Abstract
Prostate cancer (PCa) is one of the most lethal cancers in western countries. Androgen receptor (AR) signaling pathway plays a key role in PCa progression. Despite the initial effectiveness of androgen deprivation therapy (ADT)for treatment of patients with advanced PCa, most of them will develop resistance to ADT and progress to metastatic castration resistant prostate cancer (mCRPC). Constitutively transcriptional activated AR splice variants (AR-Vs) have emerged as critical players in the development and progression of mCRPC. Among AR-Vs identified to date, AR-V7 (a.k.a. AR3) is one of the most abundant and frequently found in both PCa cell lines and in human prostate tissues. Most of functional studies have been focused on AR-V7/AR3 and revealed its role in regulation of survival, growth, differentiation and migration in prostate cells. In this review, we will summarize our current understanding of regulation of expression and activity of AR-Vs in mCRPC.
Keywords: Prostate cancer, Androgen receptor splicing variants, Metastatic castration resistant prostate cancer
Prostate cancer (PCa) is the second most lethal cancer in men [1]. Patients with localized PCa do not develop a life-threatening syndrome and are curable by either surgery or radiation treatment. However, those with more advanced metastasis PCa have only very limited treatment options. Androgen deprivation therapy (ADT) is the standard treatment for metastatic PCa. Unfortunately, a majority of patients will relapse and inevitably develop more aggressive metastatic castration resistant prostate cancer (mCRPC). Though there are few FDA approved treatment options for mCRPC (i.e., taxane compound, abiraterone, enzalutamide and sipuleucel-T), drug resistance will eventually develop. There is no effective treatment for relapsed mCRPC to date [2]. Therefore, there is an urgent need to better understand molecular basis of the development, progression, and drug resistance of mCRPC. Androgen receptor (AR) plays a key role in PCa progression. AR is a transcription regulator belonging to the nuclear receptor family and is the major mediator of androgen signaling in prostate cells. It consists of an N-terminal transactivation domain, a DNA binding domain, a hinge region, and a C-terminal ligand binding domain [3]. AR was stabilized by molecular chaperone heat shock protein 90 (HSP90) in the cytoplasm. Upon androgen (i.e., testosterone and dihydrotestosterone) binding, AR dissociates from HSP90, forms a homodimer and translocates into the nucleus. Once in the nucleus, it binds to androgen response element (ARE) in the regulatory region of its target genes and modulates their expression [4], [5]. One of AR target genes prostate specific antigen (PSA) is currently the most sensitive and widely used biomaker monitoring the presence, progression and therapeutic responses in PCa [4], [6]. More importantly, it is well-known that androgen-AR pathway contributes to PCa cell proliferation, inhibits apoptosis, and promotes metastasis [4]. Thus, inhibition of AR function by ADT therapy is a first-line treatment of advanced PCa patients.
1. Expression and activity of AR splice variants (AR-Vs)
1.1. Expression of AR-Vs
In recent years, AR-Vs have emerged as potential important players in PCa progression and therapeutic resistance. Since 2008, more than a dozen of AR-Vs lacking the ligand binding domain have been identified from human PCa cell lines and xenografts [7], [8], [9], [10], [11], [12]. The updated lists of AR-Vs are summarized in previous reviews [12], [13]. These AR-Vs attracted much attention in the field due to their ligand-independent transcription activity. The presence of these AR-Vs transcripts in human prostate tissues have been validated by RT-PCR and RNA-seq [8], [10], [14]. Among them, AR-V7/AR3 is the only variant whose protein expression has been detected in human prostate tissues using isoform specific antibodies [8], [10]. The relative abundance of AR-V7/AR3 transcript appears to be highest among various AR-Vs detected in human prostate tissues [8], [14]. It should be noted that the cryptic exon utilized in AR-V7 (Exon 3-CE3) or AR3 (Exon 3b) is also detectable in benign tissues [8], [14], suggesting that this variant may also have a functional role in normal prostate. In general, AR-Vs have higher expression in more aggressive and metastatic tumor derived cell lines and xenograft models [8], [10], [11]. Among AR-Vs, AR-V7/AR3 and ARV567es appear to have relatively higher expression level than other isoforms. Hu et al.'s RT-PCR data showed that expression level of AR-V7/AR3 was significantly higher in mCRPC patients than in hormone-naive PCa patients [10]. To further characterize the expression profile of AR-V7/AR3, Guo et al. [8] developed a polyclonal antibody specific for AR3/AR-V7. In consistence with Hu et al.'s data, immunohistochemistry analysis revealed that AR3/AR-V7 expression is significantly increased in more malignant PCa tissues. Interestingly, it was observed that AR3 expression redistributed from basal and stromal cells to luminal epithelial cells with the increasing aggressiveness of PCa. In addition, the staining also showed that nuclear translocation of AR3 is significantly increased in mCRPC patient samples compared with hormone-naive PCa samples [8]. These studies suggested a potential functional activation of AR-V7/AR3 during PCa progression.
