Abstract
Immunoglobulins G (IgG) could become widespread biopharmaceuticals if cost-efficient processes for their extraction and purification are available. In this work, aqueous biphasic systems (ABS) composed of polyethylene glycols and a buffered salt, and with ionic liquids (ILs) as adjuvants, have been studied as alternative extraction and purification platforms of IgG from a rabbit serum source. Eleven ILs were investigated to provide insights on the chemical features which maximize the IgG partitioning. It is shown that in polymer-salt systems pure IgG preferentially partitions to the polymer-rich phase; yet, the complete extraction was never attained. Remarkably, after the addition of 5 wt% of adequate ILs to polymer-salt ABS, the complete extraction of pure IgG in a single-step was accomplished. The best systems and conditions were then applied to the extraction and purification of IgG directly from rabbit serum samples. The complete extraction of IgG in a single-step was maintained while its purity in the polymer-rich phase was enhanced by ca. 37% as compared to the IL-free ABS. The antibody stability was also evaluated revealing that appropriate ILs are able to maintain the IgG stability and can be used as phase-forming components of ABS when envisaging the purification of high-cost biopharmaceuticals.
Keywords: Aqueous biphasic system, Ionic liquids, Adjuvants, Antibody, Extraction, Purification
1. Introduction
In the past few years, progresses in pharmaceutical-related sciences have led to a new era of therapeutic-based drugs – biopharmaceuticals (Martínez-Aragón et al., 2009). The demand for purified proteins, such as specific antibodies, has increased considerably, and not only for therapeutic purposes but also for advanced diagnosis (Martínez-Aragón et al., 2009). Antibodies or immunoglobulins, known as Ig’s, are glycoproteins present in plasma and extracellular fluids (humors) which constitute the humoral branch of the animal immune system (Leenaars and C. F. M. Hendriksen, 2005). Currently, the immunoglobulins G (IgG) isotype is one of the most used in a variety of scientific applications (Zhiqiang, 2008) and as alternative therapies to treat many diseases (Wang et al., 2013) such as inflammatory diseases (e.g., Myesthenia gravis, Crohn’s disease, Multiple sclerosis), as well as in patients with asthma, cardiovascular and infectious diseases (Rosa et al., 2007; Nimmerjahn and Ravetch, 2008). An additional relevant application of IgG is in oncology, where immunoglobulins act as carrier agents of toxins or radiolabeled isotopes to cancerous cells (Zhiqiang, 2008; Silva et al., 2014; Schuster et al., 2006). Antibodies are also suitable for use in indirect flow cytometry assays, for instance in ELISA or cytotoxicity studies (Andew and Titus, 2001).
The production of therapeutic antibodies must meet high efficiency and safety standards, which translate into the requirement of high purity levels (Azevedo et al., 2007). The high cost of the currently used downstream technologies is the key problem which has been preventing the widespread use of antibodies. Therefore, there is a crucial need to develop efficient, economic, and fast methods for their purification (Rosa et al., 2013). Typical steps in monoclonal and polyclonal antibodies recovery and processing comprise: (i) harvest, (ii) clarification, (iii) concentration, (iv) purification, (v) clearance, and (vi) validation and quality control (Rosa et al., 2007). Purification costs are responsible for between 20 and 60 % and, in some special cases, up to 90 % of the total product costs (Rosa et al., 2007). The purification is usually achieved by expensive techniques, such as chromatography, that are not viable when envisaging a large-scale production. In order to suppress these and other shortcomings related with traditional methods, the extraction and purification using aqueous biphasic systems (ABS) constitutes an interesting alternative (Rosa et al., 2007; Rosa et al., 2009; Soares et al., 2015).
ABS consist on two immiscible aqueous-rich phases based on polymer/polymer, polymer/salt or salt/salt combinations dissolved in aqueous media that may be used in liquid-liquid extraction processes (Azevedo et al., 2009). On the whole, both phases are mostly composed of water, which means that they can offer a biocompatible medium for biologically active molecules (Raghavarao st al., 2003). Due to this advantage, ABS have been successfully used for the recovery of biological products, such as proteins/enzymes (Kammoun et al., 2009; Dreyer and Kragl, 2008), antibiotics (Bora et al., 2005; Porto et al., 2008), antibodies (Rosa et al., 2007; Azevedo et al., 2009), among others (Benavides and Rito-Palomares, 2008). Moreover, ABS can be viewed as more efficient strategies than traditional chromatographic methods since clarification, concentration and partial purification can be combined in a single-step (Silva et al., 2014). Polymer-polymer ABS have been studied for the purification of IgG (Silva et al., 2014; Rosa et al., 2009). However, these systems display high viscosities of the coexisting phases and some polymers, e.g. dextran, are highly expensive. To overcome these drawbacks, other works described the use of polymer–salt ABS for the same purpose (Azevedo et al., 2009), since these systems use cheaper components, display a lower viscosity and present coexisting phases with a higher density difference, and thus provide faster separation rates. The most used polymers in the formulation of ABS are polyethylene glycols (PEGs), because they present some attractive properties, namely enhanced biodegradability, high water solubility, low toxicity, negigible volatility, low melting temperature, and low cost (Pereira et al., 2010).
To overcome some of the limitations of PEG-based ABS, in particular regarding the polarity differences between the phases and their selectivity, Zijlstra et al. (1996) proposed the use of functionalized-PEG-dextran ABS for the purification of IgG, aiming at their recovery from hybridoma cells. Asenjo and co-workers (1996) used an ABS composed of PEG, a phosphate-based salt and NaCl to successfully recover IgG from hybridoma cell supernatants in the top phase. In the same line, Rito-Palomares et al. (2000) studied PEG-potassium-phosphate ABS to process whole bovine blood. Finally, Aires-Barros and co-workers (2007; 2008; 2009) devoted a large attention to the study of ABS to the purification of IgG. The research group proposed a multi-stage equilibrium aqueous two-phase extraction for the successful purification of human antibodies from CHO cells supernatants, and demonstrated the feasibility of combining the extraction carried out by ABS with HIC (Hydrophobic Interaction Chromatography) and SEC (Size Exclusion Chromatography) for the purification of human therapeutic antibodies, without the use of any conditioning step in the three unit operations (Azevedo et al., 2008).
