Abstract
Background
Disease Activity Score in 28 Joints (DAS28) is a scoring system to evaluate disease activity and treatment response in rheumatoid arthritis (RA). A DAS28 score of greater than 3.2 is a well-described limit for treatment intensification; however, the reliability of DAS28 might be overestimated.
Objective
The aim of this study was to evaluate the reliability of DAS28 in RA, especially focusing on a subgroup of patients with a DAS28 score of greater than 3.2.
Methods
Data from RA patients registered in the local part of Danish DANBIO Registry were collected in May 2015. Patients were categorized into 2 groups: First, those with DAS28 >3.2 with at least one swollen joint (SJ) or elevated C-reactive protein (CRP) (“objective group”), and second, patients with a DAS28 >3.2 who had no SJ, and CRP values were within the reference range (“subjective group”). Disease Activity Score in 28 Joints, Clinical Disease Activity Index, and Health Assessment Questionnaire scores were calculated for each group. We defined new score, DAS28 subjective, to focus on subjective parameters.
Results
Two hundred thirty patients were included; 198 (86.1%) and 32 (13.9%) patients were in the objective and subjective groups, respectively. Patients in the subjective group had lower mean values of DAS28 (P < 0.001) and Evaluator Global Assessment (P < 0.001) with less common immunoglobulin M rheumatoid factor (P < 0.001) and anti–cyclic citrullinated peptide positivity (P = 0.02) and contrarily higher mean values of tender joints (P = 0.04) and DAS28 based on subjective parameters (P = 0.003) compared with the objective group.
Conclusions
Rheumatoid arthritis scoring systems should be used cautiously in patients who are considered for treatment intensification. Patients with central sensitization and psychological problems and those with false-positive diagnosis of RA are at high risk of overtreatment.
Key Words: CDAI, DAS28, disease activity score, HAQ, rheumatoid arthritis
Rheumatoid arthritis (RA) is a chronic inflammatory polyarthritis with a prevalence of 0.5% to 1% in the general population.1 It has been accepted that initiating RA treatment at the early stage of disease improves clinical outcomes and prevents further joint destruction.2,3
Because of the variable expressions of RA, different indices have been defined to evaluate the disease activity and response to treatment, for instance, Disease Activity Score in 28 Joints (DAS28) and Clinical Disease Activity Index (CDAI).4–6 Reaching the optimal control of RA requires regular evaluation of inflammatory activity with the aim of these scores. The different evaluations have advantages and disadvantages with respect to the monitoring of the patients. Disease Activity Score in 28 Joints–C-reactive protein (CRP) is a scoring system that is widely used to evaluate treatment efficacy and in monitoring disease activity of RA patients in daily practice.7–9 It is calculated from 4 parameters: 2 of the parameters are subjective including tender joints (TJs) (range, 0–28) and Patient Global Assessment (PGA) (range, 0–100), and 2 of them are objective components including swollen joints (SJs) (range, 0–28) and laboratory value of CRP.7,10 It is continuous and ranges from 0.96 to maximum of 9.4 if CRP up to 100 mg/L is considered.9 A DAS28 value of greater than 5.1 indicates high disease activity. The values of 3.2 < DAS28 ≤ 5.1 and DAS28 ≤ 3.2 are indicative of moderate and low disease activities, respectively. If DAS28 value is less than 2.6, the patients may be considered to be in remission phase.10–12 Clinical Disease Activity Index is a valid measure of disease activity, based only on clinical variables, calculated by the summation of TJs, SJs, PGA, and Evaluator Global Assessment (EGA), which does not require values of acute phase reactants, enhances its feasibility in routine clinical practice, and facilitates assessment of disease activity and treatment response.13 Health Assessment Questionnaire (HAQ) is a criterion standard and the most commonly used tool to evaluate functional status of RA patients.14
Disease Activity Score in 28 Joints can help clinicians to make a decision to start/change/stop treatment with disease-modifying antirheumatic drugs (DMARDs).4 Therefore, it should be calculated precisely, whereas miscalculation of DAS28 score results in incorrect patient classification and treatment plan.15 In patients with high disease activity, it is advisable to change the treatment, starting/intensifying/terminating DMARDs or initiating/changing biologics because of lack of response, and in patients with persistent low disease activity, clinicians should consider minimizing or stopping DMARD treatment.4,16
The primary objective of the study was to evaluate the reliability of DAS28 in RA. The secondary objectives are to find out how often the DAS28 score is higher than 3.2 in our patient population if the calculation is based only on subjective parameters, as well as to compare clinical characteristic of this group of patients with those who had DAS28 score of greater than 3.2 with at least 1 objective parameter. At last, we discuss our results regarding these 2 groups of patients and propose new hypotheses.
