Abstract
Many people with difficulties following conversations in noisy settings have “clinically normal” audiograms, that is, tone thresholds better than 20 dB HL from 0.1 to 8 kHz. This review summarizes the possible causes of such difficulties, and examines established as well as promising new psychoacoustic and electrophysiologic approaches to differentiate between them. Deficits at the level of the auditory periphery are possible even if thresholds remain around 0 dB HL, and become probable when they reach 10 to 20 dB HL. Extending the audiogram beyond 8 kHz can identify early signs of noise-induced trauma to the vulnerable basal turn of the cochlea, and might point to “hidden” losses at lower frequencies that could compromise speech reception in noise. Listening difficulties can also be a consequence of impaired central auditory processing, resulting from lesions affecting the auditory brainstem or cortex, or from abnormal patterns of sound input during developmental sensitive periods and even in adulthood. Such auditory processing disorders should be distinguished from (cognitive) linguistic deficits, and from problems with attention or working memory that may not be specific to the auditory modality. Improved diagnosis of the causes of listening difficulties in noise should lead to better treatment outcomes, by optimizing auditory training procedures to the specific deficits of individual patients, for example.
Keywords: Auditory development and plasticity, Auditory processing disorder, Hidden hearing loss, Speech intelligibility in noise
INTRODUCTION
Perhaps 5% of children and younger adults (<60 years old) with “clinically normal” audiograms (i.e., tone thresholds better than 20 dB HL from 0.1 to 8 kHz) have difficulties understanding speech, particularly in noisy, reverberant or otherwise challenging listening environments (Hind et al. 2011). This should not be surprising, given that understanding speech in noise taxes our auditory and cognitive capacities much more than does detecting tones in quiet (Rudner & Lunner 2014). If anything, 5% could underestimate the prevalence of listening difficulties specific to noisy environments, as such people are less likely to be referred for or seek treatment than those with more pronounced hearing loss.
There are many possible causes of hearing difficulties in noise despite clinically normal audiograms. First, tone sensitivity better than 20 dB HL does not rule out potential pathology of the cochlea or middle ear. Recent findings that both noise exposure and aging can permanently destroy synapses between the inner hair cells (IHCs) and type I auditory nerve fibers (ANFs)—leading to a gradual degeneration of the denervated ANFs but leaving the IHCs and hearing sensitivity intact—underscore the inability of the audiogram to detect certain types of peripheral loss (Kujawa & Liberman 2015). The term hidden hearing loss (HHL) has been coined with such peripheral loss in mind (Schaette & McAlpine 2011), but perhaps should extend to all possible types of hearing problems not captured by conventional clinical methods, including neurology. The term central auditory processing disorder (APD; Musiek & Chermak 2014) would then be reserved for neurologically confirmed lesions affecting the central auditory nervous system (CANS), unless specific deficits in central auditory processing (AP) could be confirmed by sensitive clinical tests that controlled for potential peripheral and cognitive confounds (Dillon et al. 2012). For example, deficits in binaural integration and sound localization (Cameron & Dillon 2008), often leading to impaired speech intelligibility in noise, could be a consequence of temporary periods of unilateral conductive hearing loss early in life, during bouts of otitis media with effusion (Kaplan et al. 2016). Likewise, AP deficits could result from chronic exposure to nontraumatic levels of noise (i.e., 50 to 70 dB SPL) during developmental sensitive periods and even in adulthood, degrading neural receptive fields and tonotopic maps in auditory cortex and subcortically (de Villers-Sidani & Merzenich 2011; Gourévitch et al. 2014). Finally, listening difficulties might not be due to hearing problems at all, but to deficits in language comprehension, termed specific language impairment (Bishop 1997), or to deficits in attention or working memory that may not be particular to the auditory modality (Cacace & McFarland 2005). In this review, I discuss these possible causes of listening difficulties in spite of clinically normal audiograms, and examine established as well as promising new psychoacoustic and electrophysiologic approaches to differentiate between them, with the ultimate goal of better tailoring treatments to the specific deficits and needs of individual patients.
HIDDEN HEARING LOSS
“Clinically Normal” Audiograms
The average tone thresholds of healthy, normal-hearing young adults, measured in quiet, are defined to be 0 dB HL (hearing level) across audiometric frequency. SDs of the mean are 3 to 5 dB in the 0.1 to 8 kHz range (Wilber et al. 1988; Han & Poulsen 1998; American National Standards Institute 2010), only widening to about 10 dB at the highest audiometric frequencies (14 to 16 kHz). Thus, 95% confidence intervals (CIs) for normal tone thresholds are at most −10 to 10 dB HL up to 8 kHz. The current practice of classifying thresholds of 20 dB HL in the 0.1 to 8 kHz range (i.e., 4 to 6 SDs below average) as “clinically normal” therefore seems dubious at best.
Subjects with clinically normal audiograms typically perform very unevenly on more difficult listening tasks (Surprenant & Watson 2001; Kidd et al. 2007; Ruggles & Shinn-Cunningham 2011; Ruggles et al. 2011; Bharadwaj et al. 2015). For example, in Speech-in-Noise (SIN) tests conducted on young adults (averaging 20 years) with normal audiograms, Surprenant and Watson (2001) obtained a 95% CI of 6 dB SNR (signal to noise ratio) for the 50% correct identification of low redundancy sentences, which corresponded to SIN scores of 82% at an SNR of −1.6 dB in the best 10% of listeners and only 38% at the same SNR in the worst 10%. In such young people, SIN scores tend not to correlate with tests of cognitive function (Akeroyd 2008), suggesting a potential HHL component instead. Highly variable performance on difficult listening tasks further weakens the case for using 20 dB HL as the cutoff for clinically normal hearing, especially for the purpose of establishing other clinical norms. Indeed, many people with listening difficulties and suspected APD (on the basis of normal audiograms and no apparent cognitive disorders) have SIN scores that are below average but within the wide normative range (i.e., z scores between −1 and −2; Ferguson et al. 2011).
The majority of people with thresholds between 10 and 20 dB HL have distortion product otoacoustic emission (DPOAE) amplitudes that are outside of the DPOAE normative range (z < −2), indicative of some degree of outer hair cell (OHC) dysfunction (Zhao & Stephens 2006; Job et al. 2007; Dhar & Hall 2011). DPOAEs are by-products of the frequency-specific and compressively nonlinear amplification of sound-induced cochlear vibrations by the OHCs, a process which increases both the sensitivity and the frequency resolution of hearing (Robles & Ruggero 2001; Moore 2007). Thus, even modest losses of OHCs, which are likely to leave audiometric thresholds within their wide clinically normal range, could contribute to hearing difficulties in noisy settings, where fine frequency resolution is especially important (Festen & Plomp 1983; Houtgast & Festen 2008; Badri et al. 2011). DPgrams can be more sensitive to OHC dysfunction (and to conductive hearing loss) than the audiogram, especially if individual baseline or pre damage DPgrams are available (see Fig. 1 for an example), and should always be performed to test for a potential OHC contribution to listening difficulties in noise. However, DPgrams and audiograms correlate only modestly with each other and with self-reported hearing loss (Dorn et al. 2001; Engdahl et al. 2013), in large part because OAEs are not very sensitive to damage to the IHCs or ANFs.
Fig. 1.
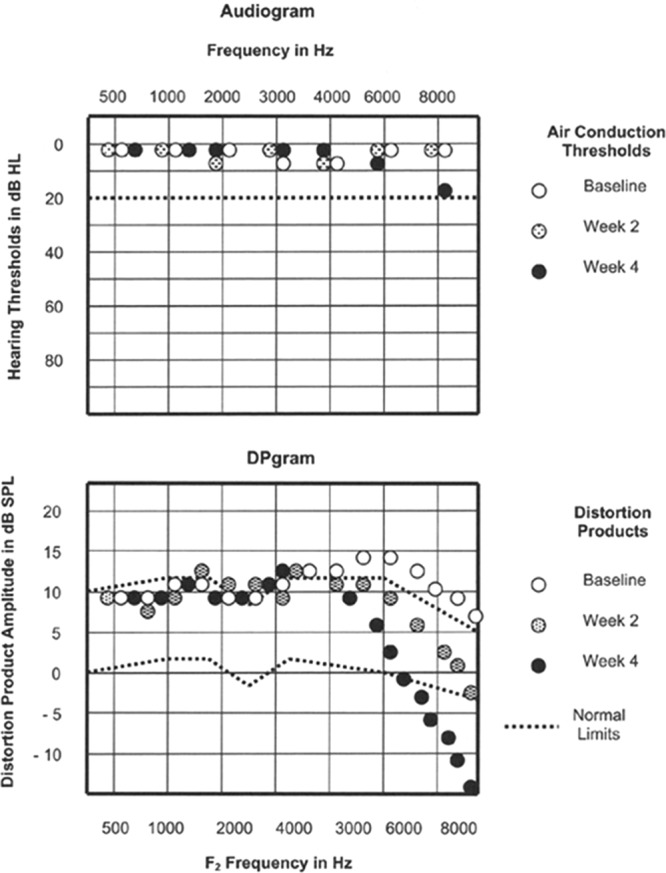
Audiograms (top) and DPgrams (bottom; i.e., distortion product otoacoustic emission amplitudes vs. primary tone frequency) in a child with a brainstem tumor who underwent chemotherapy that included the ototoxic drug cisplatin. Adapted from Figure 7–4 in Otoacoustic Emissions: Principles, Procedures, and Protocols. San Diego, CA: Plural Publishing; 2011.
The potential utility of OAEs in uncovering HHL can be extended by presenting moderately loud noise to the contralateral ear during OAE recording, which normally decreases DPOAE amplitudes by 1 or 2 dB (Kim et al. 2001; Butler et al. 2011), by activating the crossed medial olivocochlear (MOC) efferent pathway to suppress the OHC amplifier (Murugasu & Russell 1996). A recent study has suggested that the MOC efferents are activated by input from type II ANFs, which originate from the OHCs, rather than from type I ANFs, which originate from the IHCs (Froud et al. 2015). Thus, if DPOAEs are normal but are not contralaterally suppressible, this could indicate a problem with either the MOC efferent system or with type II ANFs, and not necessarily with type I ANFs as previously thought. However, the Froud et al. (2015) study remains controversial and more work is generally needed to identify the potential contributions of MOC dysfunction to hearing problems, both with and without threshold shifts (Mishra 2014). One possibility is that both the MOC and the LOC efferent systems (the lateral olivocochlear or LOC efferents contact the input terminals of the ANFs) play an oto-protective role, without which the cochlea becomes more vulnerable to both noise- (Maison et al. 2013) and age-related synaptopathy (Liberman et al. 2014), as described in the “Cochlear Synaptopathy” section.
If “clinically normal” thresholds of 10 to 20 dB HL are in fact likely indicators of some degree of peripheral loss, do thresholds better than 10 dB HL guarantee normal peripheral hearing? The first and most obvious possibility is that measuring the audiogram to only 8 kHz (let alone to 4 kHz) may miss damage to the ultra-high-frequency basal turn of the cochlea. It has long been suspected that basal-turn hair cells, which at high SPLs are strongly stimulated not only by high-frequency but also low frequency sounds, are most vulnerable to noise trauma (Bredberg 1968). In a recent longitudinal study of teenagers who frequented discos, it was found that permanent threshold shifts (PTSs) were most common and pronounced at 14 to 16 kHz (Biassoni et al. 2005; Serra et al. 2005). As an example, consider the “extended” audiogram of a young adult amateur musician (Fig. 2): thresholds mostly in the −5 to 5 dB HL range up to 10 kHz give way to a sloping bilateral loss of 45 to 50 dB at 16 kHz. While elevated thresholds above 10 kHz may have little direct impact on speech intelligibility (Boothroyd & Medwetsky 1992), they likely triggered a chronic, bilateral, hissing tinnitus, which this subject reliably pitch-matched to narrowband noise with a center frequency of 9 kHz, near the edge of the loss. In addition to potentially triggering a high-pitched tinnitus, noise-induced damage at ultra-high frequencies could increase the severity of future age-related losses at lower frequencies (Kujawa & Liberman 2006). More speculatively, damage at ultra-high frequencies might point to already present but still hidden losses in the speech frequency range, as discussed below.
Fig. 2.
