the cat model. Fertil Steril. 2009;91:2051–2060. doi: 10.1016/j.fertnstert.2008.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faerge I, Terry B, Kalous J, Wahl P, Lessl M, Ottesen JL, Hyttel P, Grondahl C. Resumption of meiosis induced by meiosis-activating sterol has a different signal transduction pathway than spontaneous resumption of meiosis in denuded mouse oocytes cultured in vitro. Biol Reprod. 2001;65:1751–1758. doi: 10.1095/biolreprod65.6.1751. [DOI] [PubMed] [Google Scholar]
- 58.Mayes MA, Sirard MA. Effect of type 3 and type 4 phosphodiesterase inhibitors on the maintenance of bovine oocytes in meiotic arrest. Biol Reprod. 2002;66:180–184. doi: 10.1095/biolreprod66.1.180. [DOI] [PubMed] [Google Scholar]
- 59.Miyano T, Ebihara M, Goto Y, Hirao Y, Nagai T, Kato S. Inhibitory action of hypoxanthine on meiotic resumption of denuded pig follicular oocytes in vitro. J Exp Zool. 1995;273:70–75. doi: 10.1002/jez.1402730109. [DOI] [PubMed] [Google Scholar]
- 60.Zhou B, Ann DK, Li X, Kim KJ, Lin H, Minoo P, Crandall ED, Borok Z. Hypertonic induction of aquaporin-5: novel role of hypoxia-inducible factor-1α. Am J Physiol Cell Physiol. 2007;292:C1280–C1290. doi: 10.1152/ajpcell.00070.2006. [DOI] [PubMed] [Google Scholar]
- 61.Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Feraille E. Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol. 2005;16:1571–1582. doi: 10.1681/ASN.2004110930. [DOI] [PubMed] [Google Scholar]
- 62.Sales AD, Duarte AB, Rodrigues GQ, Lima LF, Silva GM, Carvalho AA, Brito IR, da Maranguape RM, Lobo CH, Aragao JA, Moura A, Figueiredo JR, Rodrigue AP. Steady-state level of messenger RNA and immunolocalization of aquaporins 3, 7, and 9 during in vitro growth of ovine preantral follicles. Theriogenology. 2015;84:1–10. doi: 10.1016/j.theriogenology.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 63.Findlay JK, Britt K, Kerr JB, O’Donnell L, Jones ME, Drummond AE, Simpson ER. The road to ovulation: the role of oestrogens. Reprod Fertil Dev. 2001;13:543–547. doi: 10.1071/rd01071. [DOI] [PubMed] [Google Scholar]
- 64.Sales AD, Duarte AB, Santos RR, Alves KA, Lima LF, Rodrigues GQ, Brito IR, Lobo CH, Bruno JB, Locatelli Y, Figueiredo JR, Rodrigues AP. Modulation of aquaporins 3 and 9 after exposure of ovine ovarian tissue to cryoprotectants followed by in vitro culture. Cell Tissue Res. 2016;365:415–424. doi: 10.1007/s00441-016-2384-z. [DOI] [PubMed] [Google Scholar]