Abstract
Background
Type 2 diabetes (T2D) is an established risk factor for dementia, but evidence for T2D and memory decline is less consistent. Understanding how T2D and blood glucose relate to memory decline is crucial to elucidating the mechanisms linking T2D and dementia.
Methods
For 8,888 Health and Retirement Study participants aged 50+, glycosylated hemoglobin (HbA1c) was measured in either 2006 or 2008 and physician's diagnosis of diabetes was self-reported in the same year. Composite memory (z-scored) was assessed biennially through 2012 using immediate and delayed word list recall or the Informant Questionnaire for Cognitive Decline. Marginal mean regression models for repeated outcomes were specified to predict memory decline as a function of diabetes or HbA1c, using age as the timescale and adjusting for health and social confounders.
Results
Diabetes was associated with a 10% faster rate of memory decline (β=−0.04 per decade; 95% CI: −0.06,−0.01). A 1-unit increase in HbA1c corresponded with a 0.05 SD decrease in memory score per decade (95% CI: −0.08,−0.03). Even among individuals with HbA1c <6.5% (threshold for diabetes), higher HbA1c was associated with memory decline (β=−0.05 per decade; 95% CI: −0.08,−0.03).
Discussion
Diabetes accelerated memory loss and higher HbA1c predicted memory decline even in non-diabetics.
Keywords: diabetes, blood glucose, glycosylated hemoglobin, memory, cognition, cognitive decline, memory decline, dementia
INTRODUCTION
The relationship between type 2 diabetes (T2D) and increased dementia risk is well-characterized 1-3, but the biological mechanisms linking the two are not well understood. Although memory loss is a hallmark of dementia, diagnostic criteria include both cognitive and functional indicators. Dementia incidence is therefore influenced by both rate of cognitive decline and pre-decline level of cognitive function. Risk factors for dementia may impact either level of cognitive function or rate of cognitive decline, or both. Since rate of memory decline is closely aligned with the development and progression of diseases such as Alzheimer's and cerebrovascular pathology 4, understanding how T2D and blood glucose levels relate to memory decline is crucial to elucidating the mechanisms driving higher dementia rates among individuals with T2D. To date, some studies have found an association between diabetes and memory decline 5-8, while others have not 9-12. Understanding this relationship is crucial for identifying people who would benefit from potential interventions.
Chronic hyperglycemia, measured via glycosylated hemoglobin (HbA1c), is one possible biological mechanism linking T2D with increased risk of dementia and memory decline (Figure 1). Other than hyperglycemia, possible explanations for the association between T2D and dementia include both causal and noncausal factors 3. Proposed causal mechanisms include insulin dysregulation 13, expression of insulin-degrading enzyme 3,13, and severe hypoglycemic events among individuals with T2D 3,14. The primary noncausal explanation is that shared determinants of T2D and dementia confound the association, including health behaviors, socioeconomic factors, as well as obesity, hypertension, and other comorbidities. Analyses of change in memory are less vulnerable to such potential confounders.
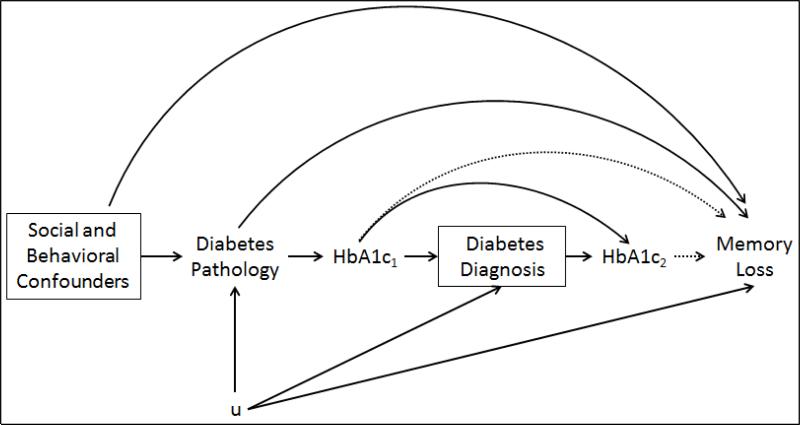
Figure 1.
Directed Acyclic Graph (DAG) of the hypothesized relationship between diabetes, HbA1c, and cognitive decline.
We show HbA1c at two different time points (subscripts 1 and 2) to clarify that for some people we have a measure of HbA1c that precedes self-reported diabetes diagnosis and for others we have a measure that follows self-reported diabetes diagnosis. Diabetes pathology may influence cognitive decline either via chronic hyperglycemia (HbA1c) or via other mechanisms, such as hyperinsulinemia. Alternatively, it is possible that unmeasured confounders induce a spurious, non-causal association between diabetes and cognitive decline. The “u” represents possible variables that confound the relationship between diabetes diagnosis and decline, such as socioeconomic factors like late life income. If there were many unmeasured “u” variables, we would be concerned about collider stratification bias, but we believe that most of the variables in “u” are likely to be the same social and behavioral confounders already included in the study.