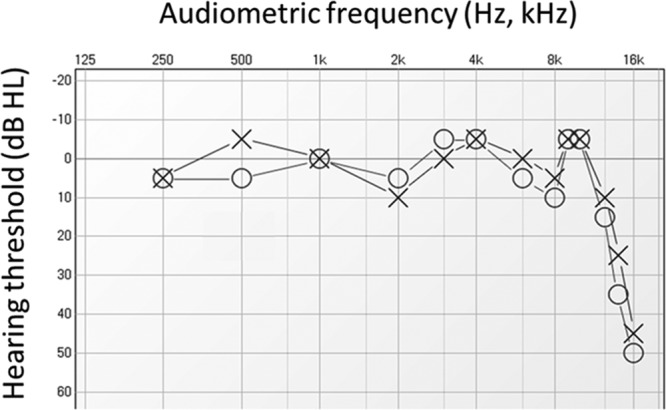
Previously unpublished extended audiogram of a young adult amateur musician, recorded in our University clinic, showing elevated thresholds only above 10 kHz. This person had a chronic, bilateral, hissing tinnitus, which she reliably pitch-matched to narrowband noise with a center frequency of 9 kHz, near the edge of the hearing loss.
Cochlear Synaptopathy
The audiogram is even less sensitive to partial losses of the IHCs or ANFs than to partial losses of OHCs. By partial losses, I mean that in every tonotopic region of the cochlea, there remain some normally functioning hair cells and nerve fibers; in other words, there are no “dead regions” (Vinay & Moore 2007). Schuknecht and Woellner (1953) reported long ago that cats could retain normal hearing sensitivity after a nearly complete bilateral sectioning of the auditory nerve, despite ANF losses of up to 80%. More recently, Lobarinas et al. (2013) found normal behavioral audiograms in chinchillas with carboplatin-induced IHC losses of also up to 80%; importantly however, tone thresholds in noise were elevated in these animals (Lobarinas et al. 2016). Similar findings were reported by Chambers et al. (2016) in ouabain-treated mice with up to 95% ANF loss. In all of these studies, the losses were distributed more or less evenly along the length of the cochlea, indicating that only a small percentage of surviving IHCs or ANFs is required for the preservation of normal tone thresholds in quiet, as long as the OHCs remain intact. A hallmark of human auditory neuropathy is that speech intelligibility, especially in noise, is substantially poorer than expected on the basis of the audiogram, which can remain in the clinically normal range (Starr et al. 1996; Zeng et al. 2005; Giraudet & Avan 2012). Again, understanding speech in noise can be challenging even for “normally-hearing” people (Ruggero 1992; Surprenant & Watson 2001; Joris et al. 2004; Kidd et al. 2007; Lopez-Poveda & Barrios 2013), whereas only about 20% of IHCs or ANFs appear to suffice for normal sensitivity to tones in quiet, providing that the losses are distributed along the cochlea and the OHCs are unaffected (Schuknecht & Woellner 1953; Lobarinas et al. 2013; Chambers et al. 2016).
A seminal recent finding was that mice or guinea pigs exposed for 2 hr to octave-band noise at about 100 dB SPL suffered an irreversible loss of up to 50% of IHC synapses in frequency regions peaking just above the noise band, while hearing sensitivity decreased only temporarily (i.e., a temporary threshold shift or TTS) and recovered completely within a week or so of the 2-hr exposure (Kujawa & Liberman 2009; Lin et al. 2011). Furthermore, this synaptopathy led to a gradual degeneration of the denervated spiral ganglion cells, the cell bodies of the ANFs, whereas both IHCs and OHCs remained intact. (Of course, after louder and/or longer exposures, IHCs and especially OHCs would also be lost.) Each successive 2 hr, ~100 dB SPL noise dose, delivered a few weeks apart (to allow for complete recovery from TTS), added to the severity of the synaptopathy, and PTS did develop after the third exposure (Wang & Ren 2012). Longer exposures to 8 to 16 kHz noise at lower SPLs also caused significant (but less pronounced) synaptopathy in mice; this was shown after a 1-week exposure at 84 dB SPL (Maison et al. 2013), and a 10-week exposure at 75 dB SPL (Pienkowski 2016). Fortunately, a recent mouse study showed that not all TTS-inducing noise exposures cause synaptopathy: 2 hr of 8 to 16 kHz noise at 91 dB SPL (versus 100 dB SPL) did not (Fernandez et al. 2015). In older mice never exposed to loud noise, synaptic and neural losses were also observed well before hair cell losses or PTS (Sergeyenko et al. 2013). Similar age-related (if not also noise-related) losses of IHC synapses and spiral ganglion cells were seen in human temporal bones, again in the absence of hair cell loss (Makary et al. 2011; Viana et al. 2015). All of these findings imply that IHC synapses are more vulnerable to both noise exposure and aging than the hair cells themselves. Synaptopathy is thus a likely candidate for at least some of the hearing difficulties experienced in noisy settings by people with a history of noise exposure, particularly the elderly, in spite of normal or near-normal hearing sensitivity (Kujala et al. 2004; Brattico et al. 2005; Humes et al. 2012; Kumar et al. 2012; Füllgrabe et al. 2014).
At present, an 8-hr daily exposure limit of about 85 dB A (A-weighted SPL) is thought to safeguard normal-hearing people from PTS (NIOSH 1998; OSHA 2002), with an additional 3 to 5 dB of noise allowed per halving of the exposure duration (i.e., 88 to 90 dB A for a 4-hr exposure, 91 to 95 dB A for a 2-hr exposure, etc., so that 100 dB SPL for 2 hr is in fact considered unsafe, whereas 91 dB SPL is not [cf. Fernandez et al. 2015]). However, as mentioned above, synaptopathy in mice can occur at noise levels in the 75 to 85 dB SPL range over longer exposure durations (Maison et al. 2013; Pienkowski 2016). Furthermore, mild TTS can be induced at relatively low exposure levels in humans (Ward et al. 1976): for example, 76 dB A for 8 hr of broadband noise, or just 65 dB A for 4 hr of octave-band noise centered at 4 kHz, the frequency range to which we are normally most sensitive. It is thus unclear if present occupational noise standards do in fact adequately safeguard against synaptopathy and eventual PTS.
Given that clinically normal audiograms do not necessarily rule out synaptopathy as a possible contributor to listening difficulties in noise, how can this be done? Detecting IHC/ANF loss and differentiating it from OHC loss (e.g., to diagnose auditory neuropathy) has traditionally been the purview of the auditory brainstem response (ABR; Starr et al. 1996; Harrison 1998; Mills 2006; Picton 2011; Pienkowski & Ulfendahl 2011; Hall 2015). However, ABR audiograms can be unaffected in rodents with confirmed synaptic losses, despite the fact that ABR wave I amplitudes at mid to high SPLs are reduced (Kujawa & Liberman 2009; Lin et al. 2011; Furman et al. 2013). This can be explained by the finding that noise apparently destroys synapses mostly with high-threshold (low spontaneous rate) ANFs, while largely sparing synapses with low-threshold (high-spontaneous rate) ANFs (Furman et al. 2013). A loss of predominantly high-threshold ANFs has also been observed in aged gerbils raised in a quiet vivarium (Schmiedt et al. 1996). Unfortunately, wave I amplitudes are much less robust in scalp-recorded human ABRs than subcutaneously-recorded animal ABRs, especially at low to moderate stimulus levels. Nonetheless, two recent studies reported reduced ABR wave I amplitudes at high SPLs in groups of tinnitus patients with normal audiograms, compared with nontinnitus controls (Schaette & McAlpine 2011; Gu et al. 2012). Most recently, Stamper and Johnson (2015) studied people with normal audiograms and varying degrees of self-reported chronic exposure to moderately loud noise in the 67 to 83 dB A range. They found significant negative correlations between the level of noise exposure and the ABR wave I amplitude evoked by high-level clicks and 4 kHz tone bursts, but not between the level of noise exposure and DPOAE amplitudes, suggesting that they may have been tracking the severity of noise-induced synaptopathy in their subjects.
Given the high variability of scalp-recorded human ABR wave I, a more reliable clinical metric of synaptopathy may be the wave V to wave I amplitude ratio, which is more consistent between normal-hearing subjects. The wave V/I ratio is predicted to increase in synaptopathy because whereas wave I amplitude should be reduced, wave V is likely to remain normal as a result of compensatory increases in auditory brainstem activity (Turrigiano 1999; Salvi et al. 2000; Schaette & McAlpine 2011; Chambers et al. 2016). Another approach is to record the amplitude of the auditory nerve compound action potential (CAP) with a tiptrode in the ear canal or a tymptrode at the eardrum. The CAP is more robust than its ABR wave I counterpart (by virtue of being recorded closer to its cochlear source), and can often be measured reliably down to 0 dB nHL (normalized hearing level) in normal-hearing subjects. An interesting recently-revived scheme is to measure chirp-evoked CAPs in the presence of high-pass masking noise, using progressively higher noise cutoff frequencies to track the frequency specificity of the loss (Eggermont 1976; Earl & Chertoff 2012; Earl 2015). One could also use tip- or tymptrodes to measure the hair cell-generated summating potential (SP), and compare it to the amplitude of the neural CAP. (This SP/CAP ratio is currently used in the diagnosis of Meniere’s disease [Lamounier et al. 2014]). A significant increase in the SP/CAP ratio, indicative of normal hair cells but impaired ANFs, was recently observed in a group of noise-exposed subjects with normal thresholds, compared with nonexposed controls (Epstein et al. 2016). However, an important complication here are the recent findings of Bourien et al. (2014), who showed that substantial ouabain-induced ANF losses in guinea pigs can coexist with both normal CAP thresholds and suprathreshold CAP amplitudes. These data suggest that the contribution of high-threshold ANFs to CAP (or ABR wave I) amplitudes might be small, and that a substantial reduction of the CAP implies that low-threshold fibers must also have been denervated, which is surprising given that CAP/ABR thresholds remain normal.
Another window to a partial loss of high-threshold (and perhaps also low threshold) ANFs could be impaired temporal encoding fidelity. Individual ANFs can phase lock to sound pressure fluctuations of at most a few hundred Hz, a limit imposed by their maximal rate of spike firing. However, groups of ANFs can collectively phase lock to sound frequencies an order of magnitude higher (several kHz), as seen in cochlear microphonic recordings (Palmer & Russell 1986; Ruggero 1992). This collective phase-locking would suffer with a partial ANF loss (Joris et al. 2004; Lopez-Poveda & Barrios 2013). A recent study recorded ABRs to sinusoidally amplitude-modulated (AM) tones in noise-exposed mice, and found that decreases in envelope-following response parameters were better indicators of synaptopathy than decreases in tone-evoked ABR wave I (Shaheen et al. 2015). Amplitude modulation detection can also be assessed psychophysically, by measuring temporal modulation transfer functions for example (Viemeister 1979). Temporal modulation transfer functions show AM detection thresholds versus the modulation frequency, and are always low pass in character (i.e., it is easier to detect low- than high-frequency AM); this low-pass cutoff frequency should decrease with synaptopathy, although this remains to be tested. The most common behavioral metric of the auditory system’s temporal acuity is gap detection in noise (Green 1971), variants of which can be studied electrophysiologically to facilitate comparisons with animal studies. For example, ABRs measured in a tone-on-tone burst forward-masking paradigm with a variable gap duration (2 to 64 msec) showed prolonged wave V latencies for short but not for long-duration gaps in older subjects with normal thresholds compared with younger controls (Walton et al. 1999). However, in seeming contrast to this finding, differences in wave V latencies between older and younger subjects with normal thresholds were not seen in standard click ABRs when the click presentation rate was increased from 11 to 75 Hz, although the older subjects did have significantly reduced wave I amplitudes (Burkard & Sims 2001). Most recently, Mehraei et al. (2016) demonstrated that noise-exposed synaptopathic mice showed smaller ABR wave 4 (analogous to human wave V) latency increases when measured in noise, compared with nonexposed controls, as would be predicted from a selective loss of high-threshold ANFs (Furman et al. 2013). In normal-hearing humans, smaller wave V latency increases in noise were correlated with poorer performance on a timing-based sound localization task, suggesting that masked ABRs could be another useful clinical metric of synaptopathy (Mehraei et al. 2016).