In addition to the functionalization of polymers, additions of salts, such as NaCl, and multi-stage approaches, ionic liquids (ILs) can be also used as phase-forming components of ABS to promote the tailoring of the phases’ polarities and affinities (Freire et al., 2012). ILs are composed of ions of low-charge density instead of high-charge density ions characteristic of high melting temperature salts, and thus display lower melting temperatures than conventional salts (Marsh et al., 2004). ILs have attractive properties, namely a negligible volatility and non-flammability, under ambient conditions, which contributed to their “green solvents” characterization (Freire et al., 2012; Chen et al., 2010). Moreover, there is a large number of possible variations in the cation and anion chemical structures which further allow the fine-tuning of their physicochemical properties (Freire et al., 2012). The use of ILs in ABS leads also to the possibility of controlling the phases’ polarities by an adequate choice of the constituting ions (Passos et al., 2012), and thus, this tunable characteristic makes of them a desirable class of solvents in liquid–liquid extraction processes. In addition to their use as phase-forming components of IL-salt ABS, it was already shown that ILs can be used as adjuvants, and thus in lower amounts, to tailor the systems’ selectivity for target biomolecules (Pereira et al., 2010). Furthermore, several researchers have reported the capacity of enzymes and proteins to remain stable and active in presence of IL aqueous solutions (Pei et al., 2009; Desai et al., 2014). Hence, it seems likely that appropriate ILs could act as adjuvants in typical polymer-based ABS while envisaging the extraction and purification of high-value biopharmaceuticals/proteins, such as antibodies.
In this work, ILs were investigated as adjuvants (at 5 wt%) in polymer-salt ABS to allow the tailoring of the phases’ polarities and affinities aiming at purifying high-cost biopharmaceuticals. Extractions with pure/commercial IgG were initially performed to evaluate the best systems/conditions and ILs to be used. The best systems were then applied to the extraction and purification of IgG from rabbit serum samples, and the stability of the antibody evaluated. Promising results were obtained with most of the ILs investigated which allowed the complete extraction of IgG in a single-step and an enhancement on its purity of ca. 37%, while being able at the same time to maintain the IgG stability.
2. Experimental Section
2.1. Materials
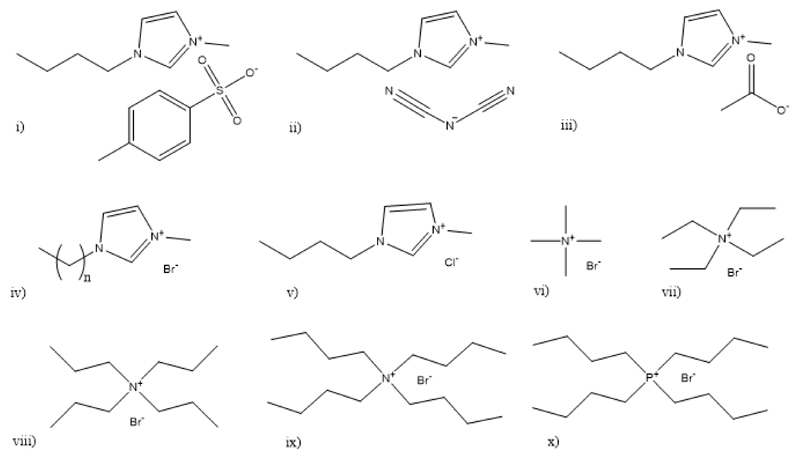
The ABS studied in this work were established using PEGs of different molecular weights, namely 400, 600, 1000, 2000, 4000 and 8000 g·mol-1 (herein abbreviated as PEG 400, PEG 600, PEG 1000, PEG 2000, PEG 4000 and PEG 8000, respectively). They were from Sigma–Aldrich, with the exception of PEG 1000 that was from Fluka. K3C6H5O7·H2O (purity ≥ 99 wt%) was acquired from Sigma–Aldrich Chemical, and C6H8O7.H2O, from Panreac. Different ILs namely: 1-ethyl-3-methylimidazolium bromide, [C2mim]Br; 1-butyl-3-methylimidazolium bromide, [C4mim]Br; 1-butyl-3-methylimidazolium chloride, [C4mim]Cl; 1-butyl-3-methylimidazolium tosylate, [C4mim][TOS]; 1-butyl-3-methylimidazolium dicyanamide, [C4mim][N(CN)2]; 1-butyl-3-methylimidazolium acetate, [C4mim][CH3CO2]; tetramethylammonium bromide, [N1111]Br; tetraethylammonium bromide, [N2222]Br; tetrapropylammonium bromide, [N3333]Br; tetrabutylammonium bromide, [N4444]Br; tetrabutylphosphonium bromide, [P4444]Br. The purity level of all ILs were >98 wt%. Imidazolium-ILs were purchased from Iolitec; all ammonium-based ILs were from Aldrich, with the exception of [N4444]Br that was supplied by Fluka AG; and the [P4444]Br was kindly offered by Cytec Industries Inc. Before use, all ILs were purified and dried for a minimum of 24 h, under constant agitation, at moderate temperature (≈ 50°C) and under vacuum (to reduce their volatile impurities to negligible values). After this step, the purity of each IL was confirmed by 1H and 13C NMR spectra and found to be in accordance with the purity levels given by the suppliers. The chemical structures of the investigated ILs are depicted in Fig. 1. Phosphate buffered saline (PBS) pellets, from Sigma-Aldrich, were used to prepare the solutions of IgG from rabbit serum (reagent grade, ≥ 95%) obtained as a lyophilized powder from Sigma-Aldrich. The rabbit serum used was obtained from Sigma Aldrich (R9133 Sigma), with a total protein content between 40-70 mg/mL (determined by Biuret). The water employed was double distilled, passed across a reverse osmosis system and further treated with a Milli-Q plus 185 water purification apparatus.
Figure 1.
Chemical structures of the ILs investigated: (i) [C4mim][TOS]; (ii) [C4mim][N(CN)2]; (iii) [C4mim][CH3CO2]; (iv) [Cnmim]Br (n = 2 and 4); (v) [C4mim]Cl; (vi) [N1111]Br; (vii) [N2222]Br; (viii) [N3333]Br; (ix) [N4444]Br and (x) [P4444]Br.