Similar to the evidence on diabetes and dementia risk and rate of cognitive decline, a relationship between blood glucose levels and dementia has been established 15. However, the association between HbA1c and cognitive decline 3,6,7,16-18, and, specifically, memory decline 3,6,17, is less consistent. Therefore, whether glycemic control among diabetics, and even pre-diabetic blood glucose levels among non-diabetics, could predict the risk of memory decline remains unknown.
The majority of prior studies were limited due to short follow-ups, non-diverse and elderly populations, selective attrition and survival, and incomplete data on potential social and behavioral confounding factors. Short follow-ups are especially problematic in studies of cognitive decline because there is a low signal-to-noise ratio when identifying determinants of decline. Sample diversity is particularly important for research on diabetes because minority populations are disproportionately burdened and the association could differ in minorities versus whites 19. Selective attrition and survival is common in longitudinal studies of aging because sicker individuals are more likely to both drop out and die 20, but steps are rarely taken to minimize the potential bias 21.
We estimate the association between diabetes and memory decline, as well as the relationship between HbA1c and memory decline, among diabetics and non-diabetics aged 50+ in the U.S. Health and Retirement Study (HRS) while controlling for a comprehensive set of potential confounders. We also evaluated the possibility that HbA1c above the threshold for diabetes has worse consequences for memory than HbA1c levels in the normal or pre-diabetic range.
METHODS
Study population
HRS is a nationally representative cohort with a target population of all non-institutionalized adults age 50+ in the contiguous United States. Biomarker data was collected on two sub-samples in 2006 and 2008. Participants aged 50+ at biomarker collection were eligible for inclusion into our study 22. Biennial interviews (or proxy interviews for decedent participants) are available through 2012. Details of HRS are reported elsewhere 23. Original survey response rates varied across enrollment cohorts from 70-82%, and retention rates through 2008 ranged from 86% to 91%.
Of 12,186 possible respondents who were in the sample and age 50+ at biomarker collection (either 2006 or 2008), we excluded participants without a memory assessment at biomarker collection (N=1,094), who were missing diabetes status (N=11), or were nondiabetics with an HbA1c measure over 6.5 (the cut point for a diagnosis of diabetes 24) (N=345). In addition, we excluded anyone missing covariate information (N=1,848). These exclusions left a final analytic sample of 8,888, including 7,051 non-diabetics and 1,837 diabetics.
Memory Function and Decline Outcomes
Memory was assessed by immediate and delayed recall of a 10-word list and the Informant Questionnaire for Cognitive Decline (IQCODE). For individuals too impaired to directly participate in memory assessments, proxy informants, typically spouses, assessed the participants’ memory on a 5-item Likert scale and completed a 16-item version of the IQCODE25. We used a previously developed memory composite score combining proxy and direct memory assessments for longitudinal analyses 26. The composite score algorithm was developed in an 856-subject subsample who participated in a comprehensive neuropsychological battery as part of the Aging, Demographics, and Memory Study 27. We standardized the memory score by dividing each score by the 1995 standard deviation so that every unit change in memory score corresponds to approximately one standard deviation in the population. The potential for practice effects is minimal because everyone had taken at least one assessment prior to our baseline wave 28.
Diabetes
Diabetes is self-reported with the question: “Has a doctor ever told you that you have diabetes or high blood sugar?” 29, and we used the measure at the time of biomarker collection (2006 or 2008).
Glycosylated Hemoglobin (HbA1c)
Glycosylated hemoglobin (HbA1c) is a measure of blood glucose concentration over the past 2-3 months; it is used both for diagnosis of diabetes and for assessment of disease management among people with diagnosed diabetes 24. HbA1c was measured via dried blood spot (DBS) in either 2006 or 2008 22. In the pooled sample including both diabetics and non-diabetics, HbA1c was centered at 6.5% (the cut point for diagnosis of diabetes), and at the mean for each group in analyses stratified by diabetes status.
Age
Age was calculated as the time between self-reported date of birth and interview date, measured continuously, centered at 70 years, and converted to decades.
Covariates
All models were adjusted for hypothesized confounders of the association between diabetes/HbA1c and memory decline. These include: race (white, black, or other), gender (male vs. female), childhood socioeconomic status (continuous composite score based on measures of human and financial capital), baseline marital status (married/partnered, separated/divorced, widowed, and never married), initiated smoking before age 18 (yes/no), childhood social capital composite scale (using measures of maternal investment and created via factor analysis, continuous composite score), father absent during childhood (yes/no), childhood and baseline self-rated health (very low/low, good, very good/excellent), high school completion (yes/no), college completion (yes/no), baseline household income (log transformed), baseline household wealth (log transformed), baseline smoking (yes/no), baseline drinking (drinks per day: 0, 1-2, 3+), baseline BMI (normal weight, overweight, obese), baseline physical activity level (active/inactive), ever had hypertension at baseline (yes/no), ever had a stroke at baseline (yes/no), baseline elevated depressive symptoms (measured by the 8-item CES-D 30, depressive symptoms score > 3), and ever had heart disease at baseline (yes/no). All covariates are based on self-reports, with the exception of depression. All “baseline” covariates are measured in 2004 so that they are temporally prior to diabetes and HbA1c assessment. In sensitivity analyses, we additionally adjusted for cholesterol medication use (yes/no in 2006).