METHODS
DANBIO
The Danish DANBIO Registry was established in 2000 and provides nationwide data on the disease course of patients with inflammatory rheumatic disease including RA. Baseline variables, for example, demographic data, diagnosis, and diseases duration, and longitudinal/follow-up data, for example, treatment, functional status, and disease activity scores, are registered to DANBIO.17 DANBIO has been approved by The Danish Data Registry (j. nr. 2007-58-0014 and j. nr. 2007-58-0006) and the National Board of Health (j. nr. 7-201-03-12/1).
Study Design and Setting
This was a cross-sectional, exploratory, registry-based, single-center study. All parts of study were performed at the rheumatology outpatient clinic. Ethical approval for our local study was sought from Danish Data Protection Agency (file no. 15/25403).
Participants
Data from the last DANBIO registration of all RA patients who were registered in the local part of DANBIO were extracted in May 2015. Since 2010, diagnosis of RA has been established according to the new 2010 American College of Rheumatology/European League Against Rheumatism criteria for RA. Inclusion criteria were as follows: (a) patients who were registered in the department of rheumatology, (b) 18 years or older at diagnosis, and (c) DAS28 score of greater than 3.2 on last DANBIO registration. Data from the latest visit of patients who passed away or refereed to the other departments were also obtained. Exclusion criteria were incomplete information, that is, no CRP within 1 week prior to or after the visit or no TJs and/or SJs entered; patients unable to assess PGA (eg, severely disabled patients, dementia, etc); patients who consulted the outpatient emergency department to perform joint puncture and inject glucocorticoids as quickly as possible; and those not fully registered in DANBIO, following department policy.
After obtaining all included patients’ data, we identified 2 specific groups of patients: first, patients with at least 1 SJ or elevated CRP (“objective group”), and second, patients who had no SJ and whose CRP values were less than 6 mg/L (reference range, <6 mg/L) (“subjective group”).
Data Collection and Variables
Patients’ demographic data (age and gender), disease characteristics (TJs, SJs, immunoglobulin M [IgM] rheumatoid factor [IgM RF], and anti–cyclic citrullinated peptide [anti-CCP]), laboratory results of CRP, PGA, EGA, and treatment plan (DMARDs, biologics) were extracted from DANBIO. The current PGA and the items in the HAQ score were entered into the database by patients in the waiting room, just before they came into the physicians’ consultation. The results of radiological evaluations for patients in the subjective group were also gathered.
A 100-mm visual analog scale technique was used to measure PGA and EGA. Disease Activity Score in 28 Joints and CDAI were calculated by using the following formulas:
DAS28 = 0.56 * √(TJ) + 0.28 * √(SJ) + 0.36 * ln(CRP + 1) + 0.014 * PGA + 0.96 (can be interpreted as low disease activity: DAS28 ≥2.6 and ≤3.2, moderate disease activity: DAS28 >3.2 and ≤5.1, and high disease activity: DAS28 >5.1).
CDAI = SJs + TJs + PGA + EGA (can be interpreted as low disease activity: CDAI >2.8 and ≤10, moderate disease activity: CDAI >10 and ≤22, and high disease activity: CDAI >22).
The Stanford Health Assessment Questionnaire was used to measure HAQ score. It ranges between 0 and 3, with 0 indicating no impairment and 3 indicating completely impaired.
In addition to the previously mentioned variables, we defined a new variable, DAS based on subjective parameters (DAS28s), to focus on the subjective parameters. It was calculated as follows:
DAS28s = 0.56 * √ (TJ) + 0.014 * PGA.