2.2. Determination of phase diagrams, tie-lines, tie-line lengths and critical points
Before the extraction and purification experiments, ternary phase diagrams were determined for each of the water-soluble PEGs (PEG 200, PEG 400, PEG 600, PEG 1000, PEG 2000, PEG 4000, PEG 6000 and PEG 8000) at pH ≈ 7 and for PEG 400 in the range from pH 5 to 9. The buffer K3C6H5O7/C6H8O7 was used to maintain the pH of the overall ABS at the desired value by different molar ratios of the two species. For this, as well as for the representation of each phase diagram, the amount of water complexed with the salt was discounted and considered in the total weight of water. The binodal curve of each ABS was determined through the cloud point titration method at 25 °C and atmospheric pressure, using aqueous solutions of salt at around 50 wt% and aqueous solutions of the different PEGs (with concentrations ranging from 60 wt % to 90 wt %). The experimental procedure was validated in a previous report (Quental et al., 2015). Further details are given in the Supporting Information.
2.3. Extraction of commercial/standard IgG using PEG-salt-based ABS
In the studied ABS, the top phase corresponds to the PEG-rich aqueous phase while the bottom phase is mainly composed of the buffered salt. The ternary mixture compositions for the IgG extraction were chosen based on the phase diagrams determined for each PEG-C6H5K3O7/C6H8O7-water mixture – cf. detailed data on phase diagrams and composition of the coexisting phases in the Supporting Information. To avoid discrepancies in the results all the partitioning studies were performed at a constant tie-line length (TLL). The mixture compositions which correspond to a TLL of ca. 35 are as follows: 25 wt% of PEG 400 + 25 wt% of C6H5K3O7/C6H8O7, 19 wt% of PEG 600 + 23 wt% of C6H5K3O7/C6H8O7, 19 wt% of PEG 1000 + 20 wt% of C6H5K3O7/C6H8O7, 18 wt% of PEG 2000 + 16 wt% of C6H5K3O7/C6H8O7, 18 wt% of PEG 4000 + 14 wt % of C6H5K3O7/C6H8O7, 17 wt% of PEG 6000 + 14 wt % of C6H5K3O7/C6H8O7, and 16 wt% of PEG 8000 + 15 wt % of C6H5K3O7/C6H8O7. The partition behavior of rabbit IgG in aqueous PEG/citrate buffer two-phase systems was investigated using IgG stock solutions prepared with a concentration at ca. 1 g.L-1 dissolved in PBS (phosphate buffered saline at 0.01 M, pH ≈ 7.4, at 25°C). In each system, a small amount of the IgG aqueous solution (≈ 0.3 g) was added to the phase-forming components to reach a total weight of the mixture of 1.5 g.
In order to elucidate the main factors that rule the partition behavior of IgG in polymer-salt ABS, two main parameters were investigated. The first parameter studied was the PEG molecular weight (namely, 400, 600, 1000, 2000. 4000, 6000 and 8000 g·mol-1). In this study, mixtures at pH ≈ 7 were prepared and centrifuged for 10 min, at 1000 rpm, and left to equilibrate for 120 min at 25°C to ensure the total phases separation. Previous optimization tests on the equilibrium conditions were carried out and the data are provided in the Supporting Information. The second parameter evaluated comprises the study of the effect of pH through the IgG extraction. In this case, mixtures at different pH values (5, 6, 7, 8, and 9) were prepared, centrifuged at 1000 rpm for 10 min and left to equilibrate for more 120 min at 25°C. Since phase diagrams depend on the pH, the mixture compositions which correspond to a TLL of ≈ 35 are as follows: 20 wt% of PEG 400 + 35 wt% of C6H5K3O7/C6H8O7, at pH ≈ 5; 22 wt% of PEG 400 + 29 wt % of C6H5K3O7/C6H8O7, at pH ≈ 6; 21 wt% of PEG 400 + 26 wt % of C6H5K3O7/C6H8O7, at pH ≈ 8; and 23 wt% of PEG 400 + 25 wt % of C6H5K3O7/C6H8O7, at pH ≈ 9. After the equilibrium conditions, both phases were carefully separated, and IgG was quantified in each phase by UV-spectroscopy, using a UV-spectrophotometry (SYNERGY|HT microplate reader, BioTek), at a wavelength of 280 nm, using calibration curves previously established with IgG. All experiments were carried out with three replicates. The interference of the salt and PEG with the quantification method was also ascertained and blank control samples were used.
The percentage extraction efficiency of IgG into to PEG-rich phase, EEIgG%, is the percentage ratio between the total weight of protein in the PEG-rich aqueous phase to that in the two aqueous phases, and is defined according to Eq. 1,
| (1) |
where, and are the total weight of IgG in the PEG-rich phase and in the salt-rich phase, respectively.
The recovery yield of IgG into to PEG-rich phase, YIgG%, is the percentage ratio between the amount of protein in the PEG-rich aqueous phase to that added in the initial mixture and is defined according to Eq. 2,
| (2) |
2.4. Extraction of commercial/pure IgG using PEG-salt-based ABS with ILs as adjuvants
After the previously described optimization procedures, a mixture point, with a composition of 25 wt% of PEG 400 + 25 wt% of C6H5K3O7/C6H8O7 at pH ≈ 7 (TLL of 35) was selected. To this mixture, 5 wt% of each IL was added, and the partition behavior of rabbit IgG was investigated. Several combinations of ILs were attempted which allow the evaluation of the IL cation and anion nature effects, as well as the increase of the alkyl side chain length. The ILs used for the study of the anion effect were [C4mim]Br, [C4mim]Cl, [C4mim][TOS], [C4mim][N(CN)2] and [C4mim][CH3CO2], while to study the effect of the cation nature and alkyl side chain length the following ILs were employed: [C2mim]Br, [C4mim]Br, [N1111]Br, [N2222]Br, [N3333]Br, [N4444]Br and [P4444]Br. The stock solutions of IgG were prepared with a concentration at ca. 1 g.L-1 in PBS (phosphate buffered saline aqueous solutions at 0.01M, pH ≈ 7.4). In each system, a small amount of the IgG aqueous solution (≈ 0.3 g) was added to a total weight of 1.5 g corresponding to the final weight of each ABS. Each mixture was then stirred, centrifuged for 10 min at 1000 rpm, and left to equilibrate for more 120 min (a time period established in previous optimization experiments) at 25°C in order to achieve the complete partitioning of IgG between the two phases. After a careful phases’ separation, the IgG content in each phase was determined as previously described. EEIgG% and YIgG% were determined using Eqs 1 and 2. At least three ABS of each type were prepared and 3 samples of each phase were quantified. Blank controls with no IgG added were always used.