Statistical Analysis
Baseline (2004) characteristics were calculated for diabetics and non-diabetics. The present analysis includes memory function measured at biennial interviews from biomarker collection (2006 or 2008) until the end of the study period (2012) or until death or dropout, for up to 4 memory assessments.
In all models, we use generalized estimating equations (GEE) to model memory decline longitudinally via marginal mean regression models for repeated outcomes with age (in decades, centered at age 70) as the timescale. First, we assess the relationship between diabetes at the time of biomarker collection and subsequent memory decline with a diabetes-by-age interaction term (Eq 1).
| (1) |
In the same sample, including both diabetics and non-diabetics, we investigated whether HbA1c predicts memory decline. We model HbA1c using two linear terms, to test whether the effect of increases in HbA1c differed above the 6.5% threshold: 1) a linear term for a percentage point increase in HbA1c; 2) a linear term for an additional effect of a percentage point increase in HbA1c for every unit greater than or equal to 6.5% (labeled HbA1cAbove6.5 in equation 2). We interacted each of these HbA1c variables with age (Eq 2). In sensitivity analyses, we estimated models that additionally controlled for self-reported cholesterol medication use since there is a hypothesis that statin use influences memory loss 31.
| (2) |
Next, we stratified by diabetes status and examined the relationship between HbA1c and memory decline separately among diabetics and non-diabetics using an HbA1c-by-age interaction term. Given our DAG in Figure 1, we concluded that stratification by diabetes diagnosis does not introduce substantial collider stratification bias because the variables that are likely to be in “u” are similar to the social and behavioral confounders we have already measured and included in the models 32. Since there is uncertainty in the field about how to control for confounding in longitudinal studies of decline, we report models both with and without interaction terms between each covariate and age. All models use inverse probability weights (IPWs) to adjust for selective survival and attrition 21. We used pooled logistic regression models to estimate the probability of mortality and attrition in order to calculate stabilized IPWs 33. Although we specified an independence working correlation structure for repeated outcomes as recommended by Tchetgen Tchetgen et al 34 in order to accommodate time updated weights, robust standard errors are reported which appropriately account for the longitudinal design and all sources of variability. The HRS is drawn from a nationally representative sample, but we did not apply the sampling weights.
Further, we conducted a sensitivity analysis where we included the 345 individuals who had HbA1c values of 6.5% or higher (the cut point for diabetes diagnosis) but self-reported never being diagnosed with diabetes. In addition, we used natural log-transformed HbA1c to predict memory decline in order to test the non-linearity of the relationship. In this analysis, we used a log-HbA1c-by-age interaction term as the main parameter of interest, and controlled for diabetes, diabetes-by-age, and all covariates. The log-HbA1c-by-age interaction term divided by 10 is interpreted as the additional decrease in memory score per decade associated with a 10% increase in HbA1c (e.g. from 6% to 6.6%). Finally, we conducted a sensitivity analysis on a subset of the sample with data on APOE-4 status (6,187 non-diabetics and 1,562 diabetics) where we included a main effect for any APOE-4 alleles (yes/no) as well as interactions between APOE-4 and diabetes/HbA1c and age.
RESULTS
Sample characteristics from 2004 are shown for the 1,837 diabetics and 7,051 non-diabetics included in our models (Table 1). Average follow-up was 5.2 years (out of 6 possible) overall, 5.3 years among non-diabetics, and 5.0 years among diabetics. Overall average number of memory assessments was 3.6 (out of 4 possible). Mean HbA1c among non-diabetics was 5.5% (SD=0.4%), and 6.8% (SD=1.3%) among diabetics. Median HbA1c among nondiabetics was 5.6%, and 6.5% among diabetics. There were 386 self-reported strokes over the follow-up period, with diabetics almost twice as likely to report a stroke.
Table 1.
