Abstract
Synergy could be an effective strategy to potentiate and recover antibiotics nowadays useless in clinical treatments against multi-resistant bacteria. In this study, synergic interactions between antibiotics and grape pomace extract that contains high concentration of phenolic compounds were evaluated by the checkerboard method in clinical isolates of Staphylococcus aureus and Escherichia coli. To define which component of the extract is responsible for the synergic effect, phenolic compounds were identified by RP-HPLC and their relative abundance was determined. Combinations of extract with pure compounds identified there in were also evaluated. Results showed that the grape pomace extract combined with representatives of different classes of antibiotics as β-lactam, quinolone, fluoroquinolone, tetracycline and amphenicol act in synergy in all S. aureus and E. coli strains tested with FICI values varying from 0.031 to 0.155. The minimal inhibitory concentration (MIC) was reduced 4 to 75 times. The most abundant phenolic compounds identified in the extract were quercetin, gallic acid, protocatechuic acid and luteolin with relative abundance of 26.3, 24.4, 16.7 and 11.4%, respectively. All combinations of the extract with the components also showed synergy with FICI values varying from 0.031 to 0.5 and MIC reductions of 4 to 125 times with both bacteria strains. The relative abundance of phenolic compounds has no correlation with the obtained synergic effect, suggesting that the mechanism by which the synergic effect occurs is by a multi-objective action. It was also shown that combinations of grape pomace extract with antibiotics are not toxic for the HeLa cell line at concentrations in which the synergistic effect was observed (47 μg/mL of extract and 0.6–375 μg/mL antibiotics). Therefore, these combinations are good candidates for testing in animal models in order to enhance the effect of antibiotics of different classes and thus restore the currently unused clinical antibiotics due to the phenomenon of resistance. Moreover, the use of grape pomace is a good and low-cost alternative for this purpose being a waste residue of the wine industry.
Introduction
Antibiotics are of immense value for fighting bacterial infections, however, their effectiveness has been threatened by the continuing emergence of bacteria resistant to these drugs [1], becoming the main cause of failure in the treatment of infectious diseases [2]. Currently, there are more than 15 kinds of antibiotics whose action sites are related to physiological or metabolic functions essential for the bacteria. Unfortunately, none have escaped the resistance phenomenon [3], increasing the number of pathogenic bacteria that show a phenotype of resistance to multiple antibiotics (MDR), as for example methicillin resistant Staphylococcus aureus (MRSA) and Escherichia coli clinical isolates [1, 4]. It is for these reasons that new alternatives need to be sought. One strategy dealing this problem is the synergy using combinations of natural compounds with antibiotics and thus enhancing or restoring the antibacterial activity of many antibiotics currently useless because of bacterial resistance mechanisms acquisition.
Different combinations may improve or facilitate the interaction of an antibiotic with its target inside the bacterial cell, and in addition, some compounds should act by a different mechanism as the known antibacterial agent. The synergy can be used to expand the antimicrobial spectrum, prevent the emergence of antibiotic resistant bacteria, and to minimize toxicity, since lower concentrations of both agents can be used. Many in vitro studies have been published which show the synergistic effect between plant extracts with antibiotics of different classes against sensitive and multi-drug resistant pathogenic strains. Betoni et al. [5] showed that plants possess antibacterial compounds that may act in synergy by sensitizing the pathogen to the antibiotic. Moreover, several studies have found that the combination of antimicrobial agents with plant extracts reduced the minimum inhibitory concentration of antibiotics in different MDR bacteria [6–8].
A rich resource of phenolic compounds is the grape pomace (Vitis vinifera), which is the main organic waste generated in the industries of wine [9] representing between 13–20% of the total weight of the processed grapes [10]. The phenolic compounds content in pomace includes phenolic acids (gallic, sinapic, protocatechuic, etc.), and flavonoids as flavan-3-ol such as (+)-catechin, (-)-epicatechin, (-)-epicatechin-3-O-gallate, quercetin, miricetin, kaempferol, luteolin, among others [11, 12].
The antibacterial activity of the phenolic compounds identified in grape has been studied in extracts obtained from fruits [13], seeds [14–16], skin [17] and pomace [15, 18, 19]. Moreover, it has been determined that phenolic compounds act in synergy with different classes of antibiotics [20–22]. Some examples are epicatechin gallate (EGC) and epigallocatechin gallate (EGCg), able to reduce 64 times the MIC of oxacillin MRSA strains [23], as well as baicalein with tetracyclines and β-lactamics [24]. Furthermore, curcumin acts in synergy with β-lactamics reducing the MIC of oxacillin and ampicillin16 times, and 25 times the MIC of ciprofloxacin [25]. Within the mechanisms by which the phenolic compounds exert their antibacterial effect, it is described that are capable of interacting with the cytoplasmic membrane, cell wall, nucleic acids and/or energy transport [2], altering or inhibiting their functions.
In this study, the synergy between grape pomace extracts with antibiotics from different classes was determined against multi-resistant clinical isolates of S. aureus and E. coli. In addition, it was evaluated if pure phenolic compounds present in grape pomace extracts exert this synergistic effect and cytotoxicity determination of synergic combinations was also included.
Materials and methods
Grape pomace extract
The grape pomace of Cabernet Sauvignon variety was obtained from Viña Miguel Torres (Curicó, Chile). Samples (900 g) were ground and extracted with methanol/HCl 1% (v/v) for 18 h at 4°C under constant agitation (100 rpm). Samples were concentrated on a rotary evaporator at 50°C and subjected to liquid-liquid extraction with ethyl acetate [12]. Finally, the samples were concentrated to dryness and kept at -20°C.
Compounds identification by chromatographic analysis
Phenolic compounds present in the grape pomace extract were separated through RP-HPLC as described by Mendoza et al. [12] using a Waters 600 HPLC chromatograph (Waters, Mildford, MA, USA) equipped with a Waters 2990 diode detector and a C-18 column (3,9 mm x 150 mm; Waters, Mildford, MA, USA). A gradient program consisting of solvent A (acetic acid 1% (v/v) in distilled water) and solvent B (acetonitrile 100%) was applied at a flow rate of 0.8 mL/min as follows: a linear gradient of 10 to 20% B in 20 min; this proportion was maintained for the following 20 min; and then, a linear gradient from 20 to 50% B in 5 min; this last proportion was maintained up to 60 min. Grape pomace extract and individual phenolic compounds were prepared at 5 mg/mL in 1 mL methanol and 20 μL of sample solution was injected. The detector was set at 280 and 360 nm. Identification of phenolic compounds was done by comparison of UV—vis spectra and retention times with standards using Millenium 3.20 software. All standards, gallic acid, protocatechuic acid, syringic acid, vanillic acid, p-coumaric acid, ellagic acid, kaempferol, quercetin, luteolin, (+)-catechin and (-)-epicatechin, were obtained from Sigma Aldrich. Each sample was analyzed in triplicate.