Bone is the primary site where metastatic PCa spreads. A recent clinical report showed that transcripts of AR variants (AR-V1, AR-V7/AR3, and ARV567es) were increased in PCa bone metastasis. The higher expression of AR-V7/AR3 and ARV567es in bone metastasis was associated with poorer prognosis [15]. The correlation of AR-Vs and other important parameters in PCa were also monitored. It is well known that PSA level after castration is a prognostic biomarker for distal metastasis. Kaplan–Meier analysis indicated that patients with higher AR3 cytoplasmic staining usually have higher PSA recurrence and lower overall survival probability [8]. A more recent study on a larger cohort of patients further validated the importance of AR-Vs, especially AR-V7/AR3, in metastatic PCa and CPRC [16]. They compared AR-V7/AR3 expression among three patient groups (localized PCa n = 100, newly diagnosed metastatic PCa n = 104, and mCRPC n = 46). The number of AR-V7/AR3 positive patients was significantly larger in newly diagnosed metastatic PCa group compared to localized PCa group. This rate increased more dramatically when comparing mCRPC to localized PCa. More importantly, the data revealed that the presence of AR-V7/AR3 was associated with shorter survival [16]. Thus, these studies suggest a strong link between AR-Vs and PCa progression. A recent study further support that AR-V7/AR3 is related to and valuable for clinical application. It showed that AR-V7/AR3 can be detected in circulating tumor cells from mCRPC patients. Patients with higher AR-V7/AR3 expression in circulating tumor cells had shorter PSA progression-free survival [17]. These findings suggest that AR-V7/AR3 may serve as a prognostic and predictive biomarker for metastasis PCa patients.
It has been well documented that transcription of AR genes is elevated upon androgen derivation possibly due to relief of negative feed-back regulation by AR–FL [18], [19]. The relative increase of AR-Vs compared to AR–FL in CRPC could be resulted from change of splicing factors, RNA binding proteins, microRNAs [19], [20], [21], [22]. RNA splicing factors U2AF65 and ASF/SF2 were shown to be critical for AR-V7/AR3 splicing under androgen deprivation condition [19]. RNA binding protein Sam68 could distinctly regulate AR–FL and AR-Vs transcription. Exogenous expression of Sam68 was significantly elevated AR-V7/AR3, but not AR–FL transcript levels [21]. In addition, epigenetic factors such as microRNAs are also involved AR-Vs transcript production. MiR-124 directly targeted AR-V4 and AR-V7/AR3 and downregulated their expression [22]. Thus, the expression of AR-Vs are likely regulated at multiple levels including transcriptional, posttranscriptional as well as posttranslational.
1.2. Regulation of AR-Vs activity
Although AR-Vs are shown to be able to regulate canonical AR target genes, it is still under debate whether AR-Vs have distinct functions or just serve as a substitute for AR-FL under androgen depleted condition [8], [10], [23]. Some studies suggest that the presence of AR–FL is critical for activation of AR-Vs. It has been shown that expression of AR-Vs regulated genes could be repressed by either ligand binding domain-targeted compound or AR–FL siRNA knockdown [23]. A recent mechanistic study also supports a role of AR–FL in AR-Vs’ function [24]. It was showed that AR–FL and AR-Vs formed a heterodimer mediated by DNA-binding domain and by N- and C-termini of AR-V and AR–FL, respectively. The dimerization seemed to be required for the function of AR-V. Mutants disrupting AR-V and AR–FL interaction diminished AR-V's transactivation and reduced growth rate of AR-V positive androgen-independent PCa cells [24]. This study suggests that though AR-Vs are constitutively active, they might still need the presence of AR–FL to exert their functions. However, such kind of study could not exclude the possibility that mutation of the FxxLF motif of AR-V may also compromise its ability to interact with other co-factors essential for its transcriptional activity. On the other hand, some studies suggest that AR-Vs may also exert its unique function independent of AR–FL in addition to their overlapping activity. Two studies have shown that selective knock-down of AR variants in CWR-R1 cells caused the changes of a unique subset of genes that are not affected by selective knockdown of AR–FL [8], [25]. It is also reported that AR-Vs is resistant to pharmacological inhibition of ligand binding domain of AR–FL. Specific knocking down of AR-Vs, but not AR–FL, had dramatically effects on canonical AR pathway genes expression under pharmacological inhibition [25]. A more recent report revealed that AR-V7/AR3 was capable of promoting PCa migration and sphere-formation under the condition that exogenous expression of AR3/AR-V7 alone in AR-negative PCa cells (PC3/DU145) by upregulating genes involved in epithelial-mesenchymal transition (EMT) [26]. This is consistent with a study in genetic modified mouse model in which AR3/AR-V7 is targeted expressed in mouse epithelial cells using the ARR2PB promoter [27]. In this mouse model, overexpression of AR3/AR-V7 in luminal epithelial cells appeared to antagonize the terminal differentiation process driven by AR–FL by promoting EMT. Perhaps, whether AR-Vs require AR–FL for their activity may very likely depend on cell context.