Characteristics of our Health and Retirement Study sample in 2004.
| Diabetics N=1,837 |
Non-Diabetics N=7,051 |
|
|---|---|---|
| Male (%) | 848 (46%) | 2753 (39%) |
| Race (ref=White) | ||
| Black (%) | 375 (20.41%) | 737 (10.45%) |
| Other (%) | 56 (3.05%) | 123 (1.74%) |
| Age (mean, std) | 67.4 (8.8) | 66.9 (9.6) |
| Southern birth place (%) | 347 (18.89%) | 1008 (14.3%) |
| Started smoking before age 18 (%) | 1649 (25.53%) | 1649 (25.53%) |
| Childhood self-rated health (ref=good) | ||
| Low (%) | 167 (9.09%) | 530 (7.52%) |
| High (%) | 619 (33.7%) | 2235 (31.7%) |
| Rural childhood (%) | 3657 (56.62%) | 3657 (56.62%) |
| Father absent (%) | 525 (8.13%) | 525 (8.13%) |
| Childhood Social Capital (mean, std) | −0.01 (1.14) | 0.01 (1.05) |
| Not physically active (%) | 1527 (83.12%) | 5149 (73.03%) |
| Drinks per day (ref=0) | ||
| 1-2 drinks (%) | 1726 (93.96%) | 6202 (87.96%) |
| 3+ drinks (%) | 27 (1.47%) | 225 (3.19%) |
| Current smoker (%) | 247 (13.45%) | 1131 (16.04%) |
| Body mass index (ref=normal) | ||
| Overweight (%) | 658 (35.82%) | 2798 (39.68%) |
| Obese (%) | 893 (48.61%) | 1549 (21.97%) |
| Current hypertension (%) | 1300 (70.77%) | 3281 (46.53%) |
| Current stroke (%) | 176 (9.58%) | 388 (5.5%) |
| Current heart disease (%) | 589 (32.06%) | 1364 (19.34%) |
| Current depression (%) | 329 (17.91%) | 769 (10.91%) |
| Current self-rated health (ref=good) | ||
| Low (%) | 718 (39.09%) | 1276 (18.1%) |
| High (%) | 399 (21.72%) | 3555 (50.42%) |
| Current marital status (ref=married) | ||
| Divorced/separated | 196 (10.67%) | 712 (10.1%) |
| Widowed | 343 (18.67%) | 1217 (17.26%) |
| Never married | 44 (2.4%) | 185 (2.62%) |
| Childhood SES (mean, std) | −0.09 (0.93) | 0.15 (1.03) |
| High school completion (%) | 1356 (73.82%) | 5843 (82.87%) |
| College completion (%) | 330 (17.96%) | 1699 (24.1%) |
| Household income (median, IQR) | $23,669 ($13,461-$41,296) | $29,792 ($16,958-$53,076) |
| Household wealth (median, IQR) | $93532 ($26,164-$266,368) | $173,242 ($60,741-$411,074) |
| Baseline HbA1c (mean, std) | 6.8 (1.3) | 5.5 (0.42) |
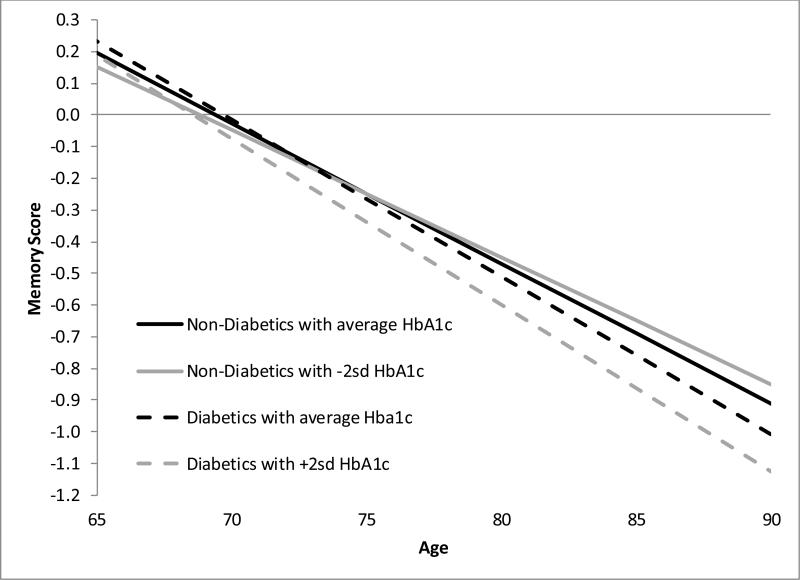
In a pooled sample including diabetics and non-diabetics, in the model without adjustment for covariate-by-age interaction terms, the average rate of memory decline was 0.443 memory score units per decade for people without diabetes and 0.480 memory score units per decade for people with diabetes (β for the difference=−0.037; 95% CI: −0.064, −0.010; p=0.007) (Table 2, Model 1). The association between diabetes and decline remained when the covariate-by-age interactions were included. We tested whether the relationship between HbA1c and memory decline differed for individuals with HbA1c greater than and less than 6.5% (the cutoff for diabetes diagnosis). A percentage point increase in HbA1c corresponded to an estimated 0.052 unit decrease in memory per decade (95% CI: −0.078, −0.026; p<0.0001) (Table 2, Model 2). Figure 2 depicts linear memory decline curves for diabetics and non-diabetics at different levels of HbA1c. Although results without covariate-by-age interaction terms suggested effects of HbA1c were smaller for individuals with HbA1c greater than 6.5% (β=0.044 for the difference in slope for a percentage point increase in HbA1c; 95% CI: 0.005, 0.082; p=0.026), in a fully adjusted model with covariate-by-age interaction terms, there was no evidence of a difference in slope for individuals with an HbA1c over 6.5% (p=0.440). Diabetes did not predict memory decline once HbA1c was included in the model. Estimated associations were qualitatively similar when cholesterol medication use was included in the model (Table 2, Model 3).