One obstacle to the development of clinical tests of synaptopathy is difficulty quantifying a person’s noise exposure history. This is easier in musicians, for example, but their hearing loss could be especially well hidden by their more “practiced ears” (or rather auditory brains; Moreno & Bidelman 2014; Alain et al. 2014), allowing them to hear comparatively well in noise in spite of synaptopathy. A better initial study group might be nonmusicians with normal audiograms in the speech range but elevated thresholds at ultra-high frequencies, as in Figure 2. Such audiograms may be more reliable indicators of noise trauma than questionnaires, and could prove to be simple but effective screeners for hidden losses at lower frequencies.
These or other ideas (Plack et al. 2014; Bharadwaj et al. 2014; Kujawa & Liberman 2015; Valero et al. 2016) could soon be used to diagnose noise- and age-related cochlear synaptopathy, and to correlate it with the extent of patients’ listening difficulties in noise. Until then, the task of dissociating peripheral from central contributions to listening difficulties in noise will remain fraught with at least some uncertainty. Finally, although the prevalence of synaptopathy should increase with age, children unfortunately often listen to music or play video games at high volumes through earphones, and attend noisy school dances, concerts, sporting events, etc. (Keith et al. 2011; Taljaard et al. 2013; Carter et al. 2014), making them potentially vulnerable to noise-induced synaptopathy as well.
Central HHL
Hearing difficulties in spite of normal audiograms can also arise in the absence of any damage to the ear, including the hidden varieties discussed above, and in the absence of neurologically detectable CANS lesions. Animal studies have shown that exposure to moderately loud broadband noise during developmental sensitive periods can lastingly degrade cortical tonotopic maps and block the emergence of cortical neural tuning for complex sound features (Zhang et al. 2002; Chang & Merzenich 2003; Chang et al. 2005; de Villers-Sidani et al. 2008; Insanally et al. 2010; de Villers-Sidani & Merzenich 2011; Bureš et al. 2016). Less clear are the perceptual consequences of these cortical (and often also subcortical) changes. In one of the few studies addressing this important issue, Pan et al. (2011) exposed juvenile rats to pulses of broadband noise at 65 dB SPL and found that this led to impaired sound localization in adulthood. Very similar noise exposures early in life did not affect the adult rat’s ability to discriminate human speech sounds (Ranasinghe et al. 2012), but did impair working memory as assessed in T-maze tasks (Ruvalcaba-Delgadillo et al. 2015). Note that although these studies only reported pre- and post-exposure ABR audiograms and not ABR wave I amplitudes, noise-induced synaptopathy is unlikely because exposure levels were only in the 60 to 70 dB SPL range. Furthermore, in these and other studies (Zhou & Merzenich 2007, 2009; Zhu et al. 2014), intensive perceptual training or environmental acoustic enrichment in adulthood was able to completely reverse the degraded cortical representations and sensory deficits resulting from developmental noise exposure and also from aging (de Villers-Sidani et al. 2010), encouraging the use of auditory training (AT) as a clinical treatment for central HHL (see the “Implications for AT” section).
The pinnacle of human auditory system development is language acquisition, which requires both passive listening (resulting in CANS specializations for discriminating phonetic contrasts in the native language, for example) and active engagement with language “tutors,” a process reminiscent of song learning in birds (Kuhl 2004; Woolley 2012). Although the disruption of normal sound input (or of social contact) during sensitive periods can delay or even lastingly impair language and song acquisition (Kuhl 2004; Woolley 2012; Amin et al. 2013), it is presently unknown if moderate noise exposure during human infancy could lead to listening difficulties persisting into the grade school years. Neonatal intensive care units are one source of such nontraumatic but potentially CANS-altering noise (Brown 2009; McMahon et al. 2012). Another are broadband noise generators intended to help babies sleep, which many manufacturers recommend leaving on through the night during the first year of life. Despite concerns about the high noise levels put out by some of these machines (Hugh et al. 2014), and the possibility that they could impair CANS development (e.g., http://www.webmd.com/baby/news/20030417/white-noise-may-delay-infant-development), their popularity appears to be on the rise.
Another example of abnormal sound input early in life that could potentially lead to longer-term listening difficulties is temporary unilateral conductive loss due to infection of the middle ear, a condition named amblyaudia in analogy to the better known amblyopia (Whitton & Polley 2011; Kaplan et al. 2016). Animal studies have shown that short periods of such conductive loss can alter CANS development and disrupt binaural integration and hearing in noise even after a complete reversal of the loss (Knudsen et al. 1984; Popescu & Polley 2010; Polley et al. 2013; Gay et al. 2014). While some residual conductive loss sometimes remains in older children and adults with a history of middle ear infection, especially those who required multiple intubations (typically 5 to 10 dB, especially above 4 kHz; Hunter et al. 1996), the bulk of the lingering difficulties hearing in noise is likely the result of impaired binaural hearing, as evidenced by poor performance on the masking level difference test (Lynn et al. 1981), for example (Teele et al. 1984; Pillsbury et al. 1991; Wilmington et al. 1994; Hall et al. 1995; Hogan & Moore 2003; Gray et al. 2009).
Abnormal sound input can also trigger long-lasting changes in the mature auditory brain, which maintains a lifelong capacity not only for perceptual learning but also for passive sound-driven plasticity (Pienkowski & Eggermont 2011, 2012; Eggermont 2014; Gourévitch et al. 2014). For example, adult cats exposed for several weeks or months to sharply band-limited, moderately loud noise showed a long-term suppression of auditory cortical responses to sound frequencies within the noise band, and an enhancement of responses to frequencies just above and below the noise band, in the apparent absence of hearing loss (Noreña et al. 2006; Pienkowski & Eggermont 2009, 2010a, b; Pienkowski et al. 2011). These cortical changes may be the neural correlates of the loudness rescaling demonstrated in normal-hearing human subjects after several weeks of wearing a noise generator or earplugs (Formby et al. 2003; Fournier et al. 2014).
Could working or living in moderately noisy environments lead to listening difficulties over time, even in the absence of synaptopathic-type losses? Kujala et al. (2004) reported electrophysiologic evidence of impaired syllable discrimination in quiet, but interestingly, not in noise, in 8 shipyard workers and 2 preschool teachers (23 to 36 years old) with an average 6 years of moderate occupational noise exposure but normal audiograms. The fact that syllable discrimination was impaired in quiet but not in noise suggests that synaptopathy was not a factor. Similar findings were described in a recent animal study by Zheng (2012): adult rats exposed to moderately loud broadband noise had impaired frequency discrimination in quiet but better discrimination in noise. These results suggest that while persistent exposure to moderately loud noise can lead to difficulties hearing in quiet (see also Zhou & Merzenich 2012; Kamal et al. 2013), noise at nonsynaptopathic levels can actually be useful for improving listening in noise. Such low level “sound therapy” has also been shown to reduce the impact of a traumatic noise exposure, and to potentially ameliorate tinnitus and hyperacusis (Noreña & Eggermont 2005, 2006; Noreña & Chery-Croze 2007; Schaette et al. 2010), providing that the noise spectrum is matched to the frequency range of the hearing loss and contains the pitch of the tinnitus.
PERIPHERAL HEARING LOSS, APD, OR COGNITIVE DEFICIT?
Lesions of the CANS, caused by neurodegenerative disease, stroke, tumor growth, head injury, etc., can lead to APDs without impacting hearing sensitivity (Musiek & Chermak 2014). Audiologic assessment for APD is presently indicated when listening difficulties occur in the apparent absence of peripheral hearing loss (but see the “Hidden Hearing Loss” section) and cognitive disorders. The American Academy of Audiology (2010) recommends that relevant psychologic/psychiatric assessments be performed first, so that information about potential attention, memory, and linguistic deficits is available to the audiologist before APD testing. However, as this is not always practical, the audiologist can perform basic tests of attention, memory, and linguistic abilities, such as continuous performance tests (Riccio et al. 2001) and the Second Comprehensive Test of Phonological Processing (CTOPP2; Wagner et al. 2013). If cognitive deficits are found, they should be controlled for in subsequent APD testing, as discussed further below.
Audiologic assessment for APD has traditionally consisted of psychoacoustic tests with reasonably good sensitivity and specificity for detecting frank lesions of the CANS (Musiek 1983a; Musiek & Pinheiro 1987; Jerger et al. 1988; Musiek et al. 1991, 2005, 2011; Rappaport et al. 1994; Hurley & Musiek 1997; Boscariol et al. 2011; Musiek & Weihing 2011; Bamiou et al. 2012; Musiek & Chermak 2015). For example, in a recent study on 20 adult patients with neurologically confirmed bilateral cortical lesions that included auditory cortex (caused by epilepsy, stroke, tumors, or head trauma), and 29 age-matched controls, Musiek et al. (2011) reported a sensitivity of 80% and specificity of 93% for the dichotic digits (Musiek 1983b) and frequency patterns tests (Musiek 1994) when used as a two-test battery (i.e., failure required performance below the control group 95% CI on both tests). While these are useful numbers for screening purposes, justifying further psychoacoustic, electrophysiologic, and neurologic testing, it is unclear whether such a test battery could effectively predict the existence of CANS lesions in the general population of children and adults with listening difficulties, many or most of whom may instead have hidden peripheral losses or cognitive deficits.
A key factor influencing the diagnostic utility of any clinical test is the prevalence of the condition the test is designed to detect. If the prevalence is low, then the predictive power of a test with even high sensitivity and specificity may be poor. At present, the general prevalence of the types of CANS lesions used to validate the dichotic digits and frequency patterns test battery by Musiek et al. (2011) is difficult to estimate. As a hypothetical example, consider a prevalence of 10%, with the remaining 90% of cases of hearing problems despite normal audiograms attributable to HLL or to cognitive deficits. The conditional probability, P(L F), of a frank CANS lesion (L), given a failed APD test or test battery (F), also known as the positive predictive value, is given by Bayes’ rule:
where P(L∩F) is the joint probability of having a lesion and failing the test battery. If test sensitivity and specificity, as established in patients with confirmed CANS lesions, is 80% and 93%, respectively (Musiek et al. 2011), then P(F) = 0.1(0.8) + 0.9(0.07) = 0.143 (i.e., you can fail the test as a true positive or a false positive, respectively), and P(L∩F) = 0.1(0.8) = 0.08 (i.e., the joint probability is just the probability of being a true positive). Finally, P(L F) = 0.08/0.143 = 56%. Thus, if a person failed the dichotic digits and frequency patterns test battery, he would have a roughly 50/50 chance of actually having a CANS lesion. On the other hand, if the prevalence of CANS lesions among people with listening difficulties and normal audiograms was only 1%, the positive predictive value of Musiek et al.’s (2011) test battery would drop to just 10%. The predictive value would also drop if HHL or cognitive deficits also resulted in poorer performance on such tests of “AP” (Smurzynski & Probst 1999; Neijenhuis et al. 2004; Glyde et al. 2013; Musiek & Chermak 2015).
A number of studies have reported that children who fail tests of AP are also likely to fail tests of cognition (Sharma et al. 2009, 2014; Miller 2011; Ahmmed et al. 2014). For example, Sharma et al. (2009) found that in a sample of 68 children (7 to 12 years old) with suspected APD, almost half (47%) failed not just the AP tests but also the language and reading tests, whereas only 4% failed just the AP tests, 7% just the language tests, and 4% just the reading tests. A popular explanation for this apparent comorbidity has been that AP deficits, especially poor temporal acuity, can delay language acquisition and literacy (Tallal & Piercy 1973; Tallal 1980, 2004; Benasich & Tallal 2002; Banai et al. 2005; Ziegler et al. 2005, 2009; Boets et al. 2011; Hornickel et al. 2012; Hornickel & Kraus 2013; White-Schwoch et al. 2015). However, poor temporal acuity could also be a consequence of hidden peripheral losses (Lopez-Poveda & Barrios 2013), and cognitive deficits could drive poor performance on tests of AP. As reviewed by Rosen (2003) and substantiated by some of the more recent data (Amitay et al. 2002; Richardson et al. 2004; Dawes et al. 2009; Rosen et al. 2009; Watson & Kidd 2009; Bishop et al. 2012; Vandewalle et al. 2012), most children and adults with language and reading difficulties do not in fact appear to have impaired AP, and when they do, the deficits do not always include fast temporal processing. The current consensus seems to be that language and reading disorders more often result from impaired linguistic rather than auditory sensory processing (Bailey & Snowling 2002; Pennington & Bishop 2009). Figure 3 provides an intuitive guide to distinguishing between the two. Note that comparing performance on tests of AP that use speech versus nonspeech sounds is probably not sufficient to differentiate APD from linguistic disorders, as children with the latter tend to do poorly with both classes of stimuli (Loo et al. 2013).