Bacterial strains and culture media
Five clinical isolates of S. aureus, strains 8298–2, 8275, and methicillin-resistant S. aureus (MRSA) strains 622–4, and 97–7, kindly donated by Dr. Gino Corsini (Universidad Autónoma, Chile) were used; and as a control strain, S. aureus ATCC 6538. For E. coli, three clinical isolates (33.1, 16.1 and A2UC, kindly donated by the Instituto de Salud Pública de Chile; and the control E. coli ATCC 25922 were used. All strains were cultured on Luria-Bertani (LB) agar and incubated at 37°C for 18–24 h.
Antibiotic sensitivity assays
Antibiotic susceptibility of all bacterial strains was determined following the disc agar diffusion assay described by Clinical Laboratory Standards (CLSI) [26]. The bacterial strains were cultured overnight, diluted in Mueller Hinton (MH) broth to a McFarland turbidity of 0.5 (1 x 108 colony forming unit (CFU)/mL) and seeded homogeneously on Petri dishes containing Mueller Hinton agar. Sterile discs containing different concentrations of antibiotics were placed on the inoculated agar. After incubation for 18 h at 37°C, the inhibition diameters were measured and these values in millimeter (mm) were interpreted according to the criteria established by CLSI as resistant (R) or sensitive (S). Antibiotics used for S. aureus susceptibility determination were nalidixic acid (30 μg), oxacillin (1 μg), ciprofloxacin (5 μg), norfloxacin (10 μg), levofloxacin (5 μg) tetracycline (30 μg), and chloramphenicol (30 μg). The same antibiotics were used for E. coli, except that the oxacillin was replaced with ampicillin (10 μg).
Minimal inhibitory concentration determination
Minimal inhibitory concentration (MIC) of four different classes of antibiotics against all used strains was determined. As representative of the β-lactam class, oxacillin and ampicillin; nalidixic acid for quinolone; the fluoroquinolones class represented by ciprofloxacin, norfloxacin and levofloxacin; tetracycline representing tetracycline family; and finally, chloramphenicol as representative of the antibiotic class amphenicol. All these drugs were obtained from Sigma Aldrich. The MIC was also determined for grape pomace extract and the following phenolic compounds identified in pomace in this work as gallic acid, syringic acid, vanillic acid, quercetin, kaempferol, luteolin, (+)-catechin, and (-)-epicatechin.
For all the above-mentioned drugs, compounds and extract, the MIC determination assay was followed as established by CLSI using the micro-broth dilutions method in 96-well plates (Nunc) at different concentration ranges. To each well, 188 μL of MH broth, 10 μL of pomace extract (300 to 3,000 μg/mL diluted in dimethyl sulfoxide (DMSO)), antibiotic (0.75 to 3,000 μg/mL) or pure phenolic compound (200 to 10,000 μg/mL diluted in DMSO), and finally 2 μL bacterial suspension at McFarland 0.5 (1 x 108 CFU/mL) to complete a final volume of 200 μL. In addition, some wells were used as solvent and sterility controls. The plates were incubated at 37°C for 24 h and the optical density was measured at 600 nm in an Elisa lector (Thermos Labsystems Multiskan FC Model). Results are expressed in μg/mL and all experiments were done in triplicates in three individual experiments.
Checkerboard assays
Synergy between grape pomace extract and 8 different antibiotics, as well as between grape pomace extract and 10 pure phenolic components identified therein were evaluated by the checkerboard method described by Motyl et al. [27] with minor modifications. Results of the combination between pure compounds and the whole extract should give a clue which component is responsible of the synergic effect against the multi-resistant clinical isolates and control strains. Briefly, eight serial, twofold dilutions of grape pomace extract and antibiotic were prepared. In a 96-well plate, 25 μL of each dilution of grape pomace extract was added in each vertical row, and 25 μL of antibiotic or pure compound dilution was added in each horizontal row. Both first horizontal and vertical rows were left with only one agent and the following rows contained a fixed amount of one agent and increasing concentrations of the second agent. In the selection of the range of concentrations, the MICs obtained for each tested agent and tested bacteria were considered. Grape pomace extract concentrations used ranged from 47 to 3,000 μg/mL and 3 to 3,000 μg/mL for antibiotic. To each well, 100 μL of MH broth and 10 μL bacterial suspension (1 x 108 CFU/mL) were added. The plates were incubated at 37°C for 24 h and measured at 600 nm in an Elisa lector (Thermos Labsystems Multiskan FC Model). All tests were performed in triplicate in three different experiments. Fractional inhibitory concentrations (FIC) were calculated by the formula FICextract = (MIC extract + antibiotic / MIC extract) or FICantibiotic = (MIC extract + antibiotic / MIC antibiotic). The FIC index (FICI; [3]) for each combination was calculated by the sum of both FIC values, and results were interpreted as follows: FICI ≤ 0.5 synergic effect, 0.5 < FICI ≤ 4 additive effect and FICI ˃ 4 antagonistic effect [3]. These same formula were used for the calculation of the combinations of grape pomace extract with each phenolic compound.
Cytotoxicity
In order to determine the cytotoxic potential of the synergistic combinations between grape pomace extract with antibiotics of different types, the cervical cancer cell line HeLa (ATCC CCL-2, USA) was used.
HeLa cell line culture
HeLa cells were grown in Dulbecco's modified medium (DMEM; Corning, USA) with bovine fetal serum 10% (FBS) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin (Corning Eagle, USA) in a modified Thermo Scientific air incubator (5% CO2 at 37°C). The medium was renewed every 2 days to reach 80% confluence, then the cells were transferred to T75 flasks, grown to reach 80% confluence, and finally the culture was divided into sterile 24-well plates.
Cytotoxicity evaluation
Approximately 10,000 cells per well in a 24-well plate were seeded in 100 μL of DMEM. The cells were treated with different concentrations of the grape pomace extract (750, 375, 188, 94 and 47 μg/mL, in 20 μL DMSO). Simultaneously, growth controls were performed, which consisted of cells incubated with culture medium alone and with 20 μL DMSO as solvent control. Finally, treated HeLa cells and the respective controls were incubated for 24 h in 5% CO2 at 37°C. All tests were carried out in triplicate. This same procedure was performed with the combinations between grape pomace extracts and the representatives of different classes of antibiotics that showed a synergistic effect at the minimal concentration. Concentrations used for ciprofloxacin and chloramphenicol were 20, 10, 5, 2.5, 1.25 and 0.62 μg/mL, for ampicillin were 750, 375, 188, 94, 47 and 23 μg/mL, while the concentration of grape pomace extract was 47 μg/mL, the lowest concentration that showed synergic effect. Cell viability was determined using propidium iodide as a marker of dead cells at a concentration of 1 μg/mL. Samples were analyzed on a FACSCanto II flow cytometer (Becton Dickinson). The analysis of the results was performed using the FACSDiva software.