In IL-based ABS, the partitioning behavior of ILs between the coexisting phases was also evaluated. The amount of aromatic imidazolium-based ILs in each phase was quantified by UV-spectroscopy, using a SYNERGY|HT microplate reader, BioTek, at a wavelength of 211 nm. The extraction efficiency of the IL, EEIL%, is defined as the weight of the IL in the PEG-rich to that in the two phases.
2.5. Extraction and purification of IgG from rabbit serum using PEG-salt-based ABS with ILs as adjuvants
After the identification of the best systems to extract and recover IgG, ABS formed by 25 wt% of PEG 400 + 25 wt% of C6H5K3O7/C6H8O7 + 5 wt% of IL + 45 wt% of rabbit serum diluted at 1:10 (v:v) at pH ≈ 7 (TLL of 35) were investigated to extract and purify IgG from the real matrix. Several ILs were investigated to infer on the IL cation and anion nature effects, as well as on the increase of the IL cation alkyl side chain length. Each mixture was stirred, centrifuged for 10 min at 1000 rpm, and left to equilibrate for more 120 min at 25°C in order to achieve the complete partitioning of IgG and remaining proteins between the two phases. After the phases’ separation, the IgG and remaining proteins contents were determined by size exclusion high performance liquid chromatography (SE-HPLC). At least three ABS of each type were prepared and 3 samples of each phase were quantified. Blank controls with no IgG added were always used. Each phase was diluted at a 1:10 (v:v) ratio in PBS 10 mM before injection in the SE-HPLC. A Chromaster HPLC (VWR, Hitachi) coupled to a DAD detector was used. SE-HPLC was performed with an analytical column Shodex Protein KW-802.5 (8 mm x 300 mm). A 100 mM phosphate buffer pH 7.0 with NaCl 0.3 M was run isocratically with a flow rate of 0.5 mL.min-1. The temperature of the column and autosampler was kept at 25ºC. The injection volume was of 25 µL. The wavelength was set at 280 nm whereas the retention time of IgG (confirmed with the commercial and pure sample) was found to be ca. 15.5 min within an analysis time of 30 min. The quantification of IgG in each phase was carried out by the use of a calibration curve established in the SE-HPLC at the conditions described before. The percentage extraction efficiency and recovery yield of IgG to the PEG-rich phase were determined according to Eqs. 1 and 2, while the percentage purity of IgG was calculated dividing the HPLC peak area corresponding to IgG by the total area of all peaks corresponding to all proteins present at the PEG-rich phase.
2.6. Stability of IgG
In addition to the analysis of the SE-HPLC chromatograms used to quantify IgG and remaining contaminant proteins on the extraction studies of IgG from rabbit serum samples, and which allow to infer on the IgG integrity, the structural stability of IgG was also studied by Fourier Transform Infrared Spectroscopy (FT-IR) and Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE). Aqueous solutions containing IgG (0.1 wt%), PEG 400 (35 wt%) and different ILs (5 wt%) were prepared and used to perform the stability studies. 0.1 wt% of IgG in an aqueous solution of PBS buffer at 10 mM, pH 7.4, was taken as the control sample for comparative issues. FT-IR spectra were obtained in the wavelength range from 1800 to 1200 cm−1, and recorded using a Perkin Elmer Spectrum Bx spectrophotometer with a resolution of 4 cm−1 and 64 scans. All spectra were fitted at amide I region (1600-1700 cm-1) with the Origin 8.5 software. Prior to loading in the gel wells to carry out the SDS-PAGE, all samples were diluted in Laemmli sample buffer in order to load 200 μg of IgG/mL per lane. This solution was heated at 95 ºC for 5 min.These solutions were then subjected to SDS-PAGE in 20% polyacrylamide gels. Gels were electrophoresed for 1.5 h at 135 V on polyacrylamide gels (stacking 4%; resolving 20%) with a running buffer constituted by 250 mM Tris-HCl, 1.92 M glycine, and 1% SDS, and then stained with Coomassie Brilliant Blue G-250 0.1% (w/v), methanol 50% (v/v), acetic acid 7% (v/v) and water 42.9% (v/v), for 3-4 h in an orbital shaker at room temperature. Gels were further distained in a solution of acetic acid at 7% (v/v), methanol at 20% (v/v) and water at 73% (v/v) in an orbital shaker at a moderate speed during 3–4 h at room temperature. SDS-PAGE Molecular Weight Standards, namely marker molecular weight full-range from VWR, were used as protein standards.
3. Results and discussion
3.1. Phase diagrams, tie-lines, tie-line lengths and critical points
In this work, polymer-organic-salt-based ABS, as well as ABS employing ILs as adjuvants, were used to evaluate their extraction performance for IgG. The respective ternary phase diagrams were determined at 25°C for ABS constituted by several PEGs with different molecular weights (200, 400, 600, 1000, 2000, 4000, 6000 and 8000 g·mol-1) and the citrate-based buffer at pH ≈ 7 in aqueous media. Although phosphate buffered solutions are typically employed to maintain the pH of the coexisting phases of ABS, it should be stressed that phosphate ions can bind to metal ions, such as calcium, zinc or magnesium, which are essential to maintain the integrity of some proteins/enzymes. These high-charge density phosphate-based salts can be adequately replaced by more biocompatible and biodegradable organic salts, such as citrate-based, which combined with citric acid, also afford a wide buffered pH region. In summary, a citrate-based buffer was selected in this work aiming at improving the biodegradable and biocompatible nature of the ABS under study. Moreover, citrate-based salts also have a strong salting-out character being therefore able to provide large biphasic regions to work as ABS (Passos et al., 2012).