Currently, it is conceivable that AR-Vs are critical for maintaining AR transcriptomes under androgen deficient conditions. However, whether AR-Vs could initiate a distinct transcription program in addition to replacing AR–FL function to promote androgen independent PCa growth is still under debate. Several groups tried to identify AR-Vs regulated genes. Guo et al. [8] performed microarray analysis to compare AR–FL and AR-V7/AR3 regulated genes in PCa cells selectively knocking down AR-V or AR–FL. They showed that 71 genes were commonly regulated by both AR–FL and AR3 and 117 genes were preferentially regulated by AR-V7/AR3 but not AR–FL. Furthermore, their also identified novel ARE sites in the AKT1 regulatory regions by chromatin immunoprecipitation (ChIP) assay. These ARE sites were preferentially occupied by AR-V7/AR3 but not AR–FL. Interestingly, a study to compare AR binding sites in mCRPC and androgen-responsive PCa revealed a program shift of AR binding. Cell cycle regulator UBE2C is one of the top score targets of AR under androgen independent condition. In this study, the AR binding sites were identified by chromatin immunoprecipitation sequencing (ChIP-seq) analysis using an antibody recognizing the N-terminal part of AR which is common for both AR–FL and AR-Vs [28]. A follow up study using AR-V7/AR3 specific antibody validated the specific binding of UBE2C promoter by AR-V7/AR3 but not AR–FL under androgen depleted conditions [29]. In consistence with the ChIP assay, transient expression of exogenous AR-V7/AR3 preferentially modulated cell-cycle regulated genes. The expression of cell-cycle gene UBE2C appears to be correlated with AR-V7/AR3, but not AR–FL in both PCa cells and clinical mCRPC specimens [30]. However, in a later study, Li et al. [25] performed gene set enrichment analysis (GSEA) to examine the response of the identified “AR-V-specific” M-phase-genes in CWR-R1 cells. They found that AR-V regulated M-phase gene set was positively-enriched in androgen-induced AR gene signature derived from CWR-R1 cells. They also performed GSEA with gene expression datasets derived from LNCaP cells in response to different dose of dihydrotestosterone (DHT). Interestingly, the AR-V signature was positively enriched in the 1 nmol/L DHT dataset, but negatively enriched in the 100 nmol/L DHT dataset. They concluded that those mitotic genes appeared to be unique AR-V targets under androgen depleted conditions may reflect the proliferative events driven either by AR-Vs or androgen-stimulated AR. In addition, it has been showed that AR-Vs may bind to its unique targets independently of AR–FL, but coordinate with AR–FL on regulating canonical AR target genes [29]. In addition, distinct roles of AR-Vs might be attributed to their ability of binding of unique mediators. It has been shown that MED1 can be co-immunoprecipitated with ARV567es independently of AR–FL. ARV567es and P-MED1 co-occupies on the regulatory regions of UBE2C genes and promotes UBE2C expression independently of androgen [31]. Another report showed that RNA-binding protein Sam68 interacts with AR-V7/AR3 and enhances its transcriptional activity [21].
It is well established that ligand binding promotes AR to dissociate from chaperone HSP90 in the cytoplasm and translocate to the nucleus. However, AR-Vs only contain a portion of nuclear localization sequence (NLS) and lack the C-terminal AR dimerization domain and HSP90 binding domain. It is conceivable that the nuclear translocation mechanism of AR-Vs might differ from that of AR–FL [8], [32], [33]. It was showed that AR-Vs nuclear localization was independent on AR–FL and canonical HSP90 chaperone shuttling [34]. Recent studies revealed that microtubule-stabilizing taxane compound was capable of inhibiting nuclear translocation and transcriptional activity of AR–FL [35], [36]. The subcellular localization of AR–FL was associated with the patients' response to taxane chemotherapy [37]. On the other hand, AR-V was shown to be associated with microtubules to a much less extent than AR–FL. Several studies showed that nuclear translocation of AR-V7/AR3 was independent on microtubule motor protein dynein while AR–FL does [32], [37]. Instead, a study has shown that nuclear translocation of AR-V7/AR3 can be inhibited by an import in β inhibitor [38]. These data suggest that nuclear translocation and activation of AR-Vs might be modulated by different mechanisms. More importantly, because nuclear localization and activity of AR-V7/AR3 is not inhibited by standard taxane chemotherapy, it is likely that AR-V7/AR3 may play a greater role in mCRPC after taxane treatment [32]. Along the same line, nuclear localized AR-V7/AR3 is able to trans-active canonical and non-canonical AR pathway genes and promotes PCa growth under androgen depleted conditions [8], [10], [29], [34].
2. Functions of AR-Vs in metastasis PCs and therapeutic resistance
2.1. Transcription regulation
It is conceivable that AR-Vs play important roles in PCa progression and promote castration resistant growth. Overexpression of AR-V7/AR3 in AR-Vs negative PCa cells promoted cell growth under androgen depleted conditions. Knocking down of AR-V7/AR3 in AR-Vs positive PCa cells significantly attenuated cell growth [8]. As mentioned above, in addition to the canonical AR–FL regulated genes, AR-Vs may regulate a subset of M-phase genes under androgen deprivation conditions. Gene set enrichment analysis indicated that AR-V7/AR3 is capable of inducing the expression of cell-cycle related genes. UBE2C is one the cell-cycle genes specifically regulated by AR-V7/AR3 under androgen depleted conditions. AR-V7/AR3, but not AR–FL, binds to the regulatory regions of UBE2C and promotes it expression [29], [30]. The expression correlation between UBE2C and AR-V7/AR3 has also been confirmed in clinical samples [30]. On the other hand, genes regulated by AR–FL are primarily related to biosynthesis, metabolism and secretion [30]. In parallel with this finding, knockdown of AR-V7/AR3 significantly reduced DNA synthesis while apoptosis was unaffected. In contrast, knockdown of AR–FL promoted apoptosis [8]. The effect of AR-V7/AR3 on cell growth is partially contributed by AR-V7/AR3 specific regulation of serine/threonine kinase AKT1 [8]. A recent report also showed that AR-V7/AR3 transcriptional activity can be inhibited by multiple AKT inhibitor and restoration of PTEN activity [39]. These data suggested that AR-V7/AR3 and PI3K-AKT pathway might form a positive regulation loop that contributes to PCa progression. This feedback loop is distinct from the negative feedback loop that AR–FL and PI3K-AKT repress the activity of each other [40], [41].