DMARDs and Biologics
Disease-modifying antirheumatic drugs include methotrexate, sulfasalazine, hydroxychloroquine, azathioprine, and leflunomide, and biologics include etanercept, adalimumab, infliximab, certolizumab pegol, abatacept, tocilizumab, golimumab, and rituximab.
Statistical analysis
All statistical analyses were performed using Microsoft Excel 2010 (Version 14.0.7173.5000; Microsoft Corp, Redmond, Washington DC). Continuous data are presented as mean ± SD, and categorical data as frequencies and respective percentages. Comparisons of the previously mentioned variables between objective and subjective groups were made with Student t test. When comparing 2 binary variables, a χ2 test was performed. P ≤ 0.05 was considered significant. Correlations between variables were analyzed using correlation coefficient test. We considered the following values in interpreting correlation results: high correlation, ≥0.7; moderate correlation, ≥0.5 and <0.7; low correlation, ≥0.3 and <0.5; and no correlation, <0.3. In case of missing data, we used pairwise deletion to keep as many cases as possible for each analysis.
RESULTS
Eight hundred seventy-six RA patients were registered in the local part of DANBIO. Of 876 patients, 230 patients fulfilled the inclusion, and none of the exclusion criteria. One hundred ninety-eight (86.1%) and 32 (13.9%) patients were in the objective and subjective groups, respectively. Table 1 summarizes the demographic data and disease characteristics, as well as treatment modality, for all included patients and the objective and subjective groups (Table 1). Patients were treated with DMARDs and biologics alone or in various combinations. Immunoglobulin M RF and anti-CCP positivity were significantly less common in the subjective group.
TABLE 1.
Demographic Data and Disease Characteristics of All Included Patients and the “Objective” and “Subjective” Groups
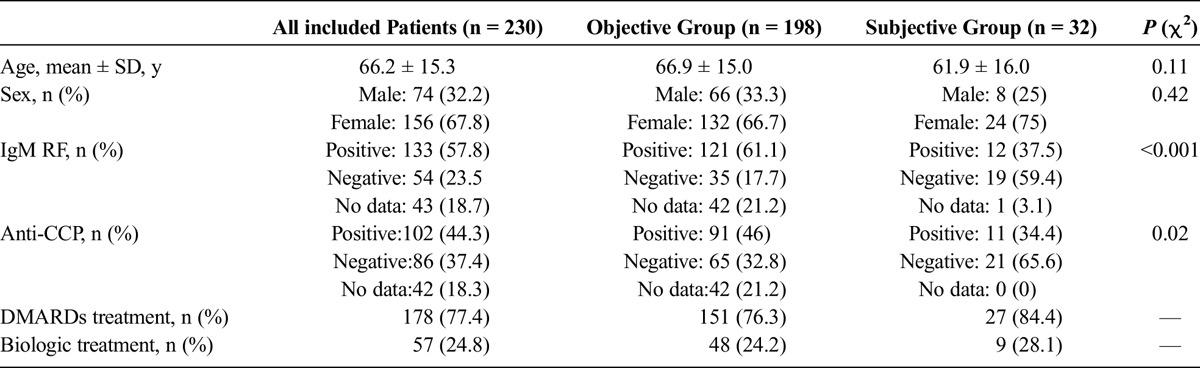
The mean values of TJs and DAS28s in the subjective group were significantly higher than those in the objective group. The mean value of PGA was higher in the subjective group; however, the difference was not statistically significant. Furthermore, DAS28 and EGA were significantly higher in the objective group. Although the mean value of CDAI in the objective group (15.4 ± 10.2) was higher than that in the subjective group (14.7 ± 6.9), the difference was not statistically significant (Table 2).
TABLE 2.