Table 2.
Diabetes and HbA1c predicting memory decline in a pooled sampled (i.e. both diabetics and non-diabetics).
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| No Confounder*Age Interactions |
Confounder*Age Interactions |
No Confounder*Age Interactions |
Confounder*Age Interactions |
No Confounder*Age Interactions |
Confounder*Age Interactions |
|
| Diabetes | 0.0001 (−0.016, 0.016) | −0.013 (−0.027, 0.001) | −0.010 (−0.028, 0.009) | −0.019 (−0.036, −0.002) | −0.016 (−0.035, 0.004) | −0.021 (−0.039, −0.004) |
| Age (centered at 70, in decades+) | −0.443 (−0.456, −0.430) | −0.527 (−0.575, −0.479) | −0.494 (−0.521, −0.467) | −0.556 (−0.609, −0.502) | −0.509 (−0.541, −0.477) | −0.588 (−0.647, −0.528) |
| Age*Diabetes | −0.037 (−0.064, −0.010) | −0.030 (−0.057, −0.004) | 0.001 (−0.032, 0.035) | −0.006 (−0.038, 0.026) | 0.010 (−0.025, 0.045) | −0.003 (−0.036, 0.031) |
| HbA1c (centered at 6.5) | 0.027 (0.012, 0.043) | 0.020 (0.006, 0.035) | 0.021 (0.004, 0.038) | 0.017 (0.001, 0.032) | ||
| Age*HbA1c | −0.052 (−0.078, −0.026) | −0.028 (−0.053, −0.004) | −0.051 (−0.080, −0.023) | −0.034 (−0.062, −0.007) | ||
| HbA1c_2 (spline term for those with HbA1c >= 6.5) | −0.044 (−0.067, −0.022) | −0.036 (−0.057, −0.015) | −0.034 (−0.059, −0.01) | −0.030 (−0.053, −0.008) | ||
| Age*HbA1c_2 | 0.044 (0.005, 0.082) | 0.014 (−0.021, 0.049) | 0.045 (0.003, 0.088) | 0.025 (−0.015, 0.064) | ||
| Cholesterol medication use | 0.026 (0.012, 0.040) | 0.016 (0.004, 0.029) | ||||
| Age*Cholesterol medication use | 0.014 (−0.009, 0.038) | 0.023 (0.001, 0.045) | ||||
All age main effects and interaction terms are measured in decades.
All models are weighted to adjust for survival and participation.
Bold indicates p<0.05
Figure 2.
Linear memory decline curves based on Model 2 in Table 2 (with no confounder*age interactions) for diabetics and non-diabetics at different levels of HbA1c.
Results restricted to non-diabetics were similar to the pooled estimates, but we found no evidence of an association between HbA1c and memory decline among diabetics alone (Table 3). However, point estimates among diabetics were suggestive of an association large enough to be clinically relevant, if we were powered to detect one of this magnitude. Results were similar in sensitivity analyses where we included the 345 individuals who had HbA1c values of 6.5% or higher (the cut point for diabetes diagnosis) but self-reported never being diagnosed with diabetes. In models controlling for APOE-4 status, while there was a main effect of APOE-4 on memory decline, we found no evidence of an interaction with either diabetes (β=0.045; 95% CI: −0.020, 0.110; p=0.173 for the interaction of APOE-4, diabetes, and age) or HbA1c (β=0.019; 95% CI: −0.044, 0.082; p=0.554 for the interaction of APOE-4, HbA1c, and age) in a pooled sample.
Table 3.
HbA1c predicting memory decline in diabetes-stratified samples.
| Diabetics | Non-Diabetics | |||
|---|---|---|---|---|
| No Confounder*Age Interactions | Confounder*Age Interactions | No Confounder*Age Interactions | Confounder*Age Interactions | |
| HbA1c (centered at mean) | −0.011 (−0.022, 0.0003) | −0.011 (−0.021, −0.0002) | 0.032 (0.014, 0.05) | 0.022 (0.005, 0.038) |
| Age (centered at 70, in decades+) | −0.492 (−0.521, −0.464) | −0.667 (−0.783, −0.551) | −0.439 (−0.453, −0.426) | −0.513 (−0.567, −0.460) |
| Age*HbA1c | −0.016 (−0.034, 0.003) | −0.013 (−0.030, 0.004) | −0.053 (−0.082, −0.025) | −0.023 (−0.051, 0.004) |
All age main effects and interaction terms are measured in decades.