In addition to cell-cycle regulation, AR-V7/AR3 is a critical factor for PCa metastasis. The AR3 transgenic (AR3Tg) mouse generated by Sun and his colleague [27] provides a new tool to study the role of AR-V7/AR3 in prostate. Their microarray analysis revealed 414 genes that were differentially expressed in AR3Tg compared to wild-type animals. Gene set analysis indicated that AR3 regulates multiple tumor-associated autocrine/paracrine factors including TGFβ2 and IGF1. TGFβ and IGF signaling are well-known positive contributor to PCa progression. Interestingly, a recent report showed that IGF-1R might be one of the upstream signaling of AR. IGF signaling modulates both AR–FL and constitutively active AR-Vs activity via receptor phosphorylation [42]. Thus, it is possible that AR-V7/AR3 and TGFβ2/IGF1 form a positive regulatory loop in androgen independent PCa. As demonstrated in another report, the link between AR-V7/AR3 and autocrine/endocrine signaling is that AR-V7/AR3 expression and activation is associated with TNF-NFκ-B signaling. NF-κB activates AR-V7/AR3 modulates ADT drug enzalutamide resistance [43]. Down-regulation of NF-κB signaling decreased AR-V7/AR3 expression and restored the growth inhibiting effect of anti-androgen drug [44]. Autocrine/paracrine pathway signaling factors have been shown to have important function on the proliferation, survival, migration and maintenance of stem cell in various types of cancer including PCa [45], [46], [47], [48]. Thus, these data suggest that the regulatory network of AR-V7/AR3 and autocrine/paracrine signaling is critical for CRPC progression.
Accompanied with the elevated autocrine/paracrine signaling, the expression of a number of EMT associated genes including N-cadherin, vimentin, snail and twist were also increased in AR-V7/AR3 overexpressed PCa cells and AR3Tg animals [27]. These finding were further validated by another group in multiple PCa cell lines. Overexpression of AR-V7/AR3 in LNCaP and DU145 cells led to upregulation of EMT markers fibronectin and ZEB1, respectively. Wound healing assay showed that AR-V7/AR3 is able to promote cell migration [26]. The correlation of AR-V7/AR3 and EMT markers highly suggest a role of AR-V7/AR3 in PCa metastasis [26], [27], [49]. In addition to EMT genes, stem cell signatures genes were also observed to be increased in AR3Tg prostate [27]. In consistence with the observations in AR3Tg, knocking down of AR-V7/AR3, but not AR–FL, reduced the expression of stem cell marker genes Nanog and Oct4 [26]. Overexpression of AR-V7/AR3 in PCa cells promote prostasphere formation [26]. In summary, these data suggest that AR-V7/AR3 contributes to PCa metastasis and mCRPC progression through promoting the process of EMT and acquiring stem cell properties.
2.2. Epigenetic regulation
Epigenetic regulation refers to dynamic alternations of the molecule function without the change of DNA sequence. Epigenetic regulation including DNA methylation, histone modification, microRNA and long non-coding RNA and other non DNA sequence alterations lead to molecular changes. The importance of epigenetics regulation in cancers including PCs has long been discovered [50]. Average DNA methylation and histone methyltransferase EZH2 level are increased in mCRPC [51], [52], [53]. This up-regulation of epigenetic elements play critical roles in PCa progression and the development of mCRPC [54]. AR has recently been shown contribute to the epigenetic regulation in multiple aspects. AR can recruit epigenetic co-activator/repressor to modulate certain genes expression. Association of AR with histone demethylase LSD1 and JMJC is critical for downstream androgen responsive genes expression [55]. Epigenetic factor EZH2 acts as a coactivator of AR and the interaction is critical for mCRPC [53]. AR is not only able to cooperate with protein. A recent finding showed that AR bound to lncRNA PRNCR1 and PCEGM1 which enhance both androgen dependent and androgen independent AR regulatory program and PCa proliferation [56]. In addition to cooperate with epigenetic factors, AR is also able to regulate gene expression via modulating epigenetic factors expression. A study showed that AR is able to regulate EZH2 expression through modulating miR-101 expression [57]. Androgen induced miR-21 upregulation was shown to play important roles in hormone-dependent and hormone-independent PCa growth [58]. The close relationship of AR and epigenetic factors opened a new window of how AR-Vs and epigenetic factors cooperate. Due to the lack of C-terminus ligand binding domain, AR-Vs may exert distinct roles in epigenetic regulation compared with AR–FL. Indeed, a few reports published in recent years gave us some clues of the role of AR-Vs in epigenetic regulation. Microarray and real-time PCR data in AR3Tg mouse model revealed that miR-29 expression was decreased when compared with the wild-type control [27]. MiR-29 is a critical tumor suppressor microRNA which was reported to be down-regulated in various types of cancer. MiR-29 serves as a tumor suppressor which is involved in multiple tumor related pathways including immune regulation, cell proliferation, cell senescence, apoptosis, and metastasis [59]. In addition to target traditional tumor-related pathways, miR-29 is also able to target epigenetic factor DNA methyltransferase (DNMT) [59]. DNMT is responsible for establishing and maintaining DNA methylation and the pattern of DNA methylation is critical for cancer progression. In consist with the hypothesis that AR-V7/AR3 may regulate DNMT expression via miR-29, a recent report showed that DNMT expression and AR-V7/AR3 expression were shown cooperatively up-regulated in 22Rv1 androgen independent cells with long term bicalutamide treatment [60]. Interestingly, this report also showed that DNMT activity was significantly decreased after addition of androgen, suggesting AR–FL and AR-Vs may have distinct roles in regulating DNMT activity. In conclusion, these data suggested that AR-V7/AR3 may regulate multiple PCa progression pathways via modulating miR-29.