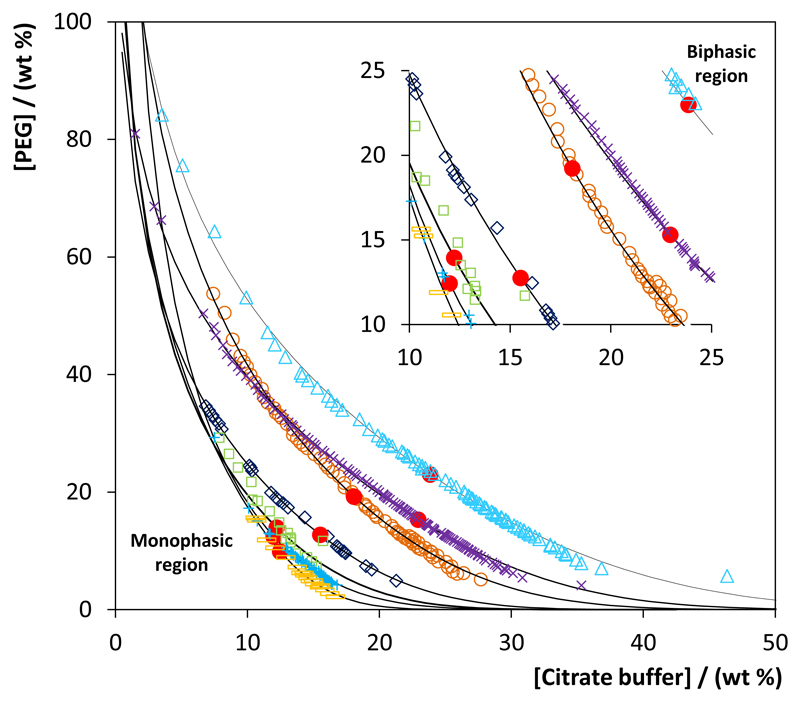
The phase diagrams obtained at 25 ºC are illustrated in Fig. 2 (the experimental weight fraction data of each phase diagram are given in the Supporting Information). The results for PEG 200 are not shown since it was found that there is no formation of a biphasic system composed of PEG 200 and C6H5K3O7/C6H8O7 at pH ≈ 7. In all phase diagrams, the biphasic region is located above the solubility curve, and the larger this region is, the higher is the ability of PEG to undergo liquid-liquid demixing in aqueous media. As can be seen from Fig. 2, the ability to form ABS increases with the PEG molecular weight. Similar trends have been observed in other ABS composed of polymer-salt or PEG-IL pairs (Azevedo et al., 2009; 2012; Lu et al., 2010). This pattern is a consequence of the higher hydrophobicity of PEGs of higher molecular weight which are more easily excluded to a second liquid phase by a salting-out species (C6H5K3O7/C6H8O7).
Figure 2.
Ternary phase diagrams of ABS composed of PEG + C6H5K3O7/C6H8O7 + H2O at pH 7 and 25 ºC: PEG 400 ( ); PEG 600 (
); PEG 600 ( ); PEG 1000 (
); PEG 1000 ( ); PEG 2000 (
); PEG 2000 ( ); PEG 4000 (
); PEG 4000 ( ); PEG 6000 (
); PEG 6000 ( ) and PEG 8000 (
) and PEG 8000 ( ). Critical point of each system (
). Critical point of each system ( ).
).
For the studied systems, the experimental binodal data were fitted by the empirical relationship originally proposed by Merchuk et al (1998), and their representation is also provided in Fig. 2. The regression parameters were estimated by the least-squares regression method, and their values and corresponding standard deviations are provided in the Supporting Information. Good correlation coefficients were obtained for all systems, indicating that these fittings can be used to predict data in a given region of the phase diagram where no experimental results are available. The experimental tie-lines (TLs), which gives the composition of each phase for a given mixture composition, along with their respective length (TLL), and detailed critical points are provided in the Supporting Information. The critical point of each system is presented in Fig. 2 and, in general, the contents of PEG at the critical point are similar, although the decrease of the amount of salt at the critical point is more visible with the increase of the PEG hydrophobicity–a consequence of their higher ability to create ABS.
The pH dependence of ABS formation was also studied using PEG 400 as phase-forming component. The respective liquid-liquid ternary phase diagrams are shown in the Supporting Information for the system composed of PEG 400 + C6H5K3O7/C6H8O7 + H2O at different pH values (5-9), using different mole ratios of potassium citrate and citric acid (C6H5K3O7/C6H8O7). The experimental weight fraction data of each phase diagram and fitting parameters are provided as part of the Supporting Information. The respective TLs, along with their respective length (TLL), pH of the coexisting phases and detailed critical points are also reported in the Supporting Information. In general, a decrease on the pH leads to a lower ability to form ABS due to the decrease of the salt salting-out ability (Shahriari et al., 2012).
3.2. Extraction of commercial/standard IgG using PEG-salt-based ABS
3.2.1. Effect of the molecular weight of PEG
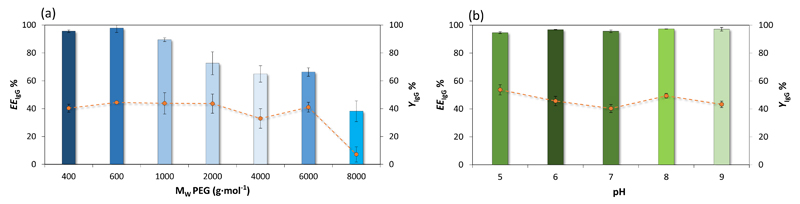
The extraction efficiency and recovery yield of IgG in ABS composed of PEGs of different molecular weights + C6H5K3O7/C6H8O7 + H2O (at a fixed pH, pH 7) were investigated and the results obtained are shown in Fig. 3a. All mixtures compositions used in this set of experiments correspond to a similar TLL (ca. 35).
Figure 3.
Extraction efficiency (EEIgG %, bars) and recovery yield (YIgG%, symbols and lines) of rabbit IgG in the systems composed of PEG + C6H5K3O7/C6H8O7 + H2O at pH 7 and 25ºC, using systems formed by PEG of different molecular weights (a), and in the systems composed of PEG 400 at different pH values (b). Error bars correspond to standard deviations obtained from three replicates.
Single-step extraction efficiencies ranging between 38 and 98% were obtained, and an increase in the polymer molecular weight leads to a decrease on the extraction efficiency of IgG to the polymer-rich phase. Indeed, for polymers of higher molecular weight, IgG preferentially partitions to the salt-rich phase. The best results, both in terms of EEIgG % and YIgG %, were achieved with ABS formed by PEG 400, PEG 600 and PEG 1000, and although some losses on the proteins were verified, no protein precipitation was macroscopically observed with these ABS. These results demonstrate that antibodies have a higher tendency to migrate to the polymer-rich phase composed of lower molecular weight polymers.