Statistical analysis
All data were analyzed using t-test using Graph Pad Prism 5 software.
Results and discussion
Minimum inhibitory concentration
Results of the susceptibility tests for S. aureus and E. coli strains are shown in Tables 1 and 2, respectively. The clinical isolates used in this study were resistant to more than three classes of antibiotics according to the criteria stablished by CLSI. This indicates that all isolates of both S. aureus and E. coli should be classified as multidrug resistant bacteria [28]. Even more, S. aureus isolates 8298–2 and 97–7 MRSA were resistant to all classes of antibiotics studied (fluoroquinolones, β-lactams, amphenicols and tetracyclines), while E. coli clinical isolates 16.1 and 33.1 showed the same trend. Three of the four S. aureus isolates and all E. coli were resistant to tetracycline. For chloramphenicol, three S. aureus and two E. coli isolates showed resistance to this drug. The minimum inhibitory concentrations (MIC) determined for each antibiotic by microdilution broth method are also shown in Tables 1 and 2. MIC values were high for all clinical isolates compared to the respective control strain. This tendency was similar for the MICs values obtained with the grape pomace extract, ranging between 1,500 to 3,000 μg/mL with the clinical isolates, compared to MIC values of 600 and 300 μg/mL obtained with the control strains S. aureus ATCC 6538 and E. coli ATCC 25922, respectively.
Table 1. Synergy analysis of grape pomace extract with different antibiotics against S. aureus.
| MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| S. aureus strain | Antibiotic (Susceptibility)* | Antibiotic alone | Grape pomace extract alone | Antibiotic plus grape pomace extract | MIC reduction fold | FICI | |
| ATCC 6538 | Nalidixic acid | (R) | 60 | 600 | 0.93 | 65 | 0.078 |
| Ciprofloxacin | 1.5 | 0.02 | 75 | 0.028 | |||
| Norfloxacin | 1.5 | 0.02 | 75 | 0.078 | |||
| Levofloxacin | 1.5 | 0.02 | 75 | 0.078 | |||
| Oxacillin | 3 | 0.05 | 64 | 0.047 | |||
| Tetracycline | 1.5 | 0.05 | 30 | 0.094 | |||
| Chloramphenicol | 16 | 0.25 | 64 | 0.047 | |||
| 8275 | Nalidixic acid | (R) | 300 | 1500 | 4.7 | 64 | 0.065 |
| Ciprofloxacin | (R) | 30 | 0.9 | 32 | 0.062 | ||
| Norfloxacin | (R) | 25 | 0.8 | 32 | 0.047 | ||
| Levofloxacin | 10 | 0.3 | 33 | 0.047 | |||
| Oxacillin | (R) | 50 | 1.6 | 31 | 0.063 | ||
| Tetracycline | 5 | 0.08 | 63 | 0.047 | |||
| Chloramphenicol | (R) | 75 | 2.3 | 33 | 0.062 | ||
| 8298–2 | Nalidixic acid | (R) | 200 | 1500 | 6.2 | 32 | 0.062 |
| Ciprofloxacin | (R) | 150 | 4.7 | 32 | 0.063 | ||
| Norfloxacin | (R) | 300 | 9.4 | 32 | 0.047 | ||
| Levofloxacin | (R) | 20 | 0.31 | 65 | 0.031 | ||
| Oxacillin | (R) | 300 | 4.7 | 64 | 0.031 | ||
| Tetracycline | (R) | 8 | 0.25 | 32 | 0.047 | ||
| Chloramphenicol | (R) | 150 | 4.7 | 32 | 0.047 | ||
| MRSA | Nalidixic acid | (R) | 300 | 3000 | 4,7 | 64 | 0.047 |
| Ciprofloxacin | (R) | 15 | 0.5 | 30 | 0.063 | ||
| Norfloxacin | (R) | 30 | 0.5 | 60 | 0.047 | ||
| Levofloxacin | 3 | 0.1 | 30 | 0.061 | |||
| Oxacillin | (R) | 150 | 2.3 | 65 | 0.047 | ||
| Tetracycline | (R) | 750 | 23 | 33 | 0.062 | ||
| Chloramphenicol | 1 | 0.02 | 50 | 0.056 | |||
| MRSA | Nalidixic acid | (R) | 150 | 1500 | 4.7 | 32 | 0.063 |
| Ciprofloxacin | (R) | 50 | 1.6 | 31 | 0.063 | ||
| Norfloxacin | (R) | 25 | 0.8 | 31 | 0.063 | ||
| Levofloxacin | (R) | 8 | 0.25 | 32 | 0.063 | ||
| Oxacillin | (R) | 300 | 4.6 | 65 | 0.047 | ||
| Tetracycline | (R) | 500 | 7.8 | 64 | 0.047 | ||
| Chloramphenicol | (R) | 5 | 0.16 | 31 | 0.063 | ||
*The susceptibility to antibiotics is indicated only if the bacterial strain is resistant (R).
Table 2. Synergy analysis of grape pomace extract with different antibiotics against E. coli.
| MIC (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|
| E. coli strain | Antibiotic (Susceptibility)* | Antibiotic alone | Grape pomace extract alone | Antibiotic plus grape pomace extract | MIC reduction fold | FICI | |
| ATCC 25922 | Nalidixic acid | (R) | 16 | 300 | 0.25 | 64 | 0.078 |
| Ciprofloxacin | 1 | 0.03 | 33 | 0.155 | |||
| Norfloxacin | 1.5 | 0.045 | 33 | 0.155 | |||
| Levofloxacin | 0.75 | 0.02 | 38 | 0.093 | |||
| Ampicillin | (R) | 15 | 0.47 | 32 | 0.063 | ||
| Tetracycline | 3 | 0.05 | 60 | 0.078 | |||
| Chloramphenicol | 8 | 0.12 | 67 | 0.078 | |||
| 16.1 | Nalidixic acid | (R) | 2000 | 1500 | 31.2 | 64 | 0.047 |
| Ciprofloxacin | (R) | 150 | 4.7 | 32 | 0.063 | ||
| Norfloxacin | (R) | 200 | 3.1 | 65 | 0.031 | ||
| Levofloxacin | (R) | 50 | 1.6 | 31 | 0.063 | ||
| Ampicillin | (R) | 1500 | 47.0 | 32 | 0.063 | ||
| Tetracycline | (R) | 100 | 3.1 | 32 | 0.047 | ||
| Chloramphenicol | (R) | 10 | 0.3 | 33 | 0.062 | ||
| 33.1 | Nalidixic acid | (R) | 3000 | 3000 | 95.0 | 32 | 0.047 |
| Ciprofloxacin | (R) | 20 | 1.25 | 16 | 0.078 | ||
| Norfloxacin | (R) | 20 | 1.25 | 16 | 0.078 | ||
| Levofloxacin | (R) | 30 | 0.47 | 64 | 0.047 | ||
| Ampicillin | (R) | 1500 | 23.4 | 64 | 0.047 | ||
| Tetracycline | (R) | 150 | 9.4 | 16 | 0.078 | ||
| Chloramphenicol | (R) | 15 | 0.47 | 32 | 0.078 | ||
| A2UC | Nalidixic acid | (R) | 150 | 3000 | 4.7 | 32 | 0.047 |
| Ciprofloxacin | (R) | 3 | 0.05 | 60 | 0.032 | ||
| Norfloxacin | (R) | 6 | 0.2 | 30 | 0.063 | ||
| Levofloxacin | 1.5 | 0.05 | 30 | 0.065 | |||
| Ampicillin | (R) | 62 | 15 | 4 | 0.281 | ||
| Tetracycline | (R) | 150 | 9.4 | 16 | 0.094 | ||
| Chloramphenicol | 10 | 0.16 | 63 | 0.047 | |||
*The susceptibility to antibiotics is indicated only if the bacterial strain is resistant (R).