2.3. AR-Vs and therapeutic response
Multiple lines of evidence indicated that AR-Vs is responsible for ADT therapy (bicalutamide and enzalutamide) resistance. Knockdown of AR-Vs, but not AR–FL, decreased androgen-independent PCa cells growth under bicalutamide or enzalutamide treatment [25]. The landmark of AR-Vs and ADT resistance in PCa patients is a study on 62 patients showed that detection of AR-V7 transcript in pooled epithelial cell adhesion molecule (EpCAM)-positive circulating tumor cells (CTCs) of men with progressive mCRPC was associated with resistance to the AR signaling inhibitors abiraterone and enzalutamide [17]. A subsequent study on 161 men with mCRPC showed that nuclear expression of AR-V7 protein were found in CTCs from 18% of patients. Patients who had AR-V7–positive CTCs were resistant to treatment of AR signaling inhibition but still responsive to taxane treatment [61]. These studies suggest that AR-V7 could represent a biomarker to guide treatment selection in mCRPC.
AR-Vs not only contribute to ADT resistance, but may also be associated with other drug resistance, such as chemotherapy drugs taxane. Taxane compounds, including paclitaxel, docetaxel and cabazitaxel, are one of the few effective chemotherapy treatments for mCRPC [62], [63]. However, taxane chemotherapy only offer a limited survival benefit (3–6 months average) due to the drug resistance developed in mCRPC patients.
The action of taxane drugs is primary by disrupting microtubule function. Taxanes stabilize tubulin polymer and affect microtubule dynamic, thereby inhibiting cell division and microtubule-dependent protein trafficking. Microtubule-dependent AR trafficking is critical for AR–FL nuclear relocalization and activation. Evidence showed that microtubule binding of AR is mediated by the C-terminal of AR [32]. Because AR-V7 lacks the C-terminal domain, the nuclear localization, transcriptional activity, and tumor-promoting function of AR-V7 is not affected by taxane treatment [32]. Thus, AR-V7 may not be sensitive to taxane-induced microtubule stabilization and partially responsible for the resistance to taxane. Despite these findings in preclinical studies, the role of AR variants in taxane resistance has yet to be established in clinical study. Two small scale studies showed that expression of AR-V7 in circulating tumor cells is not associated with taxane resistance in mCRPC patients [64], [65]. However, it is possible that AR-Vs served as an alternative pathway that is able to escape from the drug targeting mechanism thereby exerting its function on cell proliferating and metastasis. In addition to dodge the drug targeting, AR-Vs may also play a role in well-established general resistance mechanism such as increased drug efflux rate, alternation in drug metabolism [66]. Reports showed that AR-V7/AR3 is able to trigger stem-cell like feature in vitro and in vivo [26], [27]. It is conceivable that stem-cell like cancer cells generally have overexpression of drug efflux transporters and up-regulated anti-apoptotic signaling pathways thus resistant to chemotherapies. In terms of metabolic regulation, the presence of AR-V7/AR3 was shown strongly associated with the down-regulation of citrate level and gains of function in oxygen poor environment. Both factors are closely associated with cancer progression and are key characteristic of mCRPC [67].
AR-Vs expression is higher in the more aggressive androgen deprived CWR-R1 and 22Rv1 cell compared with less aggressive LNCaP and its derivative cell lines. AR-V7/AR3 is also associated with multiple drug resistance as previously mentioned. These results suggest that various stress environment may selectively enhance AR-Vs expression. Multiple mechanisms may be involved in up-regulation of AR-Vs during PCs progression given the high degree of complexity and heterogeneity in both morphological and molecular level [68]. Deregulation of AR-Vs could be either through up-regulate AR-Vs expression in all PCa population or select AR-Vs positive population under drug treatment. Thus, it is possible that drug resistance raised from minor subpopulation that contain high level of AR-Vs which already present in the tumor before treatment. In 22Rv1 androgen-independent PCa cells, AR-V7 expression increased after long term bicalutamide treatment, suggesting the enrichment of AR-V7 in drug-resistant population [60]. Recent clinical reports suggest the concept that cross-resistance is exist for patients under enzalutamide treatments who have been previously treated with taxane compound [69], [70]. These findings raised the possibility that taxane might provide a pre-selection for a drug-resistant subpopulation. Whether AR-Vs activity is responsible for the survival of the drug-resistant subpopulation needs to be further determined. The drug resistance property of AR-Vs makes it an important research topic in development of new drugs targeting AR. In recent years, much more effort was putting on the development of new compound targeting both AR–FL and AR-Vs. A new compound EPI-001 targeting N-terminal domain of AR was capable of blocking both AR–FL and AR-Vs transcriptional activity and inhibit mCRPC growth in mouse xenografts [71]. Some other new androgen signaling inhibitors, such as galeterone and ASC-J9, displayed better efficacy in preclinical models compared to enzalutamide, possibly due to that they can inhibit both AR and AR-V activity [72], [73]. Another new compound, a pan-BET degrader ARV-771, was shown effectively inhibit both AR–FL and AR-Vs signaling in PCa cells and lead to tumor regression in CRPC xenograft model [74]. These compounds may potentially become new therapeutic to overcome the AR-V induced drug resistance in mCRPC.