In the polymer-salt systems studied, the partitioning of the target proteins seems to be mainly driven by volume exclusion (of the polymer-rich phase) and salting-out effects (exerted by the salt-rich phase). However, electrostatic interactions cannot be discarded if extractions are being carried out at pH values different from the protein pI (the pI of rabbit IgG is 7.8; Berggren et al., 1995) as well as hydrogen-bonding interactions, which seem particularly relevant in water-rich media. According to the results obtained, and taking into account that the pH of the systems is fixed at 7 and that the salting-out species is always maintained, it seems that the lower partitioning of IgG for PEGs of higher molecular weight may be related with volume-exclusion effects due to the high IgG molecular weight (150 kDa). This effect can also justify the decrease on the recovery yields observed in systems constituted by polymers of higher molecular weight. Moreover, according to the composition of the phases (given in the Supporting Information, Table S6) for experiments carried out at a fixed TLL, there is a decrease on the salt amount at the salt-rich phase in the systems composed of higher molecular weight PEGs which corresponds to a lower salting-out aptitude. Consequently, the overall size-exclusion and salting-out effects seem to play the major role towards the IgG partitioning into the PEG-rich phase of the PEG-salt ABS investigated. For the remaining PEGs (2000, 4000, 6000 and 8000 g·mol-1), the EEIgG % are lower than those observed for the systems formed by PEGs of lower molecular weight. In these systems, protein precipitation and turbidity was macroscopically visible. Antibodies precipitation and lower yields in ABS composed of polymers of higher molecular weight were also previously described by Rosa et al. (2007). Taking into account the overall results obtained for EEIgG % and YIgG %, the following studies were carried out with ABS formed by the low molecular weight PEG 400. Moreover, ABS formed by lower molecular weight PEGs are by far less viscous and more soluble in water, being thus more advantageous when envisaging the scale-up of the purification process.
3.2.2. Effect of pH
Extraction efficiencies and recovery yields of IgG in ABS composed of PEG 400 + C6H5K3O7/C6H8O7 + H2O at different pH values and at 25°C are shown in Fig. 3b – detailed data are given in the Supporting Information. In all systems, EEIgG% higher than 95% and YIgG% ranging between 46 and 49% were obtained. No major differences are observed in extraction efficiencies and recovery yields at pH values ranging between 5 and 9. The pI of rabbit IgG is 7.8, meaning that at this pH the protein has a nearly zero net charge (Berggren et al., 1995). Based on the gathered results, IgG has a higher affinity to the polymer-rich phase, which seems to be independent of its charge, denoting thus that electrostatic interactions are not significant. Silva et al. (2014) evaluated the extraction and precipitation of human IgG at different pH values, ranging from 3 to 8, with PEG-3350-dextran ABS and observed more than 50% of IgG precipitation in the pH range 5–8; on the other hand, at more acidic values, the precipitation was considerable reduced. In our study, the precipitation of the protein was not macroscopically visible although the yields of IgG are always below 50%, and with no significant differences amongst the various pH values investigated. Although further studies at lower pH values should be conducted aiming at overcoming the loss of IgG, we are restricted to a minimum of pH 5 since ABS formed by PEG 400 and C6H5K3O7/C6H8O7 could not be created at lower pH values. Based on the gathered results, the remaining studies comprising the addition of ILs were carried out at pH ≈ 7.
3.3. Extraction of commercial/standard IgG using PEG-salt-based ABS with ILs as adjuvants
3.3.1. Effect of the IL anion
The capacity to extract and partition IgG in PEG 400 + C6H5K3O7/C6H8O7 + H2O (pH ≈ 7) ABS, with ILs as additives (at 5 wt% in the overall system), and at 25°C, was investigated thereafter. The effect of the IL anion nature on the IgG extraction and recovery was conducted with ILs sharing a common imidazolium cation ([C4mim]+) combined with the following anions: Cl-, Br-, [TOS]-, [CH3CO2]- and [N(CN)2]-. A common mixture composition was chosen which corresponds to the initial TLL of 35, namely 25 wt% of PEG 400 + 25 wt% of C6H5K3O7/C6H8O7 + 5 wt% of each IL.
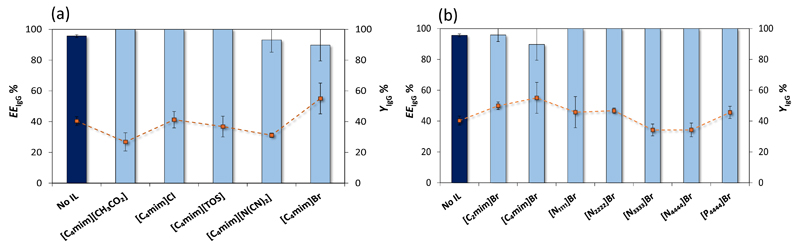
Fig. 4a depicts the extraction efficiency and recovery yields of IgG in the various systems investigated - the respective detailed data are reported in the Supporting Information. According to the obtained results, EEIgG% decreases according to the following trend: [CH3CO2]- ≈ Cl- ≈ [TOS]- > [N(CN)2]- ≈ Br-. An increase in the EEIgG% from 96 % (with no IL added) to 100% or complete extraction in a single-step was observed with the systems composed of 5 wt% of [C4mim][CH3CO2], [C4mim]Cl and [C4mim][TOS]. On the other hand, the ABS containing [C4mim]Br, although presenting a lower EEIgG% of 89%, also leads to an increase on the recovery yield of IgG (from 40 to 55%).
Figure 4.
Percentage extraction efficiencies (EEIgG %, bars) and recovery yield (YIgG%, symbols and lines) of rabbit IgG in ABS formed by PEG 400 + C6H5K3O7/C6H8O7 + H2O and [C4mim]-based ILs at 5 wt% (a) and Br-based ILs at 5 wt% (b), at pH 7 and 25ºC. Error bars correspond to standard deviations obtained from three replicates.