Fig. 3.

An intuitive guide to differentiating deficits in linguistic and auditory processing. Adapted from Richard (2013).
In contrast to Sharma et al. (2009), Gyldenkærne et al. (2014) reported that 57% of 101 children with listening difficulties (also 7 to 12 years old) failed an AP test battery, 50% failed the auditory and visual sustained attention test (Sandford & Turner 1995), while 34% failed both. If AP and attention deficits were independent, the expected failure rate for both would be 29% (57% × 50%), not much less than the observed 34%, suggesting that AP and attention deficits can exist as largely independent conditions, with each contributing roughly equally to the case count of children with listening difficulties. In an interesting study, Moore et al. (2010) randomly sampled 1469 primary school children (6 to 11 years old) and found that the correlation between numerous metrics of AP and cognition was low (0.1 < ρ < 0.3, or at most about 10% of the variance in AP scores was explained by cognitive factors), as also reported by others (Riccio et al. 2005; Rosen et al. 2010; Gyldenkærne et al. 2014). (Note that in elderly adults, these correlations are typically higher, but then secondary to the effect of audiometric hearing loss [Akeroyd 2008; Humes et al. 2013]). Nevertheless, the bottom 5% of auditory performers in the Moore et al. study also had significantly lower cognitive scores than the top 95% or “typical” auditory performers, suggesting that auditory problems in the general primary school population could indeed be caused primarily by cognitive deficits. When the auditory tests were modified to control for cognitive confounds, by taking the difference scores of pairs of subtests that kept the cognitive load constant while varying the difficulty of the auditory task (as in the masking level difference test and the low cue versus high cue subtests of the Listening in Spatialized Noise-Sentences test [LiSN-S]; see below), the bottom 5% of auditory performers no longer had significantly worse cognitive scores than the top 95%. (A complementary strategy would be to keep the auditory difficulty constant across subtests while varying the cognitive load.) Moore et al.’s message was that applying uncontrolled tests of AP to the general primary school population would tend to catch children with cognitive issues rather than sensory ones. While these findings have been criticized on several grounds (Musiek & Chermak 2015), the general approach of employing subtest difference scores to control for cognitive confounds seems to be a very useful one, as further illustrated below.
A good example of defining a subtype of APD and creating a specific, controlled test to detect it was recently provided by Dillon and colleagues (Cameron & Dillon 2007a, b, 2008; Dillon et al. 2012). Their LiSN-S test, developed to detect deficits in binaural or spatial processing, is a twist on the masking level difference test (Lynn et al. 1981). In the LiSN-S, simple target sentences are presented binaurally and in phase through earphones, so that they are localized to the front of the listener. In the four subtests of the LiSN-S, illustrated in Figure 4, two distractor voices are either co-localized to the front, or lateralized to the sides; furthermore, the two distractors are produced either by the speaker of the target sentences or by different speakers. In addition to the individual subtest scores, three difference scores can be calculated (Fig. 4): “talker advantage” gives the amount of improvement when distractor voices are different from the target voice; “spatial advantage” gives the amount of improvement when distractors are lateralized relative to the target, analogous to masking level differences; “total advantage” combines both elements, but is not necessarily equal to the summed improvement of the talker and spatial advantages. The “high cue” subtest (Fig. 4), when both spatial and speaker cues are available, should be administered first, and a normal score would rule out difficulties using these cues to hear effectively in noise (unless the ability to use one cue was enhanced by a deficit in the ability to use the other). As reported in Dillon et al. (2012), of 183 children identified by teachers or parents as having difficulties hearing in noise, only 40 (22%) failed the high cue subtest. It is thus clear that many other auditory and cognitive factors contribute to listening difficulties in children. However, in most of the children who failed the high cue LiSN-S, a subsequent normal score on the low cue subtest (Fig. 4) ruled out cognitive issues, as the cognitive demands of the low and high cue subtests are essentially the same. Running the remaining two subtests and calculating the talker and spatial advantage difference scores could then partial out the contributions of talker and spatial cues to the abnormal total advantage score. When this was done, 32 children were found to have an abnormal spatial advantage while only 4 had an abnormal talker advantage. Thus, in this sample, spatial processing deficits were eight times more likely to contribute to listening difficulties than speaker discrimination deficits. Cameron et al. (2012) went on to show, albeit only in a small-sample study of 10 of the children with spatial processing deficits as diagnosed by LiSN-S, that their LiSN & Learn auditory training program, which specifically targeted the spatial deficits, led to improved LiSN-S spatial advantage scores, whereas a nonspecific commercial auditory/reading training program (Earobics, Boston, MA) did not. These results support the strategy of subtyping APD and implementing deficit-specific remediation (see the “Implications for AT” section), a promising approach that has also been investigated and advocated by others (e.g., Moncrieff & Wertz 2008; Bellis & Bellis 2015).
Fig. 4.
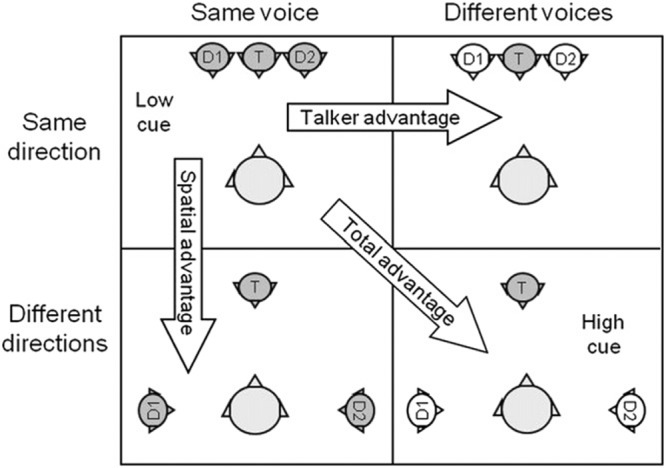
Subtests of the LiSN-S test. See text for explanation. Adapted from Figure 2 in J Am Acad Audiol, 2012;23, 97–105. LiSN-S, listening in spatialized noise-sentences.
In addition to psychoacoustic testing, electrophysiology can also be useful for differentiating auditory from cognitive contributors to listening difficulties in noise. As mentioned, the standard click- and tone ABR can be a sensitive and specific screener for lesions of the auditory nerve and brainstem (due to neuropathies, acoustic neuromas and other tumors, multiple sclerosis, infections, stroke, trauma, etc.), often even if thresholds are unaffected (Hosford-Dunn 1985; Hendrix et al. 1990; Levine et al. 1993; Starr et al. 1996; Häusler & Levine 2000; Litovsky et al. 2002; Cho et al. 2005; Church et al. 2007). In many cases, the lesions can then be confirmed by neuroimaging, particularly magnetic resonance imaging.
In recent years, the “complex” ABR (cABR), best studied using the syllable /da/, has been introduced as a potentially more sensitive metric of subcortical speech processing than the click or tone ABR (Skoe & Kraus 2010; Anderson & Kraus 2013a). The cABR normally resembles a low-pass-filtered version of the stimulus waveform, and can be analyzed to assess subcortical speech-encoding fidelity in both the time and frequency domains. For example, in a study of older adults (60 to 73 years old) with clinically normal audiograms, cABR amplitudes to /da/ presented either in quiet or in noise correlated strongly with HINT (hearing in noise test) scores (Anderson et al. 2011). The same was true in younger adults with normal self-reported hearing (Song et al. 2011), suggesting that the cABR could provide an objective, subcortical correlate of the large variability in SIN scores observed across young, apparently normal-hearing listeners (Surprenant & Watson 2001). Interestingly, in both young, normal-hearing (Song et al. 2012) and older subjects (Anderson et al. 2013), AT to improve hearing in noise led to more robust cABRs in noise, while musicians also showed better cABRs in noise compared with nonmusicians (Parbery-Clark et al. 2009; Bidelman & Krishnan 2010). The cABR could therefore potentially differentiate auditory from cognitive contributions to listening difficulties, although it could just as easily be affected by hidden deficits at the level of the cochlea and auditory nerve (see the “Cochlear Synaptopathy” section) as by deficits in brainstem processing.
Finally, while both pre- and post attentive auditory cortical evoked potentials (e.g., middle latency response, late potentials, P300) and electroencephalograms have been studied in children with listening difficulties despite normal thresholds (e.g., Jirsa & Clontz 1990; Purdy et al. 2002; Sharma et al. 2006; Schochat et al. 2010; Gilley et al. 2016), it’s not yet clear if cortical recordings could be useful in differentiating between sensory and cognitive contributions to listening difficulties in noise, given the intertwined nature of cortical processing (Medwetsky 2011; Poremba & Bigelow 2013; Steinschneider 2013).
IMPLICATIONS FOR AT
Animal studies have demonstrated that perceptual training leads to rapid improvements in task performance, initially by shifting neural receptive fields to expand the “representational area” of the trained parameter in sensory-motor cortex (Recanzone et al. 1992, 1993; Fritz et al. 2003; Bao et al. 2004; Polley et al. 2006; Atiani et al. 2009; Bieszczad & Weinberger 2010; Whitton et al. 2014), and then by consolidating a more efficient representation from the expanded one (Molina-Luna et al. 2008; Bajo et al. 2010; Reed et al. 2011). Such demonstrations of CANS plasticity have provided the rationale for the rapid emergence of commercial computer-based AT programs for people both with and without audiometric hearing loss, with considerable evidence for their efficacy (Sweetow & Palmer 2005; Moore et al. 2009; Anderson & Kraus 2013b; Henshaw & Ferguson 2013). In a recent study, Loo et al. (2016) randomized 39 children (7 to 11 years old) diagnosed with APD into an intensive (3 months, 5 days/week) AT group, consisting of a variety of SIN listening tasks, or a standard treatment-only control group. Only the AT group showed improved hearing in noise, as assessed using the LiSN-S. Furthermore, the improvement correlated with better scores on the Children’s Auditory Processing Performance Scale questionnaire (Smoski et al. 1992), and were retained for at least 3 months after the completion of training. The Loo et al. (2016) study suggests that children diagnosed with APD using conventional clinical methods can benefit from intensive speech-based AT.
An intriguing question is the extent to which AT could be even more efficacious if the specific deficits underlying the APD (or the listening difficulties more generally) are targeted. To the extent that hearing in noise involves a wide range of AP and cognitive skills, generic speech-based training could provide benefits for a wide range of patients with listening difficulties, including those with purely cognitive issues. In fact, in a study of older adults with mild hearing loss who underwent phoneme discrimination training, reductions in self-reported hearing disability best correlated with training-induced improvements in attention and working memory, not auditory skills (Ferguson et al. 2014). Nevertheless, it is reasonable to suppose that deficit-specific training should result in maximal improvement in the ability to hear in noise (Cameron et al. 2012). For example, training a person with a binaural integration deficit on sound localization tasks should improve hearing in noise more than training on phonemic discrimination tasks.
CONCLUDING SUMMARY
Many people with tone thresholds in the wide “clinically normal” range have considerable difficulty understanding speech in noisy environments. In the apparent absence of cochlear damage and of cognitive disorders, such people have traditionally been subject to psychoacoustic tests of “AP,” and diagnosed with APD on failing one or more of them (American Speech-Language-Hearing Association 2005; American Academy of Audiology 2010; British Society of Audiology 2011; Wilson & Arnott 2013). However, there is a strong possibility that certain configurations of noise- and age-related peripheral loss, “hidden” from the audiogram and other conventional clinical tests, could also account for many cases of listening difficulties and for failure on some of the tests of AP (see the “‘Clinically Normal’ Audiograms” and “Cochlear Synaptopathy” sections). The same is true of deficits in attention, working memory or linguistic abilities (see the “Peripheral Hearing Loss, APD, or Cognitive Deficit?” section). Furthermore, impaired AP could also be a consequence of abnormal patterns of sound input (e.g., auditory deprivation, persistent background noise), especially during developmental sensitive periods but also in adulthood, even in the absence of peripheral losses (including the hidden variety) and of frank lesions affecting the CANS (see the “Central HHL” section). Promising new tests to uncover hidden peripheral losses, and tests of central auditory function that better control for peripheral and cognitive confounds could soon translate to improved differential diagnosis of the causes of hearing difficulties in noise. It will then remain to determine how best to tailor the promising tool of AT to the specific deficits of individual patients (see the “Implications for AT” section).