Synergy interactions analysis
All combinations of grape pomace extract with the different classes of antibiotics were tested against each of the described clinical isolates of both S. aureus and E. coli. As shown in Table 1, against S. aureus isolates and the ATCC strain, a significant decrease of the MICs of all drugs was observed when the antibiotics were combined with the grape pomace extract reaching 30 to 75 times fold reduction, regardless if bacteria tested was or not resistant to the antibiotic.
FICI values obtained in the checkerboard assays were in the range of 0.03 to 0.094, indicating that all combinations studied have a synergistic effect (FICI ≤ 0.5) in all isolates, regardless of the mechanism of action of the tested antibiotic. Very similar results were observed in E. coli clinical isolates and the control strain (Table 2), in which the extract combinations with antibiotics (β-lactams, quinolones, tetracycline and chloramphenicol) showed reductions in the MIC of 4 to 67 times. Regarding FICI values, these ranged from 0.03 to 0.15, indicating that all tested combinations are synergistic. These results coincide with those reported in the literature in the sense that combinations of plant extracts with antibiotics belonging to different families show synergy against clinical isolates of S. aureus (MSSA, MRSA) and E. coli, significantly reducing the MIC of all antibiotics tested [8, 29]. Camellia sinensis extracts, particularly rich in polyphenols, when combined with oxacillin, decreased the MIC 256 times (from 256 μg/mL to 1 μg/mL) against MRSA strains [6]. Furthermore, this extract was able to potentiate the activity of levofloxacin in vivo against enterohemorrhagic E. coli O157 [30]. Our results indicate that the effect observed in all pomace extract—antibiotics combinations is independent of the mechanism of action of the antibiotic used, if the strain was resistant or not, and indifferent if bacteria is Gram-positive S. aureus or Gram-negative E. coli.
As grape is rich in phenolic compounds [11], their identification in the pomace extract should give a clue to determine which compound is responsible for the observed synergistic effect. Table 3 shows the list of 11 compounds identified in the extract with their relative abundance; 5 phenolic acids identified as gallic, syringic, vanillic, protocatechuic and p-coumaric, 5 flavonoids identified as quercetin, luteolin, kaempferol, (+)-catechin and (-)-epicatechin; and 1 tanin identified as ellagic acid. Different separation methods have been used for the identification of phenolic compounds in different V. vinifera varieties. Nicoletti et al. [31] analyzed nine grape varieties using RP-HPLC-MS and identified gallic acid, protocatechuic acid, catechin, epicatechin, rutin, among others. Sagdic et al. [32] determined the presence of 18 different phenolic compounds, including gallic acid, protocatechuic acid, p-coumaric acid, ferulic acid, vanillic acid, quercetin, kaempferol, (+)-catechin, (-)-epicatechin, hesperidin, among others, in 5 grape varieties cultivated in Greece. Similar results with different techniques based on HPLC were obtained by Kammerer et al. [33], Lafka et al. [34], Rockenbach et al. [9], and Mendoza et al. [12].
Table 3. Relative abundance of phenolic compounds in grape pomace extract.
| Compounds | Retention time (min) | λ (nm) | Relative abundance (%) |
|---|---|---|---|
| Quercetin | 52.1 | 255.5–369.3 | 26.3 |
| Gallic acid | 5.1 | 227.2–272.0 | 24.4 |
| Protocatechuic acid | 9.2 | 224.9–260.2 | 16.7 |
| Luteolin | 54.8 | 253.3–246.8 | 11.4 |
| (+)-Catechin | 13.5 | 228.4–279.1 | 3.7 |
| (-)-Epicatechin | 18.6 | 227.2–279.1 | 3.7 |
| Vanillic acid | 17.6 | 261.4–293.3 | 2.7 |
| Kaempferol | 53.8 | 266.1–365.7 | 2.4 |
| Syringic acid | 18.1 | 226.1–275.6 | 2.3 |
| p-Coumaric acid | 25.7 | 227.2–309.9 | 2.1 |
| Ellagic acid | 27.6 | 254.3–366.9 | 1.6 |
The relative abundance of the phenolics present in the grape pomace extract showed that gallic acid was the major component with a relative abundance of 26.3%, followed by protocatechuic acid, quercetin and luteolin (24.4, 16.7 and 11.4%, respectively; Table 3). The major components identified by Sadgic et al. [32] were gallic acid, chlorogenic acid, (+)-catechin, kaempferol, rutin and quercetin, results that are similar to those obtained in this work.
Considering the identification of phenolic compounds in the extract, checkerboard assays were performed between grape pomace extract and pure phenolic compounds to answer the question, which compound is responsible for the observed synergic effect, if the relative abundance is or not relevant, or the synergy effect is due to the presence of all components in the extract.
Tables 4 and 5 show the results obtained from the synergism analysis between grape pomace extract with each phenolic compound with S. aureus and E. coli strains, respectively. The MIC values of each of the phenolic compounds identified in the extract are high against both bacteria, including their respective control strains. For the pure phenolic acids (gallic, vanillic, syringic, p-coumaric and protocatechuic), the MIC ranged from 300 to 3,000 μg/mL for S. aureus and between 500 to 4,000 μg/mL for E. coli. The MICs obtained in the analysis of pure flavonoids (quercetin, luteolin, (-)-epicatechin and ellagic acid) ranged between 100 to 600 and 100 to 3,000 μg/mL for S. aureus and E. coli, respectively. In the case of (+)-catechin, MIC values were higher than 10,000 μg/mL in all isolates tested, indicating that it is inactive. Importantly, between control strains and the clinical isolates studied, no large differences were observed in the MICs for the identified phenolic compounds, either phenolic acids or flavonoids. Despite the high MIC values for the purified phenolic compounds, the combination of these compounds with the pomace extract reduced the MIC considerably, between 8 to 65 times for S. aureus and between 4 to125 times for E. coli.