Comparison of Tender Joint Count, Patient Global Assessment, Evaluator Global Assessment, Functional Status, and Different Disease Activity Indices Between the Objective and Subjective Groups
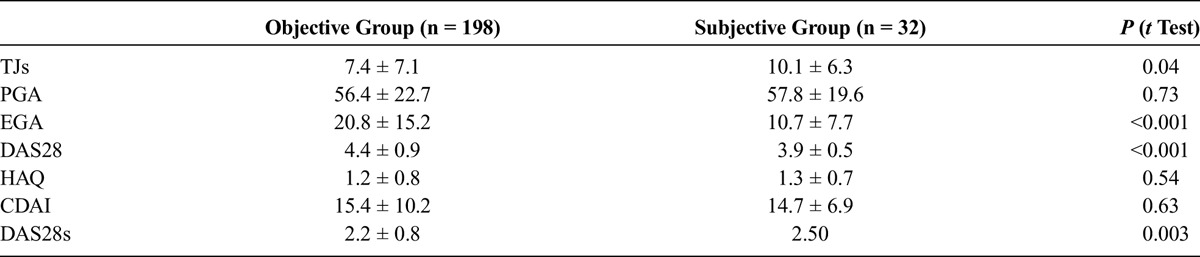
Health Assessment Questionnaire was correlated (low to almost moderate) to TJs, PGA, CDAI, DAS28, and DAS28s in the objective group; however, we did not find any correlations between HAQ and TJs, as well as PGA, CDAI, DAS28, and DAS28s, in the subjective group (Table 3).
TABLE 3.
Results of Correlation Analysis Between Different Variables in the Objective and Subjective Groups
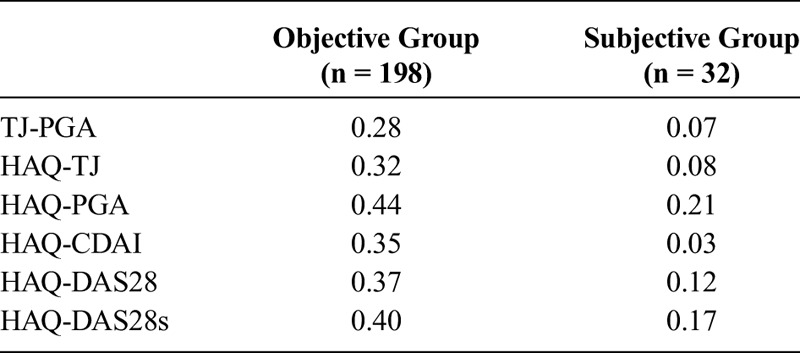
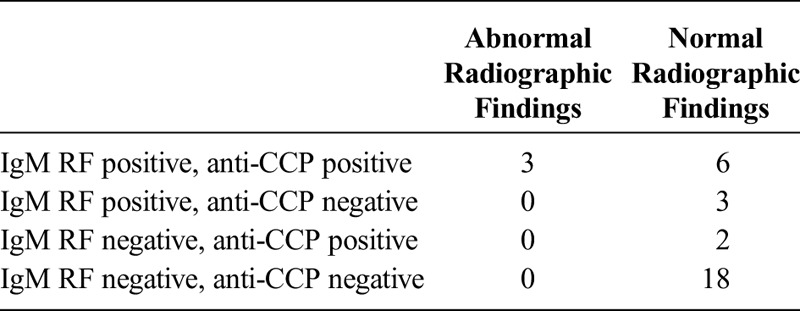
Of 32 patients in the subjective group, 29 patients had no abnormal radiographic findings (Table 4). Eighteen of 32 patients in this group were negative for both IgM RF and anti-CCP without positive findings on the radiological evaluations.
TABLE 4.
Disease Characteristics and Radiographic Findings of Patients in the Subjective Group
DISCUSSION
In the present study, of 230 included patients, 32 patients (13.91%) were categorized into the subjective group with DAS28 higher than 3.2 and no objective findings compared with 198 (86.1%) in the objective group. There were significant differences between disease characteristics (IgM RF and anti-CCP), TJs, EGA, DAS28, and DAS28s, as well as correlation analysis of patients, in the objective and subjective groups, provoking thought that these 2 groups of patients belong to different populations.