3. Perspectives
AR-Vs have emerged as critical players in PCa progression and therapeutic resistance through regulating both canonical AR signature genes as well as distinct cell cycle related genes unique for AR-Vs under androgen depleted conditions (Fig. 1). Expression of AR-V7/AR3 in circulating tumor cells could be used as a predictive biomarker for therapeutic response to guide treatment selection. However, mechanisms by which AR-Vs contribute to the development of mCRPC and drug resistance have yet to be fully understood. Further investigation is needed to elucidate the functional interaction between AR and AR-Vs given that the level of AR-Vs transcripts relative to AR–FL is extremely low. It is still unclear whether AR-Vs protein expression is correlated with their transcript level due to limited success in development of the specific antibodies for other variants besides AR-V7. It is conceivable that the expression pattern of AR-Vs could be very dynamic in response to various intracellular and extracellular cues. The dynamic repertoire of AR–FL and AR-Vs may provide a sophistic regulatory system to orchestrate cellular responses to androgens and other extracellular stimuli. In addition to their ability to directly regulate gene transcription independently, cooperatively or competitively with AR–FL, AR-Vs may play a role in epigenetic regulation by modulating non-coding RNAs and chromatin remodeling. Facilitated by cutting edge technologies (such as single-cell sequencing and imaging analysis), we would better understand the role of AR-Vs in PCa progression and therapeutic resistance.
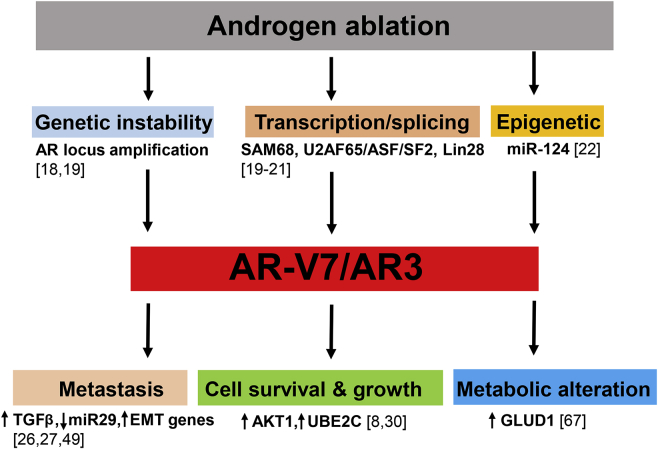
Figure 1.
Proposed model of the role of AR-V7/AR3 in mCRPC. Upon androgen ablation, changes of genetic stability, transcription/splicing and epigenetic regulation alter the expression and activity of AR-V7. In addition to canonical AR–FL regulated genes, AR-V7 is able to regulate a subset distinct target genes to promote metastasis, cell survival and growth, and metabolic alteration in prostate cancer cells mCRPC: metastatic castration resistant prostate cancer.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported by NIH (CA106504, CA169524) and DOD (W81XWH-15-1-0612) grants to YQ. The authors apologize for not being able to cite many important studies related to this subject due to limited space.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.American Cancer Society. Cancer facts & figures 2015. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/.
- 2.George D., Moul J. Emerging treatment options for patients with castration resistant prostate cancer. Prostate. 2012;72:338–349. doi: 10.1002/pros.21435. [DOI] [PubMed] [Google Scholar]
- 3.Gelmann E. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Heinlein C., Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 5.Feldman B., Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 6.Ciccarese C., Santoni M., Brunelli M., Buti S., Modena A., Nabissi M. AR-V7 and prostate cancer: the watershed for treatment selection? Cancer Treat Rev. 2016;43:27–35. doi: 10.1016/j.ctrv.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Guo Z., Qiu Y. A new trick of an old molecule: androgen receptor splice variants taking the stage?! Int J Biol Sci. 2011;7:815–822. doi: 10.7150/ijbs.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Z., Yang X., Sun F., Jiang R., Linn D., Chen H. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion–resistant growth. Cancer Res. 2009;69:2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Guo Z., Sun F., Li W., Alfano A., Shimelis H. Novel membrane-associated androgen receptor splice variant potentiates proliferative and survival responses in prostate cancer cells. J Biol Chem. 2011;286:36152–36160. doi: 10.1074/jbc.M111.265124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu R., Dunn T., Wei S., Isharwal S., Veltri R., Humphreys E. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehm S., Schmidt L., Heemers H., Vessella R., Tindall D. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu C., Luo J. Decoding the androgen receptor splice variants. Transl Androl Urol. 2013;2:178–186. doi: 10.3978/j.issn.2223-4683.2013.09.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dehm S.M., Tindall D.J. Alternatively spliced androgen receptor variants. Endocr Relat Cancer. 2011;18:R183–R196. doi: 10.1530/ERC-11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hörnberg E., Ylitalo E., Crnalic S., Antti H., Stattin P., Widmark A. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6:e19059. doi: 10.1371/journal.pone.0019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qu Y., Dai B., Ye D., Kong Y., Chang K., Jia Z. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Sci Rep. 2015;5:7654. doi: 10.1038/srep07654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonarakis E., Lu C., Wang H., Luber B., Nakazawa M., Roeser J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C., Welsbie D., Tran C., Baek S., Chen R., Vessella R. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 19.Liu L.L., Xie N., Sun S., Plymate S., Mostaghel E., Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2014;33:3140–3150. doi: 10.1038/onc.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tummala R., Nadiminty N., Lou W., Evans C., Gao A. Lin28 induces resistance to anti androgens via promotion of AR splice variant generation. Prostate. 2016;76:445–455. doi: 10.1002/pros.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stockley J., Markert E., Zhou Y., Robson C., Elliott D., Lindberg J. The RNA-binding protein Sam68 regulates expression and transcription function of the androgen receptor splice variant AR-V7. Sci Rep. 2015;5:13426. doi: 10.1038/srep13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi X.B., Ma A.H., Xue L., Li M., Nguyen H.G., Yang J.C. miR-124 and androgen receptor signaling inhibitors repress prostate cancer growth by downregulating androgen receptor splice variants, EZH2, and src. Cancer Res. 2015;75:5309–5317. doi: 10.1158/0008-5472.CAN-14-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson P., Chen Y., Balbas M., Wongvipat J., Socci N., Viale A. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A. 2010;107:16759–16765. doi: 10.1073/pnas.1012443107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu D., Zhan Y., Qi Y., Cao B., Bai S., Xu W. Androgen receptor splice variants dimerize to transactivate target genes. Cancer Res. 2015;75:3663–3671. doi: 10.1158/0008-5472.CAN-15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y., Chan S., Brand L., Hwang T., Silverstein K., Dehm S. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73:483–489. doi: 10.1158/0008-5472.CAN-12-3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong D., Sethi S., Li Y., Chen W., Sakr W.A., Heath E. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate. 2015;75:161–174. doi: 10.1002/pros.22901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun F., Chen H., Li W., Yang X., Wang X., Jiang R. Androgen receptor splice variant AR3 promotes prostate cancer via modulating expression of autocrine/paracrine factors. J Biol Chem. 2014;289:1529–1539. doi: 10.1074/jbc.M113.492140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q., Li W., Zhang Y., Yuan X., Xu K., Yu J. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao B., Qi Y., Zhang G., Xu D., Zhan Y., Alvarez X. Androgen receptor splice variants activating the full-length receptor in mediating resistance to androgen-directed therapy. Oncotarget. 2014;5:1646–1656. doi: 10.18632/oncotarget.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu R., Lu C., Mostaghel E., Yegnasubramanian S., Gurel M., Tannahill C. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 2012;72:3457–3462. doi: 10.1158/0008-5472.CAN-11-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G., Sprenger C., Wu P.J., Sun S., Uo T., Haugk K. MED1 mediates androgen receptor splice variant induced gene expression in the absence of ligand. Oncotarget. 2014;6:288–304. doi: 10.18632/oncotarget.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thadani-Mulero M., Portella L., Sun S., Sung M., Matov A., Vessella R. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Cancer Res. 2014;74:2270–2282. doi: 10.1158/0008-5472.CAN-13-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reebye V., Querol Cano L., Lavery D.N., Brooke G.N., Powell S.M., Chotai D. Role of the HSP90-associated cochaperone p23 in enhancing activity of the androgen receptor and significance for prostate cancer. Mol Endocrinol. 2012;26:1694–1706. doi: 10.1210/me.2012-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan S., Li Y., Dehm S. Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal. J Biol Chem. 2012;287:19736–19749. doi: 10.1074/jbc.M112.352930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu M.L., Horbinski C., Garzotto M., Qian D., Beer T., Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70:7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thadani-Mulero M., Nanus D., Giannakakou P. Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer Res. 2012;72:4611–4615. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darshan M.S., Loftus M.S., Thadani-Mulero M., Levy B.P., Escuin D., Zhou X.K. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–6029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang G., Liu X., Li J., Ledet E., Alvarez X., Qi Y. Androgen receptor splice variants circumvent AR blockade by microtubule-targeting agents. Oncotarget. 2015;6:23358–23371. doi: 10.18632/oncotarget.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mediwala S., Sun H., Szafran A., Hartig S., Sonpavde G., Hayes T. The activity of the androgen receptor variant AR-V7 is regulated by FOXO1 in a PTEN-PI3K-AKT-dependent way. Prostate. 2013;73:267–277. doi: 10.1002/pros.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Wang L., Lin H.K., Kan P.Y., Xie S., Tsai M.Y. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem Biophys Res Commun. 2003;305:462–469. doi: 10.1016/s0006-291x(03)00792-7. [DOI] [PubMed] [Google Scholar]
- 41.Carver B., Chapinski C., Wongvipat J., Hieronymus H., Chen Y., Chandarlapaty S. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19:575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zengerling F., Azoitei A., Herweg A., Jentzmik F., Cronauer M. Inhibition of IGF-1R diminishes transcriptional activity of the androgen receptor and its constitutively active, C-terminally truncated counterparts Q640X and AR-V7. World J Urol. 2015;34:633–639. doi: 10.1007/s00345-015-1674-5. [DOI] [PubMed] [Google Scholar]
- 43.Nadiminty N., Tummala R., Liu C., Yang J., Lou W., Evans C. NF-κB2/p52 induces resistance to enzalutamide in prostate cancer: role of androgen receptor and its variants. Mol Cancer Ther. 2013;12:1629–1637. doi: 10.1158/1535-7163.MCT-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin R., Yamashita H., Yu X., Wang J., Franco O.E., Wang Y. Inhibition of NF-kappa B signaling restores responsiveness of castrate-resistant prostate cancer cells to anti-androgen treatment by decreasing androgen receptor-variant expression. Oncogene. 2015;34:3700–3710. doi: 10.1038/onc.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritchie C.K., Andrews L.R., Thomas K.G., Tindall D.J., Fitzpatrick L.A. The effects of growth factors associated with osteoblasts on prostate carcinoma proliferation and chemotaxis: implications for the development of metastatic disease. Endocrinology. 1997;138:1145–1150. doi: 10.1210/endo.138.3.4974. [DOI] [PubMed] [Google Scholar]