Table 4. Synergy analysis between grape pomace extract and pure phenolic compounds in S. aureus.
| MIC (μg/mL) | |||||
|---|---|---|---|---|---|
| S. aureus strains | Phenolic compound | Phenolic compound alone | Phenolic compound plus grape pomace extract | MIC reduction fold | FICI |
| ATCC 6538 | Gallic acid | 1500 | 47 | 32 | 0.094 |
| Vanillic acid | 1000 | 31.2 | 32 | 0.063 | |
| Syringic acid | 625 | 39 | 16 | 0.125 | |
| p-Coumaric acid | 1500 | 24 | 63 | 0.047 | |
| Protocatechuic acid | 750 | 23 | 33 | 0.093 | |
| Ellagic acid | 500 | 16 | 31 | 0.048 | |
| Quercetin | 375 | 11.7 | 32 | 0.063 | |
| Luteolin | 100 | 3.1 | 32 | 0.047 | |
| (+)-Catechin | ˃ 10000 | - | - | - | |
| (-)-Epicatechin | 500 | 62.5 | 8 | 0.162 | |
| 8275 | Gallic acid | 1500 | 24 | 63 | 0.032 |
| Vanillic acid | 1500 | 47 | 32 | 0.061 | |
| Syringic acid | 750 | 24 | 31 | 0.060 | |
| p-Coumaric acid | 300 | 9.4 | 32 | 0.060 | |
| Protocatechuic acid | 750 | 24 | 31 | 0.048 | |
| Ellagic acid | 250 | 15.6 | 16 | 0.094 | |
| Quercetin | 600 | 9.4 | 64 | 0.032 | |
| Luteolin | 500 | 31.2 | 16 | 0.094 | |
| (+)-Catechin | ˃ 10000 | - | - | - | |
| (-)-Epicatechin | 1000 | 15.6 | 64 | 0.047 | |
| 8298–2 | Gallic acid | 3000 | 94 | 32 | 0.063 |
| Vanillic acid | 1500 | 47 | 32 | 0.063 | |
| Syringic acid | 3000 | 47 | 64 | 0.047 | |
| p-Coumaric acid | 750 | 47 | 16 | 0.078 | |
| Protocatechuic acid | 750 | 24 | 31 | 0.063 | |
| Ellagic acid | 125 | 15.6 | 8 | 0.168 | |
| Quercetin | 150 | 4.7 | 32 | 0.063 | |
| Luteolin | 500 | 31.2 | 16 | 0.094 | |
| (+)-Catechin | ˃10000 | - | - | - | |
| (-)-Epicatechin | 1000 | 15.6 | 64 | 0.047 | |
| Gallic acid | 3000 | 47 | 64 | 0.047 | |
| Vanillic acid | 1500 | 47 | 32 | 0.063 | |
| Syringic acid | 1500 | 47 | 32 | 0.047 | |
| p-Coumaric acid | 1500 | 47 | 32 | 0.063 | |
| MRSA | Protocatechuic acid | 750 | 47 | 16 | 0.094 |
| 622–4 | Ellagic acid | 250 | 3.9 | 64 | 0.031 |
| Quercetin | 300 | 9.4 | 32 | 0.063 | |
| Luteolin | 500 | 7.8 | 64 | 0.031 | |
| (+)-Catechin | ˃10000 | - | - | - | |
| (-)-Epicatechin | 1000 | 15.6 | 64 | 0.031 | |
| Gallic acid | 2000 | 31 | 65 | 0.047 | |
| Vanillic acid | 1500 | 47 | 32 | 0.063 | |
| Syringic acid | 750 | 24 | 31 | 0.047 | |
| p-Coumaric acid | 750 | 24 | 31 | 0.047 | |
| MRSA | Protocatechuic acid | 750 | 24 | 32 | 0.047 |
| 97–7 | Ellagic acid | 62.5 | 7.8 | 8 | 0.187 |
| Quercetin | 100 | 3.1 | 32 | 0.062 | |
| Luteolin | 1000 | 62.5 | 16 | 0.093 | |
| (+)-Catechin | ˃10000 | - | - | - | |
| (-)-Epicatechin | 500 | 16 | 31 | -0.063 | |
Table 5. Synergy analysis between grape pomace extract and pure phenolic compounds in E. coli.
| MIC (μg/mL) | |||||
|---|---|---|---|---|---|
| E. coli strains | Phenolic compound | Phenolic compound alone | Phenolic compound plus grape pomace extract | MIC reduction fold | FICI |
| ATCC 25922 | Gallic acid | 2000 | 16 | 125 | 0.047 |
| Vanillic acid | 750 | 23 | 32 | 0.089 | |
| Syringic acid | 1000 | 16 | 63 | 0.078 | |
| p-Coumaric acid | 750 | 47 | 16 | 0.125 | |
| Protocatechuic acid | 1000 | 16 | 63 | 0.078 | |
| Ellagic acid | 1000 | 62.5 | 16 | 0.094 | |
| Quercetin | 500 | 62.5 | 8 | 0.188 | |
| Luteolin | 200 | 25 | 8 | 0.156 | |
| (+)-Catechin | ˃10000 | - | - | - | |
| (-)-Epicatechin | 1000 | 125 | 8 | 0.188 | |
| 16.1 | Gallic acid | 2000 | 62.5 | 32 | 0.063 |
| Vanillic acid | 1000 | 62.5 | 16 | 0.125 | |
| Syringic acid | 500 | 62.5 | 8 | 0.141 | |
| p-Coumaric acid | 1000 | 62.5 | 16 | 0.125 | |
| Protocatechuic acid | 2000 | 62.5 | 32 | 0.063 | |
| Ellagic acid | 1000 | 250 | 4 | 0.281 | |
| Quercetin | 3000 | 375 | 8 | 0.156 | |
| Luteolin | 300 | 18.8 | 16 | 0.094 | |
| (+)-Catechin | ˃10000 | - | - | - | |
| (-)-Epicatechin | 3000 | 750 | 4 | 0.313 | |
| 33.1 | Gallic acid | 2500 | 150 | 16 | 0.076 |
| Vanillic acid | 4000 | 250 | 16 | 0.185 | |
| Syringic acid | 750 | 47 | 16 | 0.078 | |
| p-Coumaric acid | 1000 | 125 | 8 | 0.156 | |
| Protocatechuic acid | 4000 | 1000 | 4 | 0.500 | |
| Ellagic acid | 200 | 6.25 | 32 | 0.047 | |
| Quercetin | 500 | 15.6 | 32 | 0.062 | |
| Luteolin | 300 | 18.8 | 16 | 0.094 | |
| (+)-Catechin | ˃10000 | - | - | - | |
| (-)-Epicatechin | 3000 | 750 | 4 | 0.266 | |
| A2UC | Gallic acid | 1500 | 94 | 16 | 0.078 |
| Vanillic acid | 2000 | 62.5 | 32 | 0.047 | |
| Syringic acid | 1500 | 24 | 63 | 0.032 | |
| p-Coumaric acid | 2000 | 62.5 | 32 | 0.219 | |
| Protocatechuic acid | 2000 | 62.5 | 32 | 0.052 | |
| Ellagic acid | 500 | 31.2 | 16 | 0.156 | |
| Quercetin | 3000 | 188 | 16 | 0.078 | |
| Luteolin | 250 | 7.8 | 32 | 0.047 | |
| (+)-Catechin | ˃10000 | - | - | - | |
| (-)-Epicatechin | 5000 | 625 | 8 | 0.250 | |
The fractional inhibitory concentration index (FICI) analysis by the checkerboard method showed for all combinations and bacterial isolates tested, values below 0.5, which is indicative of synergy (Tables 4 and 5). Results show that FICI values have no correlation with the relative abundance of the compounds in the extract (Table 3), since gallic acid, one of the most abundant compound identified presented FICI values between 0.032–0.094 for S. aureus and E. coli, similar range compared to the results of less abundant compounds like vanillic, syringic and p-coumaric acids, which FICI values range between 0.031 to 0.185 with all bacteria tested. These results suggest that it is not only one compound that is responsible for the observed synergistic effect, but that each of the identified compounds contributes to this effect to a greater or lesser extent (all combinations showed synergy), resulting in a multi-objective effect of grape pomace extract. This is strongly suggested by the synergy obtained when the extract was combined with antibiotics of different kinds, regardless of their mechanism of action.