All models are weighted to adjust for survival and participation.
Since the pooled models showed a possible smaller association between a percentage point increase in HbA1c and memory decline among individuals with T2D (i.e., HbA1c > 6.5%), we conducted a sensitivity analysis investigating the relationship between log-transformed HbA1c and memory decline. A 10% increase in HbA1c (e.g. from 6.0% to 6.6%) was associated with approximately a 0.02 faster memory score decline per decade (p<0.001). Model fit was extremely similar for this model compared to a model using non-transformed HbA1c (QIC = 28082.01 vs. 28081.96). Applying IPWs to adjust for selective survival and attrition did not qualitatively change the results of any models.
DISCUSSION
Our results suggest that both diabetes and HbA1c predict memory decline in a national sample after controlling for a comprehensive set of social, behavioral, and health-related confounders. Diabetes was associated with a 0.037 unit faster decline in memory score per decade, which is about 8% of the estimated magnitude of association between a 10-year increase in age and decline. A percentage point increase in HbA1c was associated with a 0.052 unit decrease in memory score per decade, about 10% of the association between a 10-year increase in age and rate of decline. We found no consistent evidence that the association between HbA1c and decline differed based on HbA1c level or diabetes status, although point estimates for a percentage point increase in HbA1c were attenuated among individuals with T2D (i.e., HbA1c > 6.5%). Adjustment for cholesterol medication use did not meaningfully alter the results, consistent with a recent meta-analysis that showed no adverse effects of statin use on cognition 35. Our findings suggest that 1) among diabetics, poor management and higher HbA1c levels may adversely impact rate of memory decline; and 2) among non-diabetics, higher HbA1c levels – even below the threshold for diabetes diagnosis (i.e., “prediabetes” levels) – predict faster memory decline.
Although there is robust association linking diabetes and dementia, there is inconsistent evidence on the association between diabetes and cognitive decline. Many studies test the association between diabetes and rate of change in multiple cognitive domains and find an association between diabetes and one or two cognitive domains assessed, but there is no reliable association in any individual domain across studies 3. For example, memory is among the most commonly assessed cognitive domains and is of particular interest because it is a hallmark of Alzheimer's disease. While some researchers have found an association between diabetes and rate of memory decline 5-8, others have not 9-12. In particular, one study by Wu et al. used earlier data from the same cohort (HRS) and our memory scores as one of their outcomes, but did not find an association between diabetes and memory decline. However, the authors only included individuals over the age of 65. The difference may also reflect chance, as the 95% CIs reported by Wu et al., include our point estimates.
Other domains of cognition that have been assessed in multiple studies – with no consistent finding – in relation to T2D include processing speed, executive function, and verbal fluency 5,7,8,10-12,16. These discrepant findings may be due to an underlying weak association between T2D and rate of cognitive decline, high signal-to-noise ratios, or may reflect methodological differences between the studies. For example, the range of cognitive domains and neuropsychological tests used, how rate of cognitive decline was modeled, the varied length of follow-up, potential bias due to differential attrition or mortality, or differences in sample characteristics.
Establishing that diabetes predicts longitudinal change in cognition is central for ruling out confounding as the explanation for the diabetes-dementia link. Numerous social or behavioral factors that potentially influence diabetes risk, for example educational quality, may also predict lower average cognitive reserve. The previously documented association between diabetes and incident dementia may therefore reflect either confounding or mechanisms mediated by early life cognitive development and cognitive reserve. Indeed, previous Mendelian Randomization studies showed that a polygenic score combining all genetic variants confirmed to be associated with Type 2 Diabetes does not predict dementia risk 36,37. Findings in Walter et al., suggested that genetic variants related to insulin sensitivity, as opposed to polymorphisms affecting adiposity or β-cell function, might have a distinct relationship with dementia risk, however. Our finding that blood glucose levels predict longitudinal memory loss therefore substantially bolsters the evidence for an etiologic role of diabetes in dementia pathology and is consistent with the previous results on insulin sensitivity.
There is even less research investigating the relationship between HbA1c and cognitive decline to date. Many studies have been restricted to individuals diagnosed with diabetes16,18,38,39, while others included both diabetics and non-diabetics with stratification by diabetes diagnosis 6,7,17. Prior studies have found more consistent associations between blood glucose levels and decline in other domains of cognition, such as processing speed and executive function, compared to decline in memory 6. Only one other study adjusted for attrition 6, and none adjusted for selective survival; however, since our use of IPWs did not qualitatively change results, bias due to selective survival and attrition may be small. Additionally, our analyses included all participants with available data, regardless of baseline cognitive status. This approach has the advantage of allowing us to more accurately identify long-term consequences of diabetes-related processes.