The hydrogen-bond basicity is a measure of the ability of a compound to accept a proton (or donate an electron pair) in a solute-solvent hydrogen-bond (Cláudio et al., 2014). ILs with the [CH3CO2]- and Cl− anions are those with a higher hydrogen-bond basicity, which reflects their high polarity or affinity for water (Cláudio et al., 2014). These two ILs, although not favorable to increase the recovery yield, led to the complete extraction of IgG, meaning that specific hydrogen-bonding interactions play a role on this process. [C4mim][TOS] displays a lower hydrogen-bond basicity than [C4mim][CH3CO2] and [C4mim]Cl and is also capable of leading to a EEIgG% of 100%. This particular IL is constituted by an aromatic anion and further π…π interactions between aromatic amino acids in IgG may also have some effect towards the IgG partitioning. On the other hand, the ILs [C4mim]Br and [C4mim][N(CN)2] display lower hydrogen-bond basicity values (Cláudio et al., 2014), and with these, the complete extraction in a single-step was not achieved. In summary, ILs with a higher hydrogen bonding basicity are better at enhancing the extraction of IgG for the PEG-rich phase. The preferential IL partition to the top phase naturally changes the chemical and physical properties of the polymer-rich phase. These results corroborate the notion that the IL chemical structure plays a major role and that specific interactions with the IL should be governing the partitioning of IgG.
Since different ILs were added to the polymer-salt ABS, these will also partition between the coexisting phases. All imidazolium-based ILs investigated preferentially partition to the polymer-rich phase, with extraction efficiencies higher than 80% (data shown in the Supporting Information). However, there is not a correlation between the amount of IL present at the PEG-rich phase and the IgG partitioning. Therefore, the extent of the ILs migration to the polymer-rich phase has not a direct effect on the IgG partition degree. Nevertheless, from the results obtained, and considering that all ILs display extraction efficiencies higher than 80% to the PEG-rich phase, it seems that the amount of IL added to enhance the IgG partition is thus in excess and that the chemical nature of the IL plays the major role in this study. This indicates that lower amounts of IL could be enough to enhance the IgG partitioning and to optimize the purification process, which obviously brings additional advantages in terms of ABS cost and biocompatibility. Based on these results, we can conclude that the investigated ILs establish non-covalent interactions with the target protein enhancing its partition to the polymer-rich phase (the phase where the IL is also enriched), and that ILs act as “salting-in” species establishing specific interactions with the target protein.
3.3.2. Effect of the IL cation
The effect of the addition of ILs with different cations to polymer-salt ABS on the partitioning of antibodies was addressed using ILs with a fixed anion (Br-) combined with the following cations: [C2mim]+, [C4mim]+, [N1111]+, [N2222]+, [N3333]+, [N4444]+ and [P4444] +. All ILs were added at 5 wt% to the ABS formed by 25 wt% of PEG 400 + 25 wt% of C6H5K3O7/C6H8O7, pH ≈ 7. The ILs investigated allow to ascertain on the IL cation core and alkyl side chain length effects on the antibody partitioning behavior. The results obtained are shown in Fig. 4b, with the respective detailed data reported in the Supporting Information.
The extraction efficiency of the several ABS for IgG decreases in the following order of IL cations: [N1111]+ ≈ [N2222]+ ≈ [N3333]+ ≈ [N4444]+ ≈ [P4444] + > [C2mim]+ ≈ [C4mim]+. Remarkably, all tetralkylammonium- and tetraalkylphosphonium-based ILs, when added at small amounts to the original ABS, lead to the complete extraction of IgG to the polymer-rich phase. On the contrary, imidazolium-based ILs display a lower performance to increase the extraction efficiency of IgG. Even so, no major differences are observed on the IgG partitioning as a function of the alkyl side chain length in tetraalkylammonium-based ILs nor between tetrabutylammonium and tetrabutylphosphonium bromide ILs. Furthermore, the alkyl chain length (from ethyl to butyl) in imidazolium-based ILs does not have a significant impact on the IgG partitioning. These results confirm that no significant dispersive-type interactions occur between the IL cations and the protein surface.
3.4. Stability of IgG and extraction from rabbit serum
Although it was demonstrated that small amounts of ILs in conventional ABS enhance the partition and recovery yield of IgG, efforts were further carried out to evaluate the effect of ILs on the structural stability of IgG. Some “hydrated ILs” or ILs aqueous solutions have demonstrated to be extraordinary media for solubilizing and stabilizing proteins (Khono et al., 2011). Other studies (Wei and Danielson, 2011) have demonstrated that aqueous solutions of ammonium-based ILs are able to maintain the native structure of cytochrome c up to IL concentrations of 50−70% (in contrast with the denaturation of the protein observed in similar solutions of methanol or acetonitrile with water). Jha and Venkatesu (2015) also demonstrated that ammonium-based ILs act as stabilizers for globular proteins. Dreyer and Kragl (2008) have shown that ILs can be used as phase-forming components to extract and stabilize enzymes. Based on these evidences it seems plausible that proper ILs can be used to extract and to preserve the proteins integrity.
We initially evaluated the structural stability study of IgG in presence of imidazolium-, ammonium- and phosphonium-based ILs using FT-IR spectroscopy and SDS-PAGE (Fig. 5, and in the Supporting Information). The protein secondary structure was also evaluated in an aqueous solution of PBS (10 mM, pH 7.4) for comparative analysis.
Figure 5.
FT-IR spectra of IgG (amide I region): (a) no IL, (b) in presence of [C4mim][CH3CO2] and (c) in presence of [P4444]Br. (d) SDS-PAGE image of IgG at a 200 μg/mL concentration in: PBS buffer 10 mM, pH 7.4 (1), ,o IL (2), [P4444]Br (3), [N4444]Br (4), [C4mim]Cl (5), [C4mim]Br (6), [C4mim]N(CN)2] (7) and [C4mim][CH3CO2] (8). (e) SE-HPLC chromatogram of serum samples obtained from the top phases after the extraction with ABS using ILs as adjuvants.