Furthermore, it has been described that phenolic acids can break down the structure of the cytoplasmic membrane causing loss of integrity and eventual cell death [2, 35]. At sub-inhibitory concentrations, the compounds present in the extract would facilitate the entrance of the antibiotic to the cell cytoplasm, thus facilitating the entrance of fluoroquinolones, tetracycline and chloramphenicol, which have their site action within the bacterial cell, and less antibiotic dose would be needed. In this way, the multi-objective mechanism would be accomplished by disrupting the cytoplasmic membrane and some vital function as DNA replication, transcription or translation processes, depending on the antibiotic used.
Flavonoids has been described as causing cytoplasmic membrane damage, inhibition of peptidoglycan synthesis, nucleic acids synthesis inhibition, and/or energy transport inhibition [2]. Within the grape pomace extract, flavonoids luteolin, kaempferol, quercetin, (+)-catechin and (-)-epicatechin were identified. Cushnie and Lamb [36] determined the mechanism of action for (+)-catechin by cytoplasmic membrane damage, causing direct disruption of the lipid bilayers and alteration of the barrier function. Quercetin has also been shown that causes an increase in the permeability of the cytoplasmic membrane and dissipation of the membrane potential [22, 36]. Quercetin showed the highest relative abundance in our grape pomace extract. On the other hand, it has been described that flavonoids as corilagin and tellimagrandin I inhibit penicillin binding proteins (PBPs), specifically PBP2`(PBP2a) in methicillin resistant S. aureus (MRSA; [37]). This evidence support our results of synergy, compounds in grape pomace extract would interact with PBPs decreasing the effective concentration of β-lactamic antibiotics. Preliminary results obtained in our laboratory showed that grape pomace extract inhibits β-lactamases in S. aureus and E. coli (data not shown). It was also published that luteolin identified in grape pomace extract, increased the efficacy of different antibiotics, since it inhibits β-lactamase in MDR E. coli strains [21], affected the cytoplasmic membrane stability (possibly by generating hydrogen peroxide); inhibited enzymes involved in the synthesis of folic acid as dihydrofolate reductase [38]. These facts support a multi-target mechanism determined by the extract components. Therefore, the extract components could act at different targets increasing the susceptibility of bacteria and enhancing the activity of antibiotics, which result in significant reductions in the MIC of antibiotics.
However, it is important to highlight that even the grape has high concentration of phenolic compounds, it also contains other class of compounds like terpenes among which uvaol, β-amirin, palmitic acid, eicosanol, scualene and estearic acid were identified (data not shown). Antibacterial activity for these terpenes was also demonstrated [39, 40], suggesting that they could also contribute to the synergy effect.
Cytotoxicity
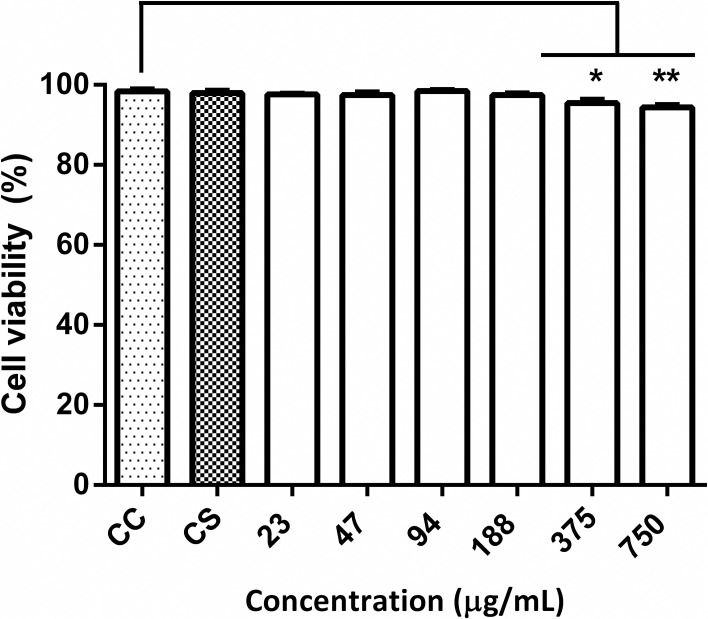
The toxicity evaluation of grape pomace extract on HeLa cells is shown in Fig 1, indicating that the cell viability at low concentrations (23–188 μg/mL) is not affected compared to the control (98.3%) with viability percentages in a range of 98.4–97.4%. However, at higher concentrations (375 and 750 μg/mL), the viability was reduced to 95.4 and 94.4%, respectively. From these results, it can be concluded that the cytotoxicity of grape pomace extract increases with concentration. This result has been previously reported with plant extracts with high content of phenolic compounds [41, 42].
Fig 1. Viability of HeLa cells exposed to different concentrations of grape pomace extract.
HeLa cells were incubated with different concentrations (μg/mL) of grape pomace extract and cell viability was determined after incubation with propidium iodide through efflux cytometry assay. As controls, HeLa cells were incubated without treatment (CC); with the solvent used with the extract (CS); *t-test *(P <0.002), ** (P <0.001) v/s CC.
The same analysis was done with combinations of grape pomace extract with ciprofloxacin, chloramphenicol and ampicillin, using the minimum concentration of extract (47 μg/mL) in which a synergistic effect was observed. Concentrations used for the tested antibiotics are shown in Table 6. Antibiotics alone or combined with the grape pomace extract were non-toxic to HeLa cell line, at the concentrations tested and after 24 h incubation, showing no significant differences in the viability compared with the control. These results are promising for possible applications in animal models.