ACKNOWLEDGMENTS
The author thanks Profs. Jos Eggermont and Condon Lau, as well as anonymous reviewers, for their helpful suggestions for improving this article.
Footnotes
The author has no conflicts of interest to disclose.
REFERENCES
- Ahmmed A. U., Ahmmed A. A., Bath J. R., et al. Assessment of children with suspected auditory processing disorder: A factor analysis study. Ear Hear, (2014). 35, 295–305.. [DOI] [PubMed] [Google Scholar]
- Akeroyd M. A.Are individual differences in speech reception related to individual differences in cognitive ability? A survey of twenty experimental studies with normal and hearing-impaired adults. Int J Audiol, (2008). 47Suppl 2S53–S71.. [DOI] [PubMed] [Google Scholar]
- Alain C., Zendel B. R., Hutka S., et al. Turning down the noise: The benefit of musical training on the aging auditory brain. Hear Res, (2014). 308, 162–173.. [DOI] [PubMed] [Google Scholar]
- American Academy of Audiology (AAA) American Academy of Audiology (AAA) American Academy of Audiology clinical practice guidelines: Diagnosis, treatment and management of children and adults with central auditory processing disorder. 2010Retrieved from http://audiology-web.s3.amazonaws.com/migrated/CAPD%20Guidelines%208-2010.pdf_539952af956c79.73897613.pdf.
- American National Standards Institute (ANSI) American National Standards Institute (ANSI) Specification for Audiometers. ANSI/ASA S3.6-2010. 2010Retrieved from http://webstore.ansi.org/RecordDetail.aspx?sku=ANSI%2FASA+S3.6-2010.
- American Speech-Language-Hearing Association (ASHA) American Speech-Language-Hearing Association (ASHA) (Central) auditory processing disorders. 2005Retrieved from http://www.asha.org/policy/TR2005-00043/.
- Amin N., Gastpar M., Theunissen F. E.Selective and efficient neural coding of communication signals depends on early acoustic and social environment. PLoS One, (2013). 8, e61417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitay S., Ahissar M., Nelken I.Auditory processing deficits in reading disabled adults. J Assoc Res Otolaryngol, (2002). 3, 302–320.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Kraus N.The potential role of the cABR in assessment and management of hearing impairment. Int J Otolaryngol, (2013a). 2013, 604729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Kraus N.Auditory training: Evidence for neural plasticity in older adults. Perspect Hear Hear Disord Res Res Diagn, (2013b). 17, 37–57.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Parbery-Clark A., Yi H. G., et al. A neural basis of speech-in-noise perception in older adults. Ear Hear, (2011). 32, 750–757.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., White-Schwoch T., Parbery-Clark A., et al. Reversal of age-related neural timing delays with training. Proc Natl Acad Sci U S A, (2013). 110, 4357–4362.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiani S., Elhilali M., David S. V., et al. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron, (2009). 61, 467–480.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri R., Siegel J. H., Wright B. A.Auditory filter shapes and high-frequency hearing in adults who have impaired speech in noise performance despite clinically normal audiograms. J Acoust Soc Am, (2011). 129, 852–863.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P. J., Snowling M. J.Auditory processing and the development of language and literacy. Br Med Bull, (2002). 63, 135–146.. [DOI] [PubMed] [Google Scholar]
- Bajo V. M., Nodal F. R., Moore D. R., et al. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci, (2010). 13, 253–260.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamiou D. E., Werring D., Cox K., et al. Patient-reported auditory functions after stroke of the central auditory pathway. Stroke, (2012). 43, 1285–1289.. [DOI] [PubMed] [Google Scholar]
- Banai K., Nicol T., Zecker S. G., et al. Brainstem timing: Implications for cortical processing and literacy. J Neurosci, (2005). 25, 9850–9857.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S., Chang E. F., Woods J., et al. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci, (2004). 7, 974–981.. [DOI] [PubMed] [Google Scholar]
- Bellis T. J., Bellis J. D.Central auditory processing disorders in children and adults. Handb Clin Neurol, (2015). 129, 537–556.. [DOI] [PubMed] [Google Scholar]
- Benasich A. A., Tallal P.Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res, (2002). 136, 31–49.. [DOI] [PubMed] [Google Scholar]
- Bharadwaj H. M., Verhulst S., Shaheen L., et al. Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci, (2014). 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj H. M., Masud S., Mehraei G., et al. Individual differences reveal correlates of hidden hearing deficits. J Neurosci, (2015). 35, 2161–2172.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biassoni E. C., Serra M. R., Richtert U., et al. Recreational noise exposure and its effects on the hearing of adolescents. Part II: Development of hearing disorders. Int J Audiol, (2005). 44, 74–85.. [DOI] [PubMed] [Google Scholar]
- Bidelman G. M., Krishnan A.Effects of reverberation on brainstem representation of speech in musicians and non-musicians. Brain Res, (2010). 1355, 112–125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad K. M., Weinberger N. M.Representational gain in cortical area underlies increase of memory strength. Proc Natl Acad Sci U S A, (2010). 107, 3793–3798.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. V., Hardiman M. J., Barry J. G.Auditory deficit as a consequence rather than endophenotype of specific language impairment: Electrophysiological evidence. PLoS One, (2012). 7, e35851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B., Vandermosten M., Poelmans H., et al. Preschool impairments in auditory processing and speech perception uniquely predict future reading problems. Res Dev Disabil, (2011). 32, 560–570.. [DOI] [PubMed] [Google Scholar]
- Boothroyd A., Medwetsky L.Spectral distribution of /s/ and the frequency response of hearing aids. Ear Hear, (1992). 13, 150–157.. [DOI] [PubMed] [Google Scholar]
- Boscariol M., Guimarães C. A., Hage S. R., et al. Auditory processing disorder in patients with language-learning impairment and correlation with malformation of cortical development. Brain Dev, (2011). 33, 824–831.. [DOI] [PubMed] [Google Scholar]
- Bourien J., Tang Y., Batrel C., et al. Contribution of auditory nerve fibers to compound action potential of the auditory nerve. J Neurophysiol, (2014). 112, 1025–1039.. [DOI] [PubMed] [Google Scholar]
- Brattico E., Kujala T., Tervaniemi M., et al. Long-term exposure to occupational noise alters the cortical organization of sound processing. Clin Neurophysiol, (2005). 116, 190–203.. [DOI] [PubMed] [Google Scholar]
- Bredberg G.Cellular pattern and nerve supply of the human organ of Corti. Acta Otolaryngol Suppl, (1968). 236. [PubMed] [Google Scholar]
- British Society of Audiology (BSA) British Society of Audiology (BSA) Position statement: Auditory processing disorder (APD). 2011Retrieved from www.thebsa.org.uk/images/stories/docs/BSA_APD_PositionPaper_31March11_FINAL.pdf.
- Brown G.NICU noise and the preterm infant. Neonatal Netw, (2009). 28, 165–173.. [DOI] [PubMed] [Google Scholar]
- Bureš Z., Popelář J., Syka J.The effect of noise exposure during the developmental period on the function of the auditory system. Hear Res, (2016in press. Doi: 10.1016/j.heares.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Burkard R. F., Sims D.The human auditory brainstem response to high click rates: aging effects. Am J Audiol, (2001). 10, 53–61.. [DOI] [PubMed] [Google Scholar]
- Butler B. E., Purcell D. W., Allen P.Contralateral inhibition of distortion product otoacoustic emissions in children with auditory processing disorders. Int J Audiol, (2011). 50, 530–539.. [DOI] [PubMed] [Google Scholar]
- Cacace A. T., McFarland D. J.The importance of modality specificity in diagnosing central auditory processing disorder. Am J Audiol, (2005). 14, 112–123.. [DOI] [PubMed] [Google Scholar]
- Cameron S., Dillon H.Development of the Listening in Spatialized Noise-Sentences Test (LISN-S). Ear Hear, (2007). 28, 196–211.. [DOI] [PubMed] [Google Scholar]
- Cameron S., Dillon H.The listening in spatialized noise-sentences test (LISN-S): Test-retest reliability study. Int J Audiol, (2007). 46, 145–153.. [DOI] [PubMed] [Google Scholar]
- Cameron S., Dillon H.The listening in spatialized noise-sentences test (LISN-S): Comparison to the prototype LISN and results from children with either a suspected (central) auditory processing disorder or a confirmed language disorder. J Am Acad Audiol, (2008). 19, 377–391.. [DOI] [PubMed] [Google Scholar]
- Cameron S., Glyde H., Dillon H.Efficacy of the LiSN & Learn auditory training software: Randomized blinded controlled study. Audiol Res, (2012). 2, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter L., Williams W., Black D., et al. The leisure-noise dilemma: Hearing loss or hearsay? What does the literature tell us? Ear Hear, (2014). 35, 491–505.. [DOI] [PubMed] [Google Scholar]
- Chambers A. R., Resnik J., Yuan Y., et al. Central gain restores auditory processing following near-complete cochlear denervation. Neuron, (2016). 89, 867–879.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. F., Merzenich M. M.Environmental noise retards auditory cortical development. Science, (2003). 300, 498–502.. [DOI] [PubMed] [Google Scholar]
- Chang E. F., Bao S., Imaizumi K., et al. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U S A, (2005). 102, 16460–16465.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho T. H., Fischer C., Nighoghossian N., et al. Auditory and electrophysiological patterns of a unilateral lesion of the lateral lemniscus. Audiol Neurootol, (2005). 10, 153–158.. [DOI] [PubMed] [Google Scholar]
- Church M. W., Parent-Jenkins L., Rozzelle A. A., et al. Auditory brainstem response abnormalities and hearing loss in children with craniosynostosis. Pediatrics, (2007). 119, e1351–e1360.. [DOI] [PubMed] [Google Scholar]
- Dawes P., Sirimanna T., Burton M., et al. Temporal auditory and visual motion processing of children diagnosed with auditory processing disorder and dyslexia. Ear Hear, (2009). 30, 675–686.. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E., Merzenich M. M.Lifelong plasticity in the rat auditory cortex: Basic mechanisms and role of sensory experience. Prog Brain Res, (2011). 191, 119–131.. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E., Simpson K. L., Lu Y. F., et al. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci, (2008). 11, 957–965.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E., Alzghoul L., Zhou X., et al. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci U S A, (2010). 107, 13900–13905.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S., Hall J. W.Otoacoustic Emissions: Principles, Procedures, and Protocols, ((2011). San Diego, CA: Plural Publishing. [Google Scholar]
- Dillon H., Cameron S., Glyde H., et al. An opinion on the assessment of people who may have an auditory processing disorder. J Am Acad Audiol, (2012). 23, 97–105.. [DOI] [PubMed] [Google Scholar]
- Dorn P. A., Konrad-Martin D., Neely S. T., et al. Distortion product otoacoustic emission input/output functions in normal-hearing and hearing-impaired human ears. J Acoust Soc Am, (2001). 110, 3119–3131.. [DOI] [PubMed] [Google Scholar]
- Earl B. R.Using SiNAPS to uncover cochlear neuropathy. Audiol Today, (2015). 27, 16–24.. [Google Scholar]
- Earl B. R., Chertoff M. E.Mapping auditory nerve firing density using high-level compound action potentials and high-pass noise masking. J Acoust Soc Am, (2012). 131, 337–352.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont J. J.Analysis of compound action potential responses to tone bursts in the human and guinea pig cochlea. J Acoust Soc Am, (1976). 60, 1132–1139.. [DOI] [PubMed] [Google Scholar]
- Eggermont J. J.Noise and the Brain. Experience Dependent Development and Adult Plasticity, (2014). San Diego, CA: Academic Press. [Google Scholar]
- Engdahl B., Tambs K., Hoffman H. J.Otoacoustic emissions, pure-tone audiometry, and self-reported hearing. Int J Audiol, (2013). 52, 74–82.. [DOI] [PubMed] [Google Scholar]