The secondary structure of IgG at native state contains 64% of β-sheet, 3% of α-helix, 28% of β-turn and 5% of random coil (Kong and Su, 2007). The amide I region in the FT-IR spectra absorbs in the region of 1600-1690 cm-1, which corresponds to the cross β-sheet (~1625 cm-1 and ~1680 cm-1), native β-sheet (~1635 cm-1), random coil ((~1650 cm-1), α-helix (~1662 cm-1) and β-turn (~1670 cm-1). The amide I region of IgG in the PEG-salt system (no IL added) shown in Fig. 5a was found to be similar to that in the PBS aqueous solution (shown in the Supporting Information). Also in the presence of [C4mim][CH3CO2] (Fig. 5b) there are no major changes in the amide I region of IgG, as well as in presence of remaining ILs at 5 wt% (results shown in the Supporting Information), demonstrating that the stability of the IgG native structure is not significantly affected. However, in presence of [P4444]Br, the changes in β conformations in the amide I region of IgG indicate structural deformation of the protein in presence of this IL (Fig. 5c). SDS-PAGE analysis was also carried out to evaluate the effect of ILs on the extraction process and possible degradation of IgG. As seen in Fig. 5d, there is no degradation of IgG, except in presence of [P4444]Br. Thus, the secondary structure of IgG got completely disturbed in presence of [P4444]Br, the IL in which the complete precipitation of the antibody was also observed macroscopically. In presence of [N4444]Br, and although some deviations in the secondary structure of IgG are also seen, still we did not observe any precipitate (macroscopically). Although similar, the phosphonium-based IL presents a slightly higher ability to form ABS than their ammonium-based counterpart, meaning that it displays a higher hydrophobicity afforded by the central atom of the cation (Passos et al., 2012). This higher hydrophobicity of phosphonium-based ILs seems thus to be the main reason behind their stronger effect on destroying the integrity of IgG. In summary, and amongst all ILs evaluated, [P4444]Br and [N4444]Br were the only found to be not suitable for the extraction of IgG since both lead to the protein denaturation, whereas the remaining ILs are appropriate for extraction and purification processes.
Finally, and aiming at gathering novel evidence on the applicability of the studied systems to real samples, the free-IL ABS and those containing 5 wt% of ILs were investigated in what concerns their extraction performance for IgG from real rabbit serum samples. A common mixture composition was chosen and which corresponds to the initial TLL of 35, namely 25 wt% of PEG 400 + 25 wt% of C6H5K3O7/C6H8O7 + 5 wt% of IL (or no IL). The SE-HPLC chromatograms are shown in Fig. 5e while data for extraction efficiency, recovery yield and purity of IgG are shown in Table 1.
Table 1.
Extraction efficiency, EEIgG %, recovery yield, YIgG %, and purity of IgG extracted from rabbit serum samples in the polymer-rich phase of ABS composed of PEG 400 + C6H5K3O7/C6H8O7 + H2O + 5 wt% of ILs at pH 7. All results were calculated from SE-HPLC data.
| IL | EEIgG% | YIgG% | Purity of IgG (%) |
|---|---|---|---|
| No IL | 95 | 44 | 19 |
| [C4mim]Cl | 100 | 44 | 22 |
| [C4mim]Br | 91 | 45 | 21 |
| [C4mim][CH3CO2] | 100 | 46 | 26 |
| [C4mim][N(CN)2] | 93 | 47 | 23 |
| [N4444]Br | 100 | 42 | 22 |
| [N1111]Br | 100 | 47 | 23 |
In all systems the extraction efficiencies were maintained at 100%, corresponding to the complete partition of IgG to the PEG-IL-rich phase attained in a single-step, even in the presence of a more complex matrix. The retention time of IgG was found to be ca. 15.5 min (according to the pure/commercial IgG sample tested in the same conditions), indicating that the remaining peaks found in serum samples correspond to other proteins while allowing us to determine the purity of the target protein in the PEG-rich phase. The purity and recovery yield of IgG in the PEG-salt system (no IL added) was found to be 19% and 42%, respectively, at the PEG-rich phase. However, for systems were ILs were added, purity levels of IgG ranging between 21 and 26%, and recovery yields ranging from 42 to 47%, were found. The best results were attained with [C4mim][CH3CO2], where a 37% enhancement in the purity of IgG was observed and with an yield of IgG of 46% (Table 1). As a result, and although demonstrating the viability of the studied systems to extract and purify IgG from serum, it was also verified that the addition of ILs as adjuvants to ABS not only enhances the extraction efficiency and the recovery yield, but also lead to an increase in the purity of IgG. It is also important to note that as observed by FT-IR (structural deformation of IgG) and SDS-PAGE (degradation of IgG) studies, in the [P4444]Br-based ABS, contrarily to remaining ABS, no peaks were observed in the respective SE-HPLC chromatogram, meaning that this IL is not appropriate to recover isolated IgG.
In previous studies reporting to the use of polymer-based ABS, the extraction efficiency of IgG in a single-step was found to be < 90% (Rosa et al., 2007; 2009; Azevedo et al., 2007; 2008). In some works extraction efficiencies higher than 90% were obtained, although in these cases multiple steps are required (Rosa et al., 2013; Ferreira et al., 2008; Borlido et al., 2010). In the systems investigated in this work, the addition of 5 wt% of ILs as adjuvants in polymer-salt ABS leads to 100% of extraction efficiency of IgG to the polymer-rich phase in a single-step. On the other hand, and although the obtained purification factors are similar to those reported in the literature for one-step extractions, the extraction efficiencies of 100% and high recovery yields obtained with the systems studied in this work will result in lower losses of the target antibody when envisaging the ABS large-scale applications. ILs appear, even at low concentrations, as promising candidates to improve extraction efficiencies, which seem to be attained by specific interactions established between the antibody and ILs. The obtained results demonstrate the possibility of using ILs as adjuvants to improve the performance of ABS when compared with other strategies previously addressed for the purification of IgG, where the functionalization of polymers, the addition of conventional salts or multistage approaches were investigated (a summary of literature results is given in the Supporting Information). In the present study, the use of adequate ILs as adjuvants (at 5 wt%) allows 100% of extraction and a 37% purity enhancement of IgG into the polymer-rich phase, in a single-step, without affecting the structural integrity of IgG.
The investigated systems are promising strategies to extract and purify IgG from serum samples while envisaging a widespread applicability of antibodies as alternative therapies. The large-scale application of the investigated ABS to purify IgG from crude samples (and since contaminant proteins migrate in some extent to the opposite phase) can be conducted by multi-step liquid-liquid equilibrium or by liquid-liquid chromatography, namely by counter current chromatography or by centrifugal partition chromatography. After the IgG purification by ABS, the target antibody can be recovered from the PEG-IL-rich phase by induced precipitation or by dialysis (Pereira et al., 2015).
4. Conclusions
The results obtained in this work reveal a high affinity of IgG to the polymer-rich phase in ABS composed of polyethylene glycols and potassium citrate/citric acid buffer; nevertheless, the complete extraction of IgG was never attained in a single-step in systems where no ILs were added (even after several optimization procedures). With the addition of 5 wt% of adequate ILs, extraction efficiencies of