Psychological diseases such as depression, anxiety, and chronic fatigue are common in patients with RA.18,19 These conditions can affect the course of RA disease and patients’ clinical function, as well as PGA.20,21 In this study, we found that patients in the subjective group had higher mean values of TJs and DAS28s; however, they had lower mean value of DAS28. Moreover, in the subjective group, there were no correlations between HAQ and TJs, PGA, CDAI, DAS28, and DAS28s, which could be due to the relatively low number of the patients in this group, but it also implies a less severe disease with better functional status. Absence of radiographic findings in 29 patients of the subjective group also supports this hypothesis. Our clinical experience showed that most of our patients in the subjective group were unresponsive to treatment intensification over time and reported more levels of pain and higher values of PGA. Therefore, we propose that reducing treatment or changing to nonsteroidal anti-inflammatory drugs in a majority of patients, after strict individual assessment, has the same effects. However, more studies are required to confirm this hypothesis.
Central sensitization is a persistent state of hyperreactivity of the nervous system. It usually presents in the forms of allodynia (experiencing pain with nonpainful triggers) or hyperalgesia (exaggerated and prolonged response to painful triggers) and contributes to different clinical syndromes, for instance, RA, osteoarthritis, fibromyalgia, and so on. Patients with RA may experience more severe amount of pain at both articular and nonarticular sites in response to different triggers.22–24 Some of the patients in the subjective group may be involved in the process of changing pain sensitivity inducing central sensitization because the high number of TJs and the poor relation between disease activity and symptoms are suggestive of the existence of central sensitization in this group of patients; however, further studies in this field are required to confirm this hypothesis. We recommend that in doubtful cases the newly introduced painDETECT questionnaire should be applied to evaluate whether a pain sensitization has occurred. It is a validated questionnaire and translated to different languages. Patients answer different questions regarding pain intensity. The painDETECT score ranges from 0 to 38, in which a score of 19 or greater indicates that central sensitization has likely happened; 13 to 18, uncertain results; and a score of 12 or less shows that central sensitization has unlikely occurred.24,25 It is particularly relevant when rheumatologists is confronted with a patient with few signs of inflammation prior to treatment initiation. A recent study, evaluating nonnociceptive pain in RA, revealed that central sensitization may occur frequently in a group of patients initiating or intensifying treatment for their RA, which can result in increased disease activity scores on a noninflammatory basis.26
In addition, earlier studies frequently showed that seropositive RA with high titers of autoantibodies and high CRP values at diagnosis are associated with more destructive disease with poorer outcome.27–29 In the present study, seropositivity was significantly less common in the subjective group. This indicates that patients in the subjective group had good prognosis in average, and there would not be a great need for treatment intensification; however, DAS28 was higher than 3.2.
The American College of Rheumatology/European League Against Rheumatism new criteria for RA were published in 2010.2 A recent meta-analysis by Radner et al30 revealed that the pooled sensitivity and specificity of the new criteria range from 79% to 84% and 59% to 64%, respectively. This is about a proportion of patients who received a diagnosis of RA but do not have the disease (false positivity). We think that, in the present study, there were some patients among the subjective group who had a misdiagnosis of RA (false-positive diagnosis of RA), while they suffer from another condition, for example, psychological diseases (depression, chronic fatigue pain, etc), as well as other inflammatory joint diseases in the differential diagnosis of RA. Thus, discordance between TJ count and PGA with disease severity and poor response to treatment intensification would be expected.
Another explanation for relatively high number of patients in the subjective group without having SJs or elevated CRP is because in the calculation of DAS28 TJ count plays a more significant role in comparison with SJ count. This is because in the previously mentioned DAS28 formula, the square root of TJ count multiplies by 0.56, whereas the square root of SJ count multiplies by 0.28. As a consequence, TJ count can affect the final amount twice as much as SJ count. Previous work from our department revealed that subjective parameters added together give a total of 58% the total DAS28. In addition, DAS28 can be as high as 5.7 without any objective findings indicating high disease activity.31 This shows the need for further improvement of DAS28 to make it more accurate. We suggest that SJs should have a more important role than TJs because it is more reliable.