- Epstein M., Cleveland S., Wang H., et al. Hidden hearing loss in young adults: audiometry, speech discrimination and electrophysiology. (2016). 39th Mid-winter Meeting of the Association for Research in Otolaryngology: (San Diego, CA), PS 781. [Google Scholar]
- Ferguson M. A., Hall R. L., Riley A., et al. Communication, listening, cognitive and speech perception skills in children with auditory processing disorder (APD) or Specific Language Impairment (SLI). J Speech Lang Hear Res, (2011). 54, 211–227.. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Henshaw H., Clark D. P., et al. Benefits of phoneme discrimination training in a randomized controlled trial of 50- to 74-year-olds with mild hearing loss. Ear Hear, (2014). 35, e110–e121.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez K. A., Jeffers P. W., Lall K., et al. Aging after noise exposure: Acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci, (2015). 35, 7509–7520.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festen J. M., Plomp R.Relations between auditory functions in impaired hearing. J Acoust Soc Am, (1983). 73, 652–662.. [DOI] [PubMed] [Google Scholar]
- Formby C., Sherlock L. P., Gold S. L.Adaptive plasticity of loudness induced by chronic attenuation and enhancement of the acoustic background. J Acoust Soc Am, (2003). 114, 55–58.. [DOI] [PubMed] [Google Scholar]
- Fournier P., Schönwiesner M., Hébert S.Loudness modulation after transient and permanent hearing loss: Implications for tinnitus and hyperacusis. Neuroscience, (2014). 283, 64–77.. [DOI] [PubMed] [Google Scholar]
- Fritz J., Shamma S., Elhilali M., et al. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci, (2003). 6, 1216–1223.. [DOI] [PubMed] [Google Scholar]
- Froud K. E., Wong A. C., Cederholm J. M., et al. Type II spiral ganglion afferent neurons drive medial olivocochlear reflex suppression of the cochlear amplifier. Nat Commun, (2015). 6, 7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllgrabe C., Moore B. C., Stone M. A.Age-group differences in speech identification despite matched audiometrically normal hearing: Contributions from auditory temporal processing and cognition. Front Aging Neurosci, (2014). 6, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman A. C., Kujawa S. G., Liberman M. C.Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol, (2013). 110, 577–586.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay J. D., Voytenko S. V., Galazyuk A. V., et al. Developmental hearing loss impairs signal detection in noise: Putative central mechanisms. Front Syst Neurosci, (2014). 8, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilley P. M., Sharma M., Purdy S. C.Oscillatory decoupling differentiates auditory encoding deficits in children with listening problems. Clin Neurophysiol, (2016). 127, 1618–1628.. [DOI] [PubMed] [Google Scholar]
- Giraudet F., Avan P.Auditory neuropathies: understanding their pathogenesis to illuminate intervention strategies. Curr Opin Neurol, (2012). 25, 50–56.. [DOI] [PubMed] [Google Scholar]
- Glyde H., Cameron S., Dillon H., et al. The effects of hearing impairment and aging on spatial processing. Ear Hear, (2013). 34, 15–28.. [DOI] [PubMed] [Google Scholar]
- Gourévitch B., Edeline J. M., Occelli F., et al. Is the din really harmless? Long-term effects of non-traumatic noise on the adult auditory system. Nat Rev Neurosci, (2014). 15, 483–491.. [DOI] [PubMed] [Google Scholar]
- Gray L., Kesser B., Cole E.Understanding speech in noise after correction of congenital unilateral aural atresia: Effects of age in the emergence of binaural squelch but not in use of head-shadow. Int J Pediatr Otorhinolaryngol, (2009). 73, 1281–1287.. [DOI] [PubMed] [Google Scholar]
- Green D. M.Temporal auditory acuity. Psychol Rev, (1971). 78, 540–551.. [DOI] [PubMed] [Google Scholar]
- Gu J. W., Herrmann B. S., Levine R. A., et al. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol, (2012). 13, 819–833.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyldenkærne P., Dillon H., Sharma M., et al. Attend to this: The relationship between auditory processing disorders and attention deficits. J Am Acad Audiol, (2014). 25, 676–687; quiz 706.. [DOI] [PubMed] [Google Scholar]
- Hall J. W.eHandbook of Auditory Evoked Responses, (2015). Boston, MA: Pearson. [Google Scholar]
- Hall J. W., 3rd, Grose J. H., Pillsbury H. C.Long-term effects of chronic otitis media on binaural hearing in children. Arch Otolaryngol Head Neck Surg, (1995). 121, 847–852.. [DOI] [PubMed] [Google Scholar]
- Han L. A., Poulsen T.Equivalent threshold sound pressure levels for Sennheiser HDA 200 earphone and Etymotic Research ER-2 insert earphone in the frequency range 125 Hz to 16 kHz. Scand Audiol, (1998). 27, 105–112.. [DOI] [PubMed] [Google Scholar]
- Harrison R. V.An animal model of auditory neuropathy. Ear Hear, (1998). 19, 355–361.. [DOI] [PubMed] [Google Scholar]
- Häusler R., Levine R. A.Auditory dysfunction in stroke. Acta Otolaryngol, (2000). 120, 689–703.. [DOI] [PubMed] [Google Scholar]
- Hendrix R. A., DeDio R. M., Sclafani A. P.The use of diagnostic testing in asymmetric sensorineural hearing loss. Otolaryngol Head Neck Surg, (1990). 103, 593–598.. [DOI] [PubMed] [Google Scholar]
- Henshaw H., Ferguson M. A.Efficacy of individual computer-based auditory training for people with hearing loss: A systematic review of the evidence. PLoS One, (2013). 8, e62836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hind S. E., Haines-Bazrafshan R., Benton C. L., et al. Prevalence of clinical referrals having hearing thresholds within normal limits. Int J Audiol, (2011). 50, 708–716.. [DOI] [PubMed] [Google Scholar]
- Hogan S. C., Moore D. R.Impaired binaural hearing in children produced by a threshold level of middle ear disease. J Assoc Res Otolaryngol, (2003). 4, 123–129.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J., Kraus N.Unstable representation of sound: A biological marker of dyslexia. J Neurosci, (2013). 33, 3500–3504.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornickel J., Zecker S. G., Bradlow A. R., et al. Assistive listening devices drive neuroplasticity in children with dyslexia. Proc Natl Acad Sci U S A, (2012). 109, 16731–16736.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosford-Dunn H.Auditory brainstem response audiometry. Applications in central disorders. Otolaryngol Clin North Am, (1985). 18, 257–284.. [PubMed] [Google Scholar]
- Houtgast T., Festen J. M.On the auditory and cognitive functions that may explain an individual’s elevation of the speech reception threshold in noise. Int J Audiol, (2008). 47, 287–295.. [DOI] [PubMed] [Google Scholar]
- Hugh S. C., Wolter N. E., Propst E. J., et al. Infant sleep machines and hazardous sound pressure levels. Pediatrics, (2014). 133, 677–681.. [DOI] [PubMed] [Google Scholar]
- Humes L. E., Dubno J. R., Gordon-Salant S., et al. Central presbycusis: A review and evaluation of the evidence. J Am Acad Audiol, (2012). 23, 635–666.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes L. E., Kidd G. R., Lentz J. J.Auditory and cognitive factors underlying individual differences in aided speech-understanding among older adults. Front Syst Neurosci, (2013). 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter L. L., Margolis R. H., Rykken J. R., et al. High frequency hearing loss associated with otitis media. Ear Hear, (1996). 17, 1–11.. [DOI] [PubMed] [Google Scholar]
- Hurley R. M., Musiek F. E.Effectiveness of three central auditory processing (CAP) tests in identifying cerebral lesions. J Am Acad Audiol, (1997). 8, 257–262.. [PubMed] [Google Scholar]
- Insanally M. N., Albanna B. F., Bao S.Pulsed noise experience disrupts complex sound representations. J Neurophysiol, (2010). 103, 2611–2617.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger S., Johnson K., Loiselle L.Pediatric central auditory dysfunction. Comparison of children with confirmed lesions versus suspected processing disorders. Am J Otol, (1988). 9 Suppl, 63–71.. [PubMed] [Google Scholar]
- Jirsa R. E., Clontz K. B.Long latency auditory event-related potentials from children with auditory processing disorders. Ear Hear, (1990). 11, 222–232.. [DOI] [PubMed] [Google Scholar]
- Job A., Raynal M., Kossowski M.Susceptibility to tinnitus revealed at 2 kHz range by bilateral lower DPOAEs in normal hearing subjects with noise exposure. Audiol Neurootol, (2007). 12, 137–144.. [DOI] [PubMed] [Google Scholar]
- Joris P. X., Schreiner C. E., Rees A.Neural processing of amplitude-modulated sounds. Physiol Rev, (2004). 84, 541–577.. [DOI] [PubMed] [Google Scholar]
- Kamal B., Holman C., de Villers-Sidani E.Shaping the aging brain: Role of auditory input patterns in the emergence of auditory cortical impairments. Front Syst Neurosci, (2013). 7, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. B., Kozin E. D., Remenschneider A., et al. Amblyaudia: Review of pathophysiology, clinical presentation, and treatment of a new diagnosis. Otolaryngol Head Neck Surg, (2016). 154, 247–255.. [DOI] [PubMed] [Google Scholar]
- Keith S. E., Michaud D. S., Feder K., et al. MP3 player listening sound pressure levels among 10 to 17 year old students. J Acoust Soc Am, (2011). 130, 2756–2764.. [DOI] [PubMed] [Google Scholar]
- Kidd G. R., Watson C. S., Gygi B.Individual differences in auditory abilities. J Acoust Soc Am, (2007). 122, 418–435.. [DOI] [PubMed] [Google Scholar]
- Kim D. O., Dorn P. A., Neely S. T., et al. Adaptation of distortion product otoacoustic emission in humans. J Assoc Res Otolaryngol, (2001). 2, 31–40.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen E. I., Esterly S. D., Knudsen P. F.Monaural occlusion alters sound localization during a sensitive period in the barn owl. J Neurosci, (1984). 4, 1001–1011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P. K.Early language acquisition: Cracking the speech code. Nat Rev Neurosci, (2004). 5, 831–843.. [DOI] [PubMed] [Google Scholar]
- Kujala T., Shtyrov Y., Winkler I., et al. Long-term exposure to noise impairs cortical sound processing and attention control. Psychophysiology, (2004). 41, 875–881.. [DOI] [PubMed] [Google Scholar]
- Kujawa S. G., Liberman M. C.Acceleration of age-related hearing loss by early noise exposure: Evidence of a misspent youth. J Neurosci, (2006). 26, 2115–2123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G., Liberman M. C.Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci, (2009). 29, 14077–14085.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G., Liberman M. C.Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res, (2015). 330Pt B191–199.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U. A., Ameenudin S., Sangamanatha A. V.Temporal and speech processing skills in normal hearing individuals exposed to occupational noise. Noise Health, (2012). 14, 100–105.. [DOI] [PubMed] [Google Scholar]
- Lamounier P., Gobbo D. A., Souza T. S., et al. Electrocochleography for Ménière’s disease: Is it reliable? Braz J Otorhinolaryngol, (2014). 80, 527–532.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. A., Gardner J. C., Fullerton B. C., et al. Effects of multiple sclerosis brainstem lesions on sound lateralization and brainstem auditory evoked potentials. Hear Res, (1993). 68, 73–88.. [DOI] [PubMed] [Google Scholar]
- Liberman M. C., Liberman L. D., Maison S. F.Efferent feedback slows cochlear aging. J Neurosci, (2014). 34, 4599–4607.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. W., Furman A. C., Kujawa S. G., et al. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol, (2011). 12, 605–616.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky R. Y., Fligor B. J., Tramo M. J.Functional role of the human inferior colliculus in binaural hearing. Hear Res, (2002). 165, 177–188.. [DOI] [PubMed] [Google Scholar]
- Lobarinas E., Salvi R., Ding D.Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hear Res, (2013). 302, 113–120.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E., Salvi R., Ding D.Selective inner hair cell dysfunction in chinchillas impairs hearing-in-noise in the absence of outer hair cell loss. J Assoc Res Otolaryngol, (2016). 17, 89–101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo J. H., Bamiou D. E., Rosen S.The impacts of language background and language-related disorders in auditory processing assessment. J Speech Lang Hear Res, (2013). 56, 1–12.. [DOI] [PubMed] [Google Scholar]