Possible biological mechanisms linking T2D and cognition include chronic hyperglycemia as well as hyperinsulinemia and functional insulin deficiency in the brain. In addition, some risk factors for dementia, such as severe hypoglycemic events and depression, are more common in individuals with T2D and could explain the association 3. This study lends support to the hypothesis that chronic hyperglycemia is a key pathway linking diabetes and memory decline, most likely via microvascular injury. It is possible that other mechanisms contribute to the explanation of the diabetes-memory relationship and they may also link diabetes with other domains of cognition. Each of these proposed mechanisms could impact cognitive decline and dementia via either contributing to Alzheimer's disease pathogenesis or vascular disease. Thus, we cannot discern whether HbA1c is associated with memory loss in our study because of chronic hyperglycemia influencing vascular disease or AD pathology.
One main limitation to this study is the lack of assessment of many important domains of cognition, other than memory. Additionally, we cannot distinguish the cause of memory decline, e.g. Alzheimer's disease versus vascular disease. Our measure of diabetes status is self-reported physician diagnosis, and therefore could suffer from bias if individuals cannot accurately report whether they have been diagnosed with diabetes. However, we excluded people whose HbA1c levels were inconsistent with their self-reported diabetes status. Further, we only have one measure of HbA1c per person, so we cannot examine trends in HbA1c levels or how time-updated HbA1c levels affect memory decline. We do not characterize duration of diabetes or duration of exposure to chronic hyperglycemia, which may be etiologically relevant. Since we did not time-update diabetes status, some individuals may have become diabetic during the follow-up period. This misclassification might have slightly attenuated effect estimates; however, we anticipate any such bias would be small because the effects of HbA1c on cognition probably take many years to manifest. Finally, we do not have as long a follow-up as Rawlings et al6, although we averaged a greater number of cognitive assessments for each participant.
The strengths of the HRS sample for evaluating this research question outweigh the above limitations. The HRS is a national sample, not a multi-site study, so we were able to control for birth region instead of only field site. Since our sample includes individuals aged 50+ at DBS collection, our younger population avoids possible bias due to selective survival 21. Finally, HRS has rich social variables, so we were able to control for a battery of hypothesized social confounders. Most other studies only include high school completion as their adjustment for SES, but childhood SES, college completion, and later life income and wealth may still confound after high school completion is included in the model 40.
In conclusion, diabetes and higher levels of HbA1c both predicted faster memory decline after controlling for an extensive battery of potential social confounders in a large, U.S. dataset. Our results add support for a causal pathway from blood glucose levels to development of dementia, although precise biological mechanisms are still unknown. Even without diabetes, elevated blood glucose appears to have adverse neurocognitive consequences.
ACKNOWLEDGEMENT
Sources of Funding:
Dr. Marden and Dr. Tchetgen Tchetgen received support from the National Institute of Allergy and Infectious Diseases (grant #: NIAID R01 AI104459).
Footnotes
Conflicts of Interest
None of the authors have any conflicts of interest or financial disclosures.
References
- 1.Cheng G, Huang C, Deng H, et al. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Internal medicine journal. 2012;42(5):484–491. doi: 10.1111/j.1445-5994.2012.02758.x. [DOI] [PubMed] [Google Scholar]
- 2.Mayeda ER, Haan MN, Kanaya AM, et al. Type 2 diabetes and 10-year risk of dementia and cognitive impairment among older Mexican Americans. Diabetes care. 2013;36(9):2600–2606. doi: 10.2337/dc12-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayeda ER, Whitmer RA, Yaffe K. Diabetes and Cognition. Clinics in geriatric medicine. 2015;31(1):101–115. doi: 10.1016/j.cger.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet neurology. 2012 Nov;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spauwen PJ, Köhler S, Verhey FR, et al. Effects of type 2 diabetes on 12-year cognitive change results from the maastricht aging study. Diabetes care. 2013;36(6):1554–1561. doi: 10.2337/dc12-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawlings AM, Sharrett AR, Schneider AL, et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Annals of internal medicine. 2014;161(11):785–793. doi: 10.7326/M14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuligenga RH, Dugravot A, Tabák AG, et al. Midlife type 2 diabetes and poor glycaemic control as risk factors for cognitive decline in early old age: a post-hoc analysis of the Whitehall II cohort study. The Lancet Diabetes & Endocrinology. 2014;2(3):228–235. doi: 10.1016/S2213-8587(13)70192-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comijs HC, Kriegsman DM, Dik MG, et al. Somatic chronic diseases and 6-year change in cognitive functioning among older persons. Archives of gerontology and geriatrics. 2009;48(2):191–196. doi: 10.1016/j.archger.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Tchetgen Tchetgen E, Osypuk T, et al. Estimating the Cognitive Effects of Prevalent Diabetes, Recent Onset Diabetes, and the Duration of Diabetes among Older Adults. Dementia and geriatric cognitive disorders. 2015;39(3-4):239–249. doi: 10.1159/000368654. [DOI] [PubMed] [Google Scholar]