A limitation of this study includes relatively low number of patients in the subjective group because we had data from only 32 patients from which to perform data analysis. Furthermore, in this study, patients in the objective group with elevated CRP might also belong to the subjective group because CRP could be increased by the reason of infection or other causes. There might contrarily be some patients in the subjective group who belong to the objective group because of the dynamic characteristic of RA, in whom swelling of the joints may fade before soreness. The strength of the study is that all RA patients at the rheumatology outpatient clinic were included in the study, which can minimize selection bias. Information bias was not a problem in this study; neither the investigating physicians nor the patients knew that the investigation was planned. The results from this study have a high degree of generalizability because of broad inclusion criteria. In addition, these 2 groups of patients can be identified in any rheumatologic departments.
In fact, there is a risk of overtreatment in RA patients with central sensitization, patients with psychological problems, and those with false-positive diagnosis of RA. Therefore, clinicians should use RA scoring systems cautiously in patients who are considered for treatment intensification. This is particularly true, once the decision to start biological treatment has been made because of relative medical expenses. To conclude, we propose that DMARD treatment should be tentatively stopped instead of being intensified if patients in 2 consecutive visits have DAS28-CRP of greater than 3.2 and the DAS28 calculation is based on subjective parameters, the CRP is normal, and there is neither SJ nor radiographic signs of arthritis, besides that IgM RF is low or negative.

ACKNOWLEDGMENT
The authors thank senior physician Steen Antonsen, PhD, for his insightful comments. They also thank DANBIO (https://danbio-online.dk/).
Footnotes
Ethical approval for this local study was sought from Danish Data Protection Agency (file no. 15/25403). DANBIO has been approved by The Danish Data Registry since the year 2000 (j. nr. 2007-58-0014 and j. nr. 2007-58-0006) and since 2006 as a national quality registry by the National Board of Health (j. nr. 7-201-03-12/1).
The authors declare no conflict of interest.
REFERENCES
- 1.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358:903–911. [DOI] [PubMed] [Google Scholar]
- 2.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–1588. [DOI] [PubMed] [Google Scholar]
- 3.Hørslev-Petersen K, Hetland ML, Ørnbjerg LM, et al. Clinical and radiographic outcome of a treat-to-target strategy using methotrexate and intra-articular glucocorticoids with or without adalimumab induction: a 2-year investigator-initiated, double-blinded, randomised, controlled trial (OPERA). Ann Rheum Dis. 2016;75:1645–1653. [DOI] [PubMed] [Google Scholar]
- 4.van Riel PL. The development of the Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28). Clin Exp Rheumatol. 2014;32:65–74. [PubMed] [Google Scholar]
- 5.Hobbs KF, Cohen MD. Rheumatoid arthritis disease measurement: a new old idea. Rheumatology (Oxford). 2012;51:21–27. [DOI] [PubMed] [Google Scholar]
- 6.Rintelen B, Sautner J, Haindl PM, et al. Comparison of three rheumatoid arthritis disease activity scores in clinical routine. Scand J Rheumatol. 2009;38:336–341. [DOI] [PubMed] [Google Scholar]
- 7.Inoue E, Yamanaka H, Hara M, et al. Comparison of Disease Activity Score (DAS)28–erythrocyte sedimentation rate and DAS28–C-reactive protein threshold values. Ann Rheum Dis. 2007;66:407–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Consolaro A, Ruperto N, Bazso A, et al. Paediatric Rheumatology International Trials Organisation. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009;61:658–666. [DOI] [PubMed] [Google Scholar]
- 9.Favalli EG, Becciolini A, Biggioggero M, et al. Is there a need for new thresholds to define remission and low disease activity by Disease Activity Score 28 calculated with C reactive protein? Real life data from a local registry. Ann Rheum Dis. 2015;74:e5. [DOI] [PubMed] [Google Scholar]