- Loo J. H., Rosen S., Bamiou D. E.Auditory training effects on the listening skills of children with auditory processing disorder. Ear Hear, (2016). 37, 38–47.. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda E. A., Barrios P.Perception of stochastically undersampled sound waveforms: A model of auditory deafferentation. Front Neurosci, (2013). 7, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn G. E., Gilroy J., Taylor P. C., et al. Binaural masking-level differences in neurological disorders. Arch Otolaryngol, (1981). 107, 357–362.. [DOI] [PubMed] [Google Scholar]
- Maison S. F., Usubuchi H., Liberman M. C.Efferent feedback minimizes cochlear neuropathy from moderate noise exposure. J Neurosci, (2013). 33, 5542–5552.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary C. A., Shin J., Kujawa S. G., et al. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol, (2011). 12, 711–717.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon E., Wintermark P., Lahav A.Auditory brain development in premature infants: The importance of early experience. Ann N Y Acad Sci, (2012). 1252, 17–24.. [DOI] [PubMed] [Google Scholar]
- Medwetsky L.Spoken language processing model: Bridging auditory and language processing to guide assessment and intervention. Lang Speech Hear Serv Sch, (2011). 42, 286–296.. [DOI] [PubMed] [Google Scholar]
- Mehraei G., Hickox A. E., Bharadwaj H. M., et al. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci, (2016). 36, 3755–3764.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. A.Auditory processing theories of language disorders: Past, present, and future. Lang Speech Hear Serv Sch, (2011). 42, 309–319.. [DOI] [PubMed] [Google Scholar]
- Mills D. M.Determining the cause of hearing loss: Differential diagnosis using a comparison of audiometric and otoacoustic emission responses. Ear Hear, (2006). 27, 508–525.. [DOI] [PubMed] [Google Scholar]
- Mishra S. K.Medial efferent mechanisms in children with auditory processing disorders. Front Hum Neurosci, (2014). 8, 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Luna K., Hertler B., Buitrago M. M., et al. Motor learning transiently changes cortical somatotopy. Neuroimage, (2008). 40, 1748–1754.. [DOI] [PubMed] [Google Scholar]
- Moncrieff D. W., Wertz D.Auditory rehabilitation for interaural asymmetry: Preliminary evidence of improved dichotic listening performance following intensive training. Int J Audiol, (2008). 47, 84–97.. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J.Cochlear Hearing Loss: Physiological, Psychological, and Technical Issues, (20072nd edChichester, UK: Wiley. [Google Scholar]
- Moore D. R., Halliday L. F., Amitay S.Use of auditory learning to manage listening problems in children. Philos Trans R Soc Lond B Biol Sci, (2009). 364, 409–420.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. R., Ferguson M. A., Edmondson-Jones A. M., et al. Nature of auditory processing disorder in children. Pediatrics, (2010). 126, e382–e390.. [DOI] [PubMed] [Google Scholar]
- Moreno S., Bidelman G. M.Examining neural plasticity and cognitive benefit through the unique lens of musical training. Hear Res, (2014). 308, 84–97.. [DOI] [PubMed] [Google Scholar]
- Murugasu E., Russell I. J.The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci, (1996). 16, 325–332.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek F. E.Results of three dichotic speech tests on subjects with intracranial lesions. Ear Hear, (1983a). 4, 318–323.. [DOI] [PubMed] [Google Scholar]
- Musiek F. E.Assessment of central auditory dysfunction: The dichotic digit test revisited. Ear Hear, (1983b). 4, 79–83.. [DOI] [PubMed] [Google Scholar]
- Musiek F. E.Frequency (pitch) and duration pattern tests. J Am Acad Audiol, (1994). 5, 265–268.. [PubMed] [Google Scholar]
- Musiek F. E., Chermak G. D.Handbook of (Central) Auditory Processing Disorder: Auditory Neuroscience and Diagnosis, (20142nd edSan Diego, CA: Plural Publishing. [Google Scholar]
- Musiek F. E., Chermak G. D.Psychophysical and behavioral peripheral and central auditory tests. Handb Clin Neurol, (2015). 129, 313–332.. [DOI] [PubMed] [Google Scholar]
- Musiek F. E., Pinheiro M. L.Frequency patterns in cochlear, brainstem, and cerebral lesions. Audiology, (1987). 26, 79–88.. [PubMed] [Google Scholar]
- Musiek F. E., Weihing J.Perspectives on dichotic listening and the corpus callosum. Brain Cogn, (2011). 76, 225–232.. [DOI] [PubMed] [Google Scholar]
- Musiek F. E., Shinn J. B., Jirsa R., et al. GIN (Gaps-In-Noise) test performance in subjects with confirmed central auditory nervous system involvement. Ear Hear, (2005). 26, 608–618.. [DOI] [PubMed] [Google Scholar]
- Musiek F. E., Chermak G. D., Weihing J., et al. Diagnostic accuracy of established central auditory processing test batteries in patients with documented brain lesions. J Am Acad Audiol, (2011). 22, 342–358.. [DOI] [PubMed] [Google Scholar]
- Musiek F. E., Gollegly K. M., Kibbe K. S., et al. Proposed screening test for central auditory disorders: Follow-up on the dichotic digits test. Am J Otol, (1991). 12, 109–113.. [PubMed] [Google Scholar]
- Neijenhuis K., Tschur H., Snik A.The effect of mild hearing impairment on auditory processing tests. J Am Acad Audiol, (2004). 15, 6–16.. [DOI] [PubMed] [Google Scholar]
- NIOSH Criteria for a recommended standard: Occupational noise exposure. National Institute for Occupational Safety and Health Publication No: 98–126, (1998Retrieved from https://www.cdc.gov/niosh/docs/98-126/pdfs/98-126.pdf. [Google Scholar]
- Noreña A. J., Chery-Croze S.Enriched acoustic environment rescales auditory sensitivity. Neuroreport, (2007). 18, 1251–1255.. [DOI] [PubMed] [Google Scholar]
- Noreña A. J., Eggermont J. J.Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J Neurosci, (2005). 25, 699–705.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreña A. J., Eggermont J. J.Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. Neuroreport, (2006). 17, 559–563.. [DOI] [PubMed] [Google Scholar]
- Noreña A. J., Gourévitch B., Gourevich B., et al. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci, (2006). 9, 932–939.. [DOI] [PubMed] [Google Scholar]
- OSHA OSHA Hearing conservation. Occupational Safety and Health Administration, U.S. Department of Labor, Publication No: OSHA 3074, (2002Retrieved from http://www.osha.gov/Publications/osha3074.pdf. [Google Scholar]
- Palmer A. R., Russell I. J.Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells. Hear Res, (1986). 24, 1–15.. [DOI] [PubMed] [Google Scholar]
- Pan Y., Zhang J., Cai R., et al. Developmentally degraded directional selectivity of the auditory cortex can be restored by auditory discrimination training in adults. Behav Brain Res, (2011). 225, 596–602.. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A., Skoe E., Kraus N.Musical experience limits the degradative effects of background noise on the neural processing of sound. J Neurosci, (2009). 29, 14100–14107.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington B. F., Bishop D. V.Relations among speech, language, and reading disorders. Annu Rev Psychol, (2009). 60, 283–306.. [DOI] [PubMed] [Google Scholar]
- Picton T. W.Human Auditory Evoked Potentials, ((2011). San Diego, CA: Plural Publishing. [Google Scholar]
- Pienkowski M.Acoustic startle reflex audiometry following (almost) nontraumatic noise exposure in CBA/CaJ Mice. (2016). 39th Mid-winter Meeting of the Association for Research in Otolaryngology: (San Diego, CA)PS 186. [Google Scholar]
- Pienkowski M., Eggermont J. J.Long-term, partially-reversible reorganization of frequency tuning in mature cat primary auditory cortex can be induced by passive exposure to moderate-level sounds. Hear Res, (2009). 257, 24–40.. [DOI] [PubMed] [Google Scholar]
- Pienkowski M., Eggermont J. J.Passive exposure of adult cats to moderate-level tone pip ensembles differentially decreases AI and AII responsiveness in the exposure frequency range. Hear Res, (2010a). 268, 151–162.. [DOI] [PubMed] [Google Scholar]
- Pienkowski M., Eggermont J. J.Intermittent exposure with moderate-level sound impairs central auditory function of mature animals without concomitant hearing loss. Hear Res, (2010b). 261, 30–35.. [DOI] [PubMed] [Google Scholar]
- Pienkowski M., Eggermont J. J.Cortical tonotopic map plasticity and behavior. Neurosci Biobehav Rev, (2011). 35, 2117–2128.. [DOI] [PubMed] [Google Scholar]
- Pienkowski M., Eggermont J. J.Reversible long-term changes in auditory processing in mature auditory cortex in the absence of hearing loss induced by passive, moderate-level sound exposure. Ear Hear, (2012). 33, 305–314.. [DOI] [PubMed] [Google Scholar]
- Pienkowski M., Ulfendahl M.Differential effects of salicylate, quinine, and furosemide on Guinea pig inner and outer hair cell function revealed by the input-output relation of the auditory brainstem response. J Am Acad Audiol, (2011). 22, 104–112.. [DOI] [PubMed] [Google Scholar]
- Pienkowski M., Munguia R., Eggermont J. J.Passive exposure of adult cats to bandlimited tone pip ensembles or noise leads to long-term response suppression in auditory cortex. Hear Res, (2011). 277, 117–126.. [DOI] [PubMed] [Google Scholar]
- Pillsbury H. C., Grose J. H., Hall J. W., 3rdOtitis media with effusion in children. Binaural hearing before and after corrective surgery. Arch Otolaryngol Head Neck Surg, (1991). 117, 718–723.. [DOI] [PubMed] [Google Scholar]
- Plack C. J., Barker D., Prendergast G.Perceptual consequences of “hidden” hearing loss. Trends Hear, (2014). 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley D. B., Steinberg E. E., Merzenich M. M.Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci, (2006). 26, 4970–4982.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley D. B., Thompson J. H., Guo W.Brief hearing loss disrupts binaural integration during two early critical periods of auditory cortex development. Nat Commun, (2013). 4, 2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu M. V., Polley D. B.Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron, (2010). 65, 718–731.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poremba A., Bigelow J. Cohen Y. E., Popper A., Fay R. R.Neurophysiology of attention and memory processing. In Neural Correlates of Auditory Cognition, (2013). New York, NY: Springer. [Google Scholar]
- Purdy S. C., Kelly A. S., Davies M. G.Auditory brainstem response, middle latency response, and late cortical evoked potentials in children with learning disabilities. J Am Acad Audiol, (2002). 13, 367–382.. [PubMed] [Google Scholar]
- Ranasinghe K. G., Carraway R. S., Borland M. S., et al. Speech discrimination after early exposure to pulsed-noise or speech. Hear Res, (2012). 289, 1–12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport J. M., Gulliver J. M., Phillips D. P., et al. Auditory temporal resolution in multiple sclerosis. J Otolaryngol, (1994). 23, 307–324.. [PubMed] [Google Scholar]
- Recanzone G. H., Merzenich M. M., Jenkins W. M., et al. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol, (1992). 67, 1031–1056.. [DOI] [PubMed] [Google Scholar]
- Recanzone G. H., Schreiner C. E., Merzenich M. M.Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci, (1993). 13, 87–103.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A., Riley J., Carraway R., et al. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron, (2011). 70, 121–131.. [DOI] [PubMed] [Google Scholar]
- Riccio C. A., Reynolds C. R., Lowe P. A.Clinical Applications of Continuous Performance Tests: Measuring Attention and Impulsive Responding in Children and Adults, ((2001). Hoboken, NJ: Wiley & Sons Inc. [Google Scholar]
- Riccio C. A., Cohen M. J., Garrison T., et al. Auditory processing measures: Correlation with neuropsychological measures of attention, memory, and behavior. Child Neuropsychol, (2005). 11, 363–372.. [DOI] [PubMed] [Google Scholar]
- Richardson U., Thomson J. M., Scott S. K., et al. Auditory processing skills and phonological representation in dyslexic children. Dyslexia, (2004). 10, 215–233.. [DOI] [PubMed] [Google Scholar]
- Robles L., Ruggero M. A.Mechanics of the mammalian cochlea. Physiol Rev, (2001). 81, 1305–1352.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S.Auditory processing in dyslexia and specific language impairment: Is there a deficit? What is its nature? Does it explain anything? J Phonetics, (2003). 31, 509–527.. [Google Scholar]
