Abstract
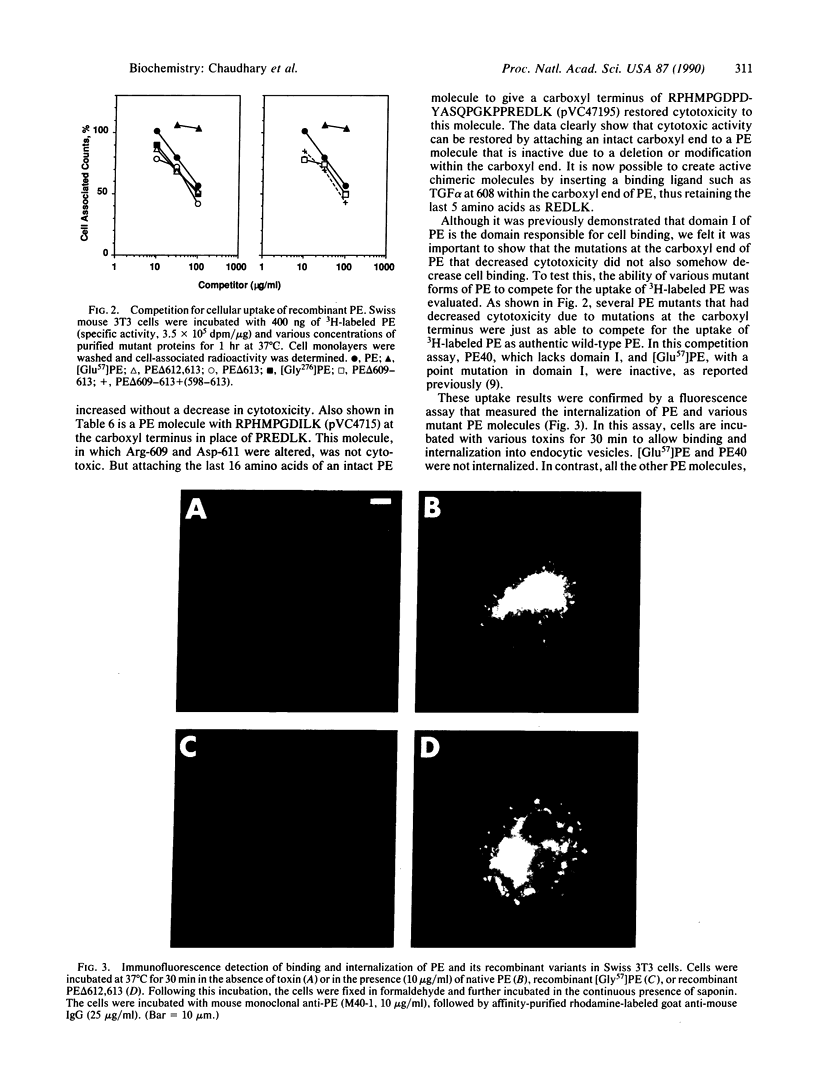
Pseudomonas exotoxin (PE), a single-chain polypeptide toxin of 613 amino acids, consists of three functional domains: an amino-terminal receptor-binding domain, a middle translocation domain, and a carboxyl-terminal ADP-ribosylation domain. Deletion of as few as 2 or as many as 11 amino acids from the carboxyl terminus of PE does not affect ADP-ribosylation activity but produces noncytotoxic molecules. Deletions and substitutions between positions 602 and 611 of PE show that the last 5 amino acids of PE are very important for its cytotoxic action. The carboxyl-terminal sequence of PE is Arg-Glu-Asp-Leu-Lys. Mutational analysis indicates that a basic amino acid at 609, acidic amino acids at 610 and 611, and a leucine at 612 are required for full cytotoxic activity. Lysine at 613 can be deleted or replaced with arginine but not with several other amino acids. Mutant toxins are able to bind normally to target Swiss mouse 3T3 cells and are internalized by endocytosis, but apparently they do not penetrate into the cytosol. A PE molecule that ends with Lys-Asp-Glu-Leu, which is a well defined endoplasmic reticulum retention sequence [Munro, S. and Pelham, R. B. (1987) Cell 48, 899-907], is fully cytotoxic, suggesting that a common factor may be involved in intoxication of cells by PE and retention of proteins in the lumen of the endoplasmic reticulum. Sequences similar to those at the carboxyl end of PE are also found at the end of Cholera toxin A chain and Escherichia coli heat-labile toxin A chain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhuber B. J., Allured V. S., Falbel T. G., McKay D. B. Mapping the enzymatic active site of Pseudomonas aeruginosa exotoxin A. Proteins. 1988;3(3):146–154. doi: 10.1002/prot.340030303. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. Amino acid sequence homology between the enzymic domains of diphtheria toxin and Pseudomonas aeruginosa exotoxin A. Mol Microbiol. 1988 Mar;2(2):293–296. doi: 10.1111/j.1365-2958.1988.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., FitzGerald D. J., Adhya S., Pastan I. Activity of a recombinant fusion protein between transforming growth factor type alpha and Pseudomonas toxin. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4538–4542. doi: 10.1073/pnas.84.13.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Xu Y. H., FitzGerald D., Adhya S., Pastan I. Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium by Escherichia coli. Proc Natl Acad Sci U S A. 1988 May;85(9):2939–2943. doi: 10.1073/pnas.85.9.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Chaudhary V. K., Kondo T., Adhya S., FitzGerald D. J., Pastan I. Mutational analysis of domain I of Pseudomonas exotoxin. Mutations in domain I of Pseudomonas exotoxin which reduce cell binding and animal toxicity. J Biol Chem. 1988 Sep 15;263(26):13203–13207. [PubMed] [Google Scholar]
- Jinno Y., Ogata M., Chaudhary V. K., Willingham M. C., Adhya S., FitzGerald D., Pastan I. Domain II mutants of Pseudomonas exotoxin deficient in translocation. J Biol Chem. 1989 Sep 25;264(27):15953–15959. [PubMed] [Google Scholar]
- Kondo T., FitzGerald D., Chaudhary V. K., Adhya S., Pastan I. Activity of immunotoxins constructed with modified Pseudomonas exotoxin A lacking the cell recognition domain. J Biol Chem. 1988 Jul 5;263(19):9470–9475. [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Sandvig K. How protein toxins enter and kill cells. Cancer Treat Res. 1988;37:39–73. doi: 10.1007/978-1-4613-1083-9_4. [DOI] [PubMed] [Google Scholar]
- Siegall C. B., Chaudhary V. K., FitzGerald D. J., Pastan I. Functional analysis of domains II, Ib, and III of Pseudomonas exotoxin. J Biol Chem. 1989 Aug 25;264(24):14256–14261. [PubMed] [Google Scholar]
- Siegall C. B., Xu Y. H., Chaudhary V. K., Adhya S., Fitzgerald D., Pastan I. Cytotoxic activities of a fusion protein comprised of TGF alpha and Pseudomonas exotoxin. FASEB J. 1989 Dec;3(14):2647–2652. doi: 10.1096/fasebj.3.14.2556314. [DOI] [PubMed] [Google Scholar]
- Spicer E. K., Noble J. A. Escherichia coli heat-labile enterotoxin. Nucleotide sequence of the A subunit gene. J Biol Chem. 1982 May 25;257(10):5716–5721. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Wozniak D. J., Hsu L. Y., Galloway D. R. His-426 of the Pseudomonas aeruginosa exotoxin A is required for ADP-ribosylation of elongation factor II. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8880–8884. doi: 10.1073/pnas.85.23.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]