- 10.Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am. 2009;35:745–757. [DOI] [PubMed] [Google Scholar]
- 11.Wells G, Becker JC, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fransen J, Creemers MC, van Riel PL. Remission in rheumatoid arthritis: agreement of the Disease Activity Score (DAS28) with the ARA preliminary remission criteria. Rheumatology. 2004;43:1252–1255. [DOI] [PubMed] [Google Scholar]
- 13.Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maska L, Anderson J, Michaud K. Measures of functional status and quality of life in rheumatoid arthritis: Health Assessment Questionnaire Disability Index (HAQ), Modified Health Assessment Questionnaire (MHAQ), Multidimensional Health Assessment Questionnaire (MDHAQ), Health Assessment Questionnaire II (HAQ-II), Improved Health Assessment Questionnaire (Improved HAQ), and Rheumatoid Arthritis Quality of Life (RAQoL). Arthritis Care Res. 2011;63:4–13. [DOI] [PubMed] [Google Scholar]
- 15.Asmussen R, Antonsen S, Jensen Hansen IM. The influence of variation in C-reactive protein values on the DAS28 score. Ann Rheum Dis. 2013;72:844.22739990 [Google Scholar]
- 16.Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hetland ML. DANBIO–powerful research database and electronic patient record. Rheumatology. 2011;50:69–77. [DOI] [PubMed] [Google Scholar]
- 18.Inanc N, Yilmaz-Oner S, et al. The role of depression, anxiety, fatigue, and fibromyalgia on the evaluation of the remission status in patients with rheumatoid arthritis. J Rheumatol. 2014;41:1755–1760. [DOI] [PubMed] [Google Scholar]
- 19.Katz PP, Yelin EH. Prevalence and correlates of depressive symptoms among persons with rheumatoid arthritis. J Rheumatol. 1993;20:790–796. [PubMed] [Google Scholar]
- 20.Margaretten M, Julian L, Katz P, et al. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumtol. 2011;6:617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smedstad LM, Kvien TK, Moum T, et al. Correlates of patients’ global assessment of arthritis impact. A 2-year study of 216 patients with RA. Scand J Rheumatol. 1997;26:259–265. [DOI] [PubMed] [Google Scholar]
- 22.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meeus M, Vervisch S, de Clerck LS, et al. Central sensitization in patients with rheumatoid arthritis: a systematic literature review. Semin Arthritis Rheum. 2012;41:556–567. [DOI] [PubMed] [Google Scholar]
- 24.Rifbjerg-Madsen S, Christensen AW, Boesen M, et al. Can the painDETECT Questionnaire score and MRI help predict treatment outcome in rheumatoid arthritis: protocol for the Frederiksberg hospital's Rheumatoid Arthritis, pain assessment and Medical Evaluation (FRAME-cohort) study. BMJ Open. 2014;4:e006058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freynhagen R, Baron R, Gockel U, et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. [DOI] [PubMed] [Google Scholar]
- 26.Christensen AW, Rifbjerg-Madsen S, Christensen R, et al. Non-nociceptive pain in rheumatoid arthritis is frequent and affects disease activity estimation: cross-sectional data from the FRAME study. Scand J Rheumatol. 2016:1–9. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulos IA, Katsimbri P, Katsaraki A, et al. Clinical course and outcome of early rheumatoid arthritis. Rheumatol Int. 2001;20:205–210. [DOI] [PubMed] [Google Scholar]
- 28.van Schaardenburg D, Hazes JM, de Boer A, et al. Outcome of rheumatoid arthritis in relation to age and rheumatoid factor at diagnosis. J Rheumatol. 1993;20:45–52. [PubMed] [Google Scholar]
- 29.Cimmino MA, Salvarani C, Macchioni P, et al. Extra-articular manifestations in 587 Italian patients with rheumatoid arthritis. Rheumatol Int. 2000;19:213–217. [DOI] [PubMed] [Google Scholar]
- 30.Radner H, Neogi T, Smolen JS, et al. Performance of the 2010 ACR/EULAR classification criteria for rheumatoid arthritis: a systematic literature review. Ann Rheum Dis. 2014;73:114–123. [DOI] [PubMed] [Google Scholar]
- 31.Asmussen Andreasen R, van Bui Hansen MN, Jensen Hansen IM. Interchangeability of 28-Joint Disease Activity Scores using the erythrocyte sedimentation rate or the C-reactive protein as inflammatory marker. Scand J Rheumatol. 2014;43:29–30. [DOI] [PubMed] [Google Scholar]