Table 6. Cytotoxicity on HeLa cells by antibiotics alone and in combination with grape pomace extract.
| Cell viability (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | Chloramphenicol | Ampicillin | ||||||
| Concentration (μg/mL) | Alone | With grape pomace extract* | Concentration (μg/mL) | Alone | With grape pomace extract* | Concentration(μg/mL) | Alone | With grape pomace extract* |
| 0 | 98.9 ± 0.9 | 99.1 ± 0.5 | 0 | 98.6 ± 0.4 | 97.5 ± 1.1 | 0 | 98.3 ± 0.8 | 98.7 ± 0.6 |
| 0.62 | 97.2 ± 1.8 | 96.6 ± 2.1 | 0.62 | 97.1 ± 0.5 | 98.4 ± 0.6 | 23 | 98.1 ± 0.6 | 97.3 ±1.8 |
| 1.25 | 97.5 ± 0.8 | 97.4 ± 2.2 | 1.25 | 96.2 ± 0.4 | 96.2 ± 0.2 | 47 | 97.3 ± 0.7 | 95.7 ± 1.9 |
| 2.5 | 98.7 ± 1.4 | 98.6 ± 0.4 | 2.5 | 93.8 ± 1.4 | 95.7 ± 0.8 | 94 | 97.8 ± 0.9 | 96.6 ± 0.4 |
| 5 | 98.4 ± 0.9 | 98.6 ± 0.8 | 5 | 93.2 ± 1.4 | 96.4 ± 0.1 | 188 | 97.6 ± 0.4 | 98.4 ± 0.4 |
| 10 | 97.6 ± 1.3 | 98.8 ± 1.3 | 10 | 95.4 ± 1.1 | 96.7 ± 0.5 | 375 | 97.4 ± 0.7 | 97.8 ± 0.5 |
| 20 | 97.0 ± 2.3 | 97.9 ± 1.1 | 20 | 96.5 ± 1.1 | 98.3 ± 0.9 | 750 | 96.7 ± 1.1 | 97.4 ± 0.8 |
*The concentration of the grape pomace extract was 47 μg/mL; t-test P ˃ 0.05.
Conclusions
Grape pomace extract obtained from Cabernet sauvignon variety, used in combination with antibiotics of different classes against S. aureus and E. coli strains, especially multi-drug resistant clinical isolates, showed synergy reducing significantly the MICs of different classes of antibiotics studied in this work. This synergistic effect may be due to the joint action of the compounds contained in the extract, and not to a particular compound. Moreover, pomace extract–antibiotic combinations are not toxic for the HeLa cell line at concentrations in which the synergistic effect was determined. Therefore, these mixtures are good candidates for animal model testing in order to enhance the effect of antibiotics of different classes and thus restore the currently unused agents due to the phenomenon of resistance. Furthermore, the use of grape pomace is a good alternative for this purpose as being a residue of the wine industry, so that extracts and/or phenolic compounds could be obtained from this waste at low cost.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported financially under Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) project 1130389 and by the Dirección de Investigación en Ciencia y Tecnología (DICYT-USACH). L. Sanhueza thanks the Comisión Nacional de Ciencia y Tecnología (CONICYT) for its support of her doctoral studies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000; 406: 775–781. 10.1038/35021219 [DOI] [PubMed] [Google Scholar]
- 2.Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr Med Chem. 2015; 22: 132–149. [DOI] [PubMed] [Google Scholar]
- 3.Garvey M, Rahman M, Gibbons S, Piddock L. Medicinal plants extracts with efflux inhibitory activity against Gram-negative bacteria. Int J Antimicrob Ag. 2011; 37: 145–151. [DOI] [PubMed] [Google Scholar]
- 4.Kuete V, Alibert-Franco S, Eyong K, Ngameni B, Folefoc G, Nuguemeving J, et al. Antibacterial activity of some natural products against bacteria expressing a multidrug–resistant phenotype. Int J Antimicrob Ag. 2011; 37: 156–161. [DOI] [PubMed] [Google Scholar]
- 5.Betoni J, Passarelli R, Nunes L, Di Stasi L, Fernandes A. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem Inst Oswaldo Cruz. 2006; 101: 387–390. [DOI] [PubMed] [Google Scholar]
- 6.Stapleton P, Shah S, Anderson J, Hara, Hamilton-Miller J, Taylor P. Modulation of β-lactam resistance in Staphylococcus aureus by catechins and gallates. Int J Antimicrob Ag. 2004; 23, 462–467. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho H, Costa J, Lima E, Silva V, Júnior J. Herbal therapy associated with antibiotic therapy: potentiation of the antibiotic activity against methicillin–resistant Staphylococcus aureus by Turnera ulmifolia L. BMC Complement Altern Med. 2009; 9: 13–16. 10.1186/1472-6882-9-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefanovic O, Stankovic M, Comic L. In vitro antibacterial efficacy of Clinopodium vulgare L extracts and their synergistic interaction with antibiotics. J Med Plants Res. 2011; 5: 4074–4079. [Google Scholar]
- 9.Rockenbach I, Rodrigues E, Gonzaga L, Caliari V, Genovese M, De Souza Schmidt Goncalves A, et al. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011; 127: 174–179. [Google Scholar]
- 10.Corrales M, Fernandez A, Vizoso G, Butz P, Franz M, Schuele E, et al. Characterization of phenolic content, in vitro biological activity and pesticide loads of extracts from white grape skins from organic and conventional cultivars. Food Chem Toxicol. 2010; 48: 3471–3476. 10.1016/j.fct.2010.09.025 [DOI] [PubMed] [Google Scholar]
- 11.Xia E, Deng G, Guo Y, Li H. Biological activities of polyphenols from grapes. Int J Mol Sci. 2010; 11: 622–646. 10.3390/ijms11020622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendoza L, Yañez K, Vivanco M, Melo R, Cotoras M. Characterization of extracts from winery by products with antifungal activity against Botrytis cinerea. Ind Crops Prod. 2013; 43: 360–364. [Google Scholar]
- 13.Thimothe J, Bonsi I, Padilla-Zakour O, Koo H. Chemical characterization of red wine grape (Vitis vinifera and Vitis interspecific hybrids) and pomace phenolic extracts and their biological activity against Streptococcus mutans. J Agric Food Chem. 2007; 55: 10200–10207. 10.1021/jf0722405 [DOI] [PubMed] [Google Scholar]
- 14.Furiga A, Lonvaud-Funel A, Badet C. In vitro study of antioxidant capacity and antibacterial activity on oral anaerobes of a grape seed extract. Food Chem. 2009; 113: 1037–1040. [Google Scholar]
- 15.Anastasiadi M, Chorianopoulos N, Nychas G, Haroutounian S. Antilisterial activities of polyphenol-rich extracts of grapes and vinification byproducts. J Agric Food Chem. 2009; 57: 457–463. 10.1021/jf8024979 [DOI] [PubMed] [Google Scholar]