- 46.Christopoulos P.F., Msaouel P., Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gennigens C., Menetrier-Caux C., Droz J.P. Insulin-like growth factor (IGF) family and prostate cancer. Crit Rev Oncol Hematol. 2006;58:124–145. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Sporn M., Roberts A. Autocrine growth factors and cancer. Nature. 1985;313:745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- 49.Cottard F., Asmane I., Erdmann E., Bergerat J.P., Kurtz J.E., Céraline J. Constitutively active androgen receptor variants upregulate expression of mesenchymal markers in prostate cancer cells. PLoS One. 2013;8:e63466. doi: 10.1371/journal.pone.0063466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albany C., Alva A.S., Aparicio A.M., Singal R., Yellapragada S., Sonpavde G. Epigenetics in prostate cancer. Prostate Cancer. 2011;2011:580318. doi: 10.1155/2011/580318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin M., Maher C., Chinnaiyan A. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29:3659–3668. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y., Yu J. EZH2, an epigenetic driver of prostate cancer. Protein Cell. 2013;4:331–341. doi: 10.1007/s13238-013-2093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu K., Wu Z., Groner A., He H., Cai C., Lis R. EZH2 oncogenic activity in castration-resistant prostate cancer cells is polycomb-independent. Science. 2012;338:1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ellis L., Ku S.Y., Lasorsa E., Pili R. Springer; 2014. Epigenetics in castration resistant prostate cancer. http://link.springer.com/chapter/10.1007/978-1-4939-1176-9_20. [Google Scholar]
- 55.Wissmann M., Yin N., Müller J., Greschik H., Fodor B., Jenuwein T. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 56.Yang L., Lin C., Jin C., Yang J., Tanasa B., Li W. lncRNA-dependent mechanisms of androgen-receptor-regulated gene activation programs. Nature. 2013;500:598–602. doi: 10.1038/nature12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao P., Deng Z., Wan M., Huang W., Cramer S., Xu J. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1α/HIF-1β. Mol Cancer. 2010;9:108. doi: 10.1186/1476-4598-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ribas J., Ni X., Haffner M., Wentzel E.A., Salmasi A.H., Chowdhury W.H. miR-21: an androgen receptor–regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res. 2009;69:7165–7169. doi: 10.1158/0008-5472.CAN-09-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y., Zhang X., Li H., Yu J., Ren X. The role of miRNA-29 family in cancer. Eur J Cell Biol. 2013;92:123–128. doi: 10.1016/j.ejcb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 60.Gravina G., Marampon F., Piccolella M., Motta M., Ventura L., Pomante R. Hormonal therapy promotes hormone-resistant phenotype by increasing DNMT activity and expression in prostate cancer models. Endocrinology. 2011;152:4550–4561. doi: 10.1210/en.2011-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scher H., Lu D., Schreiber N., Louw J., Graf R., Vargas H. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.1828. http://dx.doi.org/10.1001/jamaoncol.2016.1828. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tannock I.F., de Wit R., Berry W.R., Horti J. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 63.Bono J., Oudard S., Ozguroglu M., Hansen S., Machiels J.P., Kocak I. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 64.Antonarakis E., Lu C., Luber B., Wang H., Chen Y., Nakazawa M. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–591. doi: 10.1001/jamaoncol.2015.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Onstenk W., Sieuwerts A., Kraan J., Van M., Nieuweboer A., Mathijssen R. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68:939–945. doi: 10.1016/j.eururo.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 66.Holohan C., Schaeybroeck S., Longley D., Johnston P. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 67.Shafi A.A., Putluri V., Arnold J.M., Tsouko E., Maity S. Differential regulation of metabolic pathways by androgen receptor (AR) and its constitutively active splice variant, AR-V7, in prostate cancer cells. Oncotarget. 2015;6:31997–32012. doi: 10.18632/oncotarget.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyd L., Mao X., Lu Y.J. The complexity of prostate cancer: genomic alterations and heterogeneity. Nat Rev Urol. 2012;9:652–664. doi: 10.1038/nrurol.2012.185. [DOI] [PubMed] [Google Scholar]
- 69.Soest R.J., Royen M.E., Morrée E.S., Moll J.M., Teubel W., Wiemer E.A.C. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer. 2013;49:3821–3830. doi: 10.1016/j.ejca.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 70.Nadal R., Zhang Z., Rahman H., Schweizer M., Denmeade S., Paller C. Clinical activity of enzalutamide in Docetaxel-naïve and Docetaxel-pretreated patients with metastatic castration-resistant prostate cancer. Prostate. 2014;74:1560–1568. doi: 10.1002/pros.22874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Myung J.K., Banuelos C., Fernandez J., Mawji N., Wang J., Tien A. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123:2948–2960. doi: 10.1172/JCI66398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kwegyir-Afful A.K., Ramalingam S., Purushottamachar P., Ramamurthy V.P., Njar V.C. Galeterone and VNPT55 induce proteasomal degradation of AR/AR-V7, induce significant apoptosis via cytochrome c release and suppress growth of castration resistant prostate cancer xenografts in vivo. Oncotarget. 2015;6:27440–27460. doi: 10.18632/oncotarget.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamashita S., Lai K.P., Chuang K.L., Xu D., Miyamoto H., Tochigi T. ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia. 2012;14:74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raina K., Lu J., Qian Y., Altieri M., Gordon D., Rossi A. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc Natl Acad Sci U S A. 2016;113:7124–7129. doi: 10.1073/pnas.1521738113. [DOI] [PMC free article] [PubMed] [Google Scholar]