- Rosen S., Adlard A., van der Lely H. K.Backward and simultaneous masking in children with grammatical specific language impairment: No simple link between auditory and language abilities. J Speech Lang Hear Res, (2009). 52, 396–411.. [DOI] [PubMed] [Google Scholar]
- Rosen S., Cohen M., Vanniasegaram I.Auditory and cognitive abilities of children suspected of auditory processing disorder (APD). Int J Pediatr Otorhinolaryngol, (2010). 74, 594–600.. [DOI] [PubMed] [Google Scholar]
- Rudner M., Lunner T.Cognitive spare capacity and speech communication: A narrative overview. Biomed Res Int, (2014). 2014, 869726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero M. A. Popper A.N, Fay R.R.Physiology and coding of sound in the auditory nerve. In: The Mammalian Auditory Pathway: Neurophysiology, ((1992). New York, NY: Springer; pp. 34–93.. [Google Scholar]
- Ruggles D., Shinn-Cunningham B.Spatial selective auditory attention in the presence of reverberant energy: individual differences in normal-hearing listeners. J Assoc Res Otolaryngol, (2011). 12, 395–405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggles D., Bharadwaj H., Shinn-Cunningham B. G.Normal hearing is not enough to guarantee robust encoding of suprathreshold features important in everyday communication. Proc Natl Acad Sci U S A, (2011). 108, 15516–15521.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvalcaba-Delgadillo Y., Luquín S., Ramos-Zúñiga R., et al. Early-life exposure to noise reduces mPFC astrocyte numbers and T-maze alternation/discrimination task performance in adult male rats. Noise Health, (2015). 17, 216–226.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R. J., Wang J., Ding D.Auditory plasticity and hyperactivity following cochlear damage. Hear Res, (2000). 147, 261–274.. [DOI] [PubMed] [Google Scholar]
- Sandford J. A., Turner A.Manual for the Integrated Visual and Auditory Continuous Performance Test, (1995). Richmond, VA: Braintrain. [Google Scholar]
- Schaette R., McAlpine D.Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci, (2011). 31, 13452–13457.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaette R., König O., Hornig D., et al. Acoustic stimulation treatments against tinnitus could be most effective when tinnitus pitch is within the stimulated frequency range. Hear Res, (2010). 269, 95–101.. [DOI] [PubMed] [Google Scholar]
- Schmiedt R. A., Mills J. H., Boettcher F. A.Age-related loss of activity of auditory-nerve fibers. J Neurophysiol, (1996). 76, 2799–2803.. [DOI] [PubMed] [Google Scholar]
- Schochat E., Musiek F. E., Alonso R., et al. Effect of auditory training on the middle latency response in children with (central) auditory processing disorder. Braz J Med Biol Res, (2010). 43, 777–785.. [DOI] [PubMed] [Google Scholar]
- Schuknecht H. F., Woellner R. C.Hearing losses following partial section of the cochlear nerve. Laryngoscope, (1953). 63, 441–465.. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y., Lall K., Liberman M. C., et al. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci, (2013). 33, 13686–13694.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M. R., Biassoni E. C., Richter U., et al. Recreational noise exposure and its effects on the hearing of adolescents. Part I: an interdisciplinary long-term study. Int J Audiol, (2005). 44, 65–73.. [DOI] [PubMed] [Google Scholar]
- Shaheen L. A., Valero M. D., Liberman M. C.Towards a diagnosis of cochlear neuropathy with envelope following responses. J Assoc Res Otolaryngol, (2015). 16, 727–745.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Purdy S. C., Newall P., et al. Electrophysiological and behavioral evidence of auditory processing deficits in children with reading disorder. Clin Neurophysiol, (2006). 117, 1130–1144.. [DOI] [PubMed] [Google Scholar]
- Sharma M., Purdy S. C., Kelly A. S.Comorbidity of auditory processing, language, and reading disorders. J Speech Lang Hear Res, (2009). 52, 706–722.. [DOI] [PubMed] [Google Scholar]
- Sharma M., Dhamani I., Leung J., et al. Attention, memory, and auditory processing in 10- to 15-year-old children with listening difficulties. J Speech Lang Hear Res, (2014). 57, 2308–2321.. [DOI] [PubMed] [Google Scholar]
- Skoe E., Kraus N.Auditory brain stem response to complex sounds: a tutorial. Ear Hear, (2010). 31, 302–324.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski W. J., Brunt M. A., Tannahill J. C.Listening characteristics of children with central auditory processing disorders. Lang Speech Hear Serv Sch, (1992). 23, 145–152.. [Google Scholar]
- Smurzynski J., Probst R.Intensity discrimination, temporal integration and gap detection by normally-hearing subjects with weak and strong otoacoustic emissions. Audiology, (1999). 38, 251–256.. [DOI] [PubMed] [Google Scholar]
- Song J. H., Skoe E., Banai K., et al. Perception of speech in noise: neural correlates. J Cogn Neurosci, (2011). 23, 2268–2279.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J. H., Skoe E., Banai K., et al. Training to improve hearing speech in noise: biological mechanisms. Cereb Cortex, (2012). 22, 1180–1190.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper G. C., Johnson T. A.Auditory function in normal-hearing, noise-exposed human ears. Ear Hear, (2015). 36, 172–184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A., Picton T. W., Sininger Y., et al. Auditory neuropathy. Brain, (1996). 119Pt 3741–753.. [DOI] [PubMed] [Google Scholar]
- Steinschneider M. Cohen Y. E., Popper A., Fay R. R.Phonemic representations and categories. In. Neural Correlates of Auditory Cognition, (2013). New York, NY: Springer. [Google Scholar]
- Surprenant A. M., Watson C. S.Individual differences in the processing of speech and nonspeech sounds by normal-hearing listeners. J Acoust Soc Am, (2001). 110, 2085–2095.. [DOI] [PubMed] [Google Scholar]
- Sweetow R., Palmer C. V.Efficacy of individual auditory training in adults: A systematic review of the evidence. J Am Acad Audiol, (2005). 16, 494–504.. [DOI] [PubMed] [Google Scholar]
- Taljaard D. S., Leishman N. F., Eikelboom R. H.Personal listening devices and the prevention of noise induced hearing loss in children: the Cheers for Ears Pilot Program. Noise Health, (2013). 15, 261–268.. [DOI] [PubMed] [Google Scholar]
- Tallal P.Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang, (1980). 9, 182–198.. [DOI] [PubMed] [Google Scholar]
- Tallal P.Improving language and literacy is a matter of time. Nat Rev Neurosci, (2004). 5, 721–728.. [DOI] [PubMed] [Google Scholar]
- Tallal P., Piercy M.Defects of non-verbal auditory perception in children with developmental aphasia. Nature, (1973). 241, 468–469.. [DOI] [PubMed] [Google Scholar]
- Teele D. W., Klein J. O., Rosner B. A.Otitis media with effusion during the first three years of life and development of speech and language. Pediatrics, (1984). 74, 282–287.. [PubMed] [Google Scholar]
- Turrigiano G. G.Homeostatic plasticity in neuronal networks: The more things change, the more they stay the same. Trends Neurosci, (1999). 22, 221–227.. [DOI] [PubMed] [Google Scholar]
- Valero M. D., Hancock K. E., Liberman M. C.The middle ear muscle reflex in the diagnosis of cochlear neuropathy. Hear Res, (2016). 332, 29–38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewalle E., Boets B., Ghesquière P., et al. Auditory processing and speech perception in children with specific language impairment: Relations with oral language and literacy skills. Res Dev Disabil, (2012). 33, 635–644.. [DOI] [PubMed] [Google Scholar]
- Viana L. M., O’Malley J. T., Burgess B. J., et al. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res, (2015). 327, 78–88.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemeister N. F.Temporal modulation transfer functions based upon modulation thresholds. J Acoust Soc Am, (1979). 66, 1364–1380.. [DOI] [PubMed] [Google Scholar]
- Vinay S., Moore B. C.Prevalence of dead regions in subjects with sensorineural hearing loss. Ear Hear, (2007). 28, 231–241.. [DOI] [PubMed] [Google Scholar]
- Wagner R. K., Torgesen J. K., Rashotte C. A., et al. Comprehensive Test of Phonological Processing, (20132nd edAustin, TX: Pro-Ed. [Google Scholar]
- Walton J., Orlando M., Burkard R.Auditory brainstem response forward-masking recovery functions in older humans with normal hearing. Hear Res, (1999). 127, 86–94.. [DOI] [PubMed] [Google Scholar]
- Wang Y., Ren C.Effects of repeated “benign” noise exposures in young CBA mice: Shedding light on age-related hearing loss. J Assoc Res Otolaryngol, (2012). 13, 505–515.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward E. D., Cushing E. M., Burns E. M.Effective quiet and moderate TTS: Implications for noise exposure statndars. J Acoust Soc Am, (1976). 59, 160–165.. [DOI] [PubMed] [Google Scholar]
- Watson C. S., Kidd G. R. Cacace A. T., McFarland D. J.Associations between auditory abilities, reading, and other language skills, in children and adults. In Current Controversies in Central Auditory Processing Disorder (CAPD), (2009). San Diego, CA: Plural Publishing. [Google Scholar]
- White-Schwoch T., Woodruff Carr K., Thompson E. C., et al. Auditory processing in noise: A preschool biomarker for literacy. PLoS Biol, (2015). 13, e1002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. P., Polley D. B.Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: A comparison of basic and clinical studies. J Assoc Res Otolaryngol, (2011). 12, 535–547.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. P., Hancock K. E., Polley D. B.Immersive audiomotor game play enhances neural and perceptual salience of weak signals in noise. Proc Natl Acad Sci U S A, (2014). 111, E2606–E2615.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber L. A., Kruger B., Killion M. C.Reference thresholds for the ER-3A insert earphone. J Acoust Soc Am, (1988). 83, 669–676.. [DOI] [PubMed] [Google Scholar]
- Wilmington D., Gray L., Jahrsdoerfer R.Binaural processing after corrected congenital unilateral conductive hearing loss. Hear Res, (1994). 74, 99–114.. [DOI] [PubMed] [Google Scholar]
- Wilson W. J., Arnott W.Using different criteria to diagnose (central) auditory processing disorder: How big a difference does it make? J Speech Lang Hear Res, (2013). 56, 63–70.. [DOI] [PubMed] [Google Scholar]
- Woolley S. M.Early experience shapes vocal neural coding and perception in songbirds. Dev Psychobiol, (2012). 54, 612–631.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F. G., Kong Y. Y., Michalewski H. J., et al. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol, (2005). 93, 3050–3063.. [DOI] [PubMed] [Google Scholar]
- Ziegler J. C., Pech-Georgel C., George F., et al. Deficits in speech perception predict language learning impairment. Proc Natl Acad Sci U S A, (2005). 102, 14110–14115.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. C., Pech-Georgel C., George F., et al. Speech-perception-in-noise deficits in dyslexia. Dev Sci, (2009). 12, 732–745.. [DOI] [PubMed] [Google Scholar]
- Zhang L. I., Bao S., Merzenich M. M.Disruption of primary auditory cortex by synchronous auditory inputs during a critical period. Proc Natl Acad Sci U S A, (2002). 99, 2309–2314.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Stephens D.Distortion product otoacoustic emissions in patients with King-Kopetzky syndrome. Int J Audiol, (2006). 45, 34–39.. [DOI] [PubMed] [Google Scholar]
- Zheng W.Auditory map reorganization and pitch discrimination in adult rats chronically exposed to low-level ambient noise. Front Syst Neurosci, (2012). 6, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Merzenich M. M.Intensive training in adults refines A1 representations degraded in an early postnatal critical period. Proc Natl Acad Sci U S A, (2007). 104, 15935–15940.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Merzenich M. M.Developmentally degraded cortical temporal processing restored by training. Nat Neurosci, (2009). 12, 26–28.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Merzenich M. M.Environmental noise exposure degrades normal listening processes. Nat Commun, (2012). 3, 843. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang F., Hu H., et al. Environmental acoustic enrichment promotes recovery from developmentally degraded auditory cortical processing. J Neurosci, (2014). 34, 5406–5415.. [DOI] [PMC free article] [PubMed] [Google Scholar]