- 16.Kao T, Tu H, Chang W, Chen B, Shi Y, Chang T, et al. Grape seed extract inhibits the growth and pathogenicity of Staphylococcus aureus by interfering with dihydrofolate reductase activity and folate- mediated one-carbon metabolism. Int J Food Microbiol. 2010; 141: 17–27. 10.1016/j.ijfoodmicro.2010.04.025 [DOI] [PubMed] [Google Scholar]
- 17.Brown J, Huang G, Haley-Zitlin V, Jiang X. Antibacterial effects of grape extracts on Helicobacter pylori. Appl Environ Microbiol. 2009; 75: 848–852. 10.1128/AEM.01595-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Özkan G, Sagdiç O, Baydar N, Kurumahmutoglu Z. Antibacterial activities and total phenolic contents of grape pomace extracts. J Sci Food Agr. 2004; 84: 1807–1811. [Google Scholar]
- 19.Sanhueza L, Tello M, Vivanco M, Mendoza L, Wilkens M. Relation between antibacterial activity against food transmitted pathogens and total phenolic compounds in grape pomace extracts from Cabernet Sauvignon and Syrah. Adv Microbiol. 2014; 4: 225–232. [Google Scholar]
- 20.Eumkeb G, Siriwong S, Phitaktim S, Rojtinnakorn N, Sakdarat S. Synergistic activity and mode of action of flavonoids isolated from smaller galangal and amoxicillin combinations against amoxicillin-resistant Escherichia coli. J Appl Microbiol. 2011; 112: 55–64. 10.1111/j.1365-2672.2011.05190.x [DOI] [PubMed] [Google Scholar]
- 21.Eumkeb G, Siriwong S, Thumanu K. Synergistic activity of luteolin and amoxicillin combination against amoxicillin-resistant Escherichia coli and mode of action. J Photochem Photobiol B. 2012; 117: 247–253. 10.1016/j.jphotobiol.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 22.Amin M, Khurram M, Khattak B, Khan J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complem Altern Med. 2015; 15: 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata H, Nakano T, Parvez A, Furukawa Y, Tomoishi A, Niimi S, et al. Triple combinations of lower and longer alkyl gallates and oxacillin improve antibiotic synergy against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2009; 53: 2218–2220. 10.1128/AAC.00829-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M, Shiota S, Kuroada T, Hatano T, Yoshida T, Mizuchima T, et al. Remarkable synergies between baicalein and tetracycline and baicalein and β-lactam against methicillin resistant Staphylococcus aureus. Microbiol Immun. 2005; 49: 391–396. [DOI] [PubMed] [Google Scholar]
- 25.Mun S, Joung D, Kim Y, Kang O, Kim S, Seo Y, Kim Y, et al. Synergistic antibacterial effect of curcumin against methicillin—resistant Staphylococcus aureus. Phytomedicine. 2013; 20: 714–718. 10.1016/j.phymed.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 26.Clinical and Laboratory Standards Institute, CLSI. 2005; Performance standards for antimicrobial susceptibility testing. Weyne, PA, USA.
- 27.Motyl M, Dorso K, Barret J, Giacobbe R. Basic microbiological techniques for antibacterial drug discovery. Curr Protoc Pharmacol. 2005; 13A.3.1–13A.3.22. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Nikaido H. Efflux-mediated drug resistance in bacteria: an update. Drugs. 2009; 69: 1555–1623. 10.2165/11317030-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braga L, Kawally A, Cunha A, Silva J, Santos F, Souza C, et al. Potentiation in vitro antibiotic activity by Ocimum gratissimum L. Afr J Pharm Pharmacol. 2011; 5: 2145–2149. [Google Scholar]
- 30.Isogai E, Isogai H, Hirose K, Hayashi S, Oguma K. In vivo synergy between green tea extracts and levofloxacin against enterohemorrhagic Escherichia coli O157 infection. Curr Microbiol. 2011; 42: 248–251. [DOI] [PubMed] [Google Scholar]
- 31.Nicoletti I, Bello C, De Rossi A, Corradini D. Identification and quantification of phenolic compounds in grapes by HPLC-PDA-ESI-MS on a semimicro separation scale. J Agric Food Chem. 2008; 56: 8801–8808. 10.1021/jf801411m [DOI] [PubMed] [Google Scholar]
- 32.Sagdic O, Ozturk I, Ozcan G, Yetim H, Ekici L, Yilmaz M. RP-HPLC-DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chem. 2011; 126: 1749–1758. 10.1016/j.foodchem.2010.12.075 [DOI] [PubMed] [Google Scholar]
- 33.Kammerer D, Claus A, Carle R, Schieber A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J Agric Food Chem. 2004; 52: 4360–4367. 10.1021/jf049613b [DOI] [PubMed] [Google Scholar]
- 34.Lafka T, Sinanoglou V, Lazos E. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007; 104: 1206–1214. [Google Scholar]
- 35.Cowan M. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999; 12: 564–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cushnie T, Lamb A. Antimicrobial activity of flavonoids. Int J of Antimicrob Agents. 2005; 26: 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiota S, Shimizu M, Sugiyama J, Morita Y, Mizushima T, Tsuchita T. Mechanisms of action of corilagin and tellimagradin I that remarkably potentiate the activity of β-lactams against methicillin-resistant Staphylococcus aureus. Microbiol Immunol. 2004; 48: 67–73. [DOI] [PubMed] [Google Scholar]
- 38.Hemaiswarya S, Kumar A, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008; 15: 639–652. 10.1016/j.phymed.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 39.Wilkens M, Alarcón C, Urzúa A, Mendoza L. Characterization of the bactericidal activity of the natural diterpene kaurenoic acid. Planta Med. 2002; 68: 452–454. 10.1055/s-2002-32086 [DOI] [PubMed] [Google Scholar]
- 40.Kourtesi C, Ball A, Huang Y, Jachak S, Vera M, Khondkar P, et al. Microbial efflux systems and inhibitors: approaches to drug discovery and the challenge of clinical implementation. Open Microbiol J. 2013; 7: 34–52. 10.2174/1874285801307010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casagrande J, Benitez L, Avila K, Pereira U, Ferreira J, Dias-Júnior N, et al. Antioxidant and cytotoxic activity of hydroethanolic extract from Jacaranda decurrens leaves. PLoS ONE. 2014; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Almeida M, Correa I, Siqueira H, Justo M, Martino T, Garcia M, et al. Anti-tumor potential and acute toxicity of Jacaranda puberula Cham. (Bignoniacea). Pak J Pharm Sci. 2013; 26: 881–892. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.