- 10.Knopman DS, Mosley TH, Catellier DJ, et al. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimer's & Dementia. 2009;5(3):207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Archives of neurology. 2004;61(5):661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 12.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craft S, Cholerton B, Baker LD. Insulin and Alzheimer's disease: untangling the web. Journal of Alzheimer's disease : JAD. 2013;33(Suppl 1):S263–S275. doi: 10.3233/JAD-2012-129042. [DOI] [PubMed] [Google Scholar]
- 14.Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA : the journal of the American Medical Association. 2009;301(15):1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. New England Journal of Medicine. 2013;369(6):540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaffe K, Falvey C, Hamilton N, et al. Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Archives of neurology. 2012;69(9):1170–1175. doi: 10.1001/archneurol.2012.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christman A, Matsushita K, Gottesman R, et al. Glycated haemoglobin and cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2011;54(7):1645–1652. doi: 10.1007/s00125-011-2095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umegaki H, Kawamura T, Umemura T, et al. Factors associated with cognitive decline in older adults with type 2 diabetes mellitus during a 6-year observation. Geriatrics & gerontology international. 2015;15(3):302–310. doi: 10.1111/ggi.12273. [DOI] [PubMed] [Google Scholar]
- 19.Gaskin DJ, Thorpe RJ, Jr, McGinty EE, et al. Disparities in diabetes: the nexus of race, poverty, and place. American journal of public health. 2014;104(11):2147–2155. doi: 10.2105/AJPH.2013.301420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. Journal of the American Geriatrics Society. 2010;58(5):889–894. doi: 10.1111/j.1532-5415.2010.02818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weuve J, Proust-Lima C, Power MC, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015 Sep;11(9):1098–1109. doi: 10.1016/j.jalz.2015.06.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crimmins E, Faul J, Kim J, et al. Documentation of Biomarkers in the 2006 and 2008 Health and Retirement Study. Survey Research Center University of Michigan; Ann Arbor, MI: 2013. [Google Scholar]
- 23.Juster F, Suzman R. An overview of the health and retirement study. Journal of Human Resources. 1995;30(suppl):S7–S56. [Google Scholar]
- 24.Association AD. Standards of medical care in diabetes--2014. Diabetes care. 2014;37:S14. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 25.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994 Feb;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q, Tchetgen ET, Osypuk T, et al. Combining direct and proxy assessments to reduce attrition bias in a longitudinal study. Alzheimer's Disease and Associated Disorders. 2012 doi: 10.1097/WAD.0b013e31826cfe90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langa KM, Plassman BL, Wallace RB, et al. The aging, demographics, and memory study: Study design and methods. Neuroepidemiology. 2005;25(4):181–191. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- 28.Glymour MM, Weuve J, Chen JT. Methodological challenges in causal research on racial and ethnic patterns of cognitive trajectories: measurement, selection, and bias. Neuropsychology review. 2008;18(3):194–213. doi: 10.1007/s11065-008-9066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White K, Mondesir FL, Bates LM, et al. Diabetes risk, diagnosis, and control: do psychosocial factors predict hemoglobin A1c defined outcomes or accuracy of self-reports? Ethnicity & disease. 2014;24(1):19–27. [PubMed] [Google Scholar]
- 30.Steffick DE. Documentation of affective functioning measures in the Health and Retirement Study. University of Michigan; Ann Arbor, MI: 2000. [Google Scholar]
- 31.Feldman H, Doody R, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease LEADe. Neurology. 2010;74(12):956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 32.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004 Sep;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 33.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology (Cambridge, Mass.) 2012 Jan;23(1):119–128. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tchetgen EJT, Glymour MM, Weuve J, et al. Specifying the correlation structure in inverse-probability-weighting estimation for repeated measures. Epidemiology. 2012;23(4):644–646. doi: 10.1097/EDE.0b013e31825727b5. [DOI] [PubMed] [Google Scholar]
- 35.Ott BR, Daiello LA, Dahabreh IJ, et al. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. Journal of general internal medicine. 2015;30(3):348–358. doi: 10.1007/s11606-014-3115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Østergaard SD, Mukherjee S, Sharp SJ, et al. Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study. PLoS Med. 2015;12(6):e1001841. doi: 10.1371/journal.pmed.1001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter S, Marden JR, Kubzansky LD, et al. Diabetic Phenotypes and Late-Life Dementia Risk: A Mechanism-specific Mendelian Randomization Study. Alzheimer disease and associated disorders. 2015 doi: 10.1097/WAD.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luchsinger JA, Palmas W, Teresi JA, et al. Improved diabetes control in the elderly delays global cognitive decline. The journal of nutrition, health & aging. 2011;15(6):445–449. doi: 10.1007/s12603-011-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. The Lancet Neurology. 2011;10(11):969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA : the journal of the American Medical Association. 2005;294(22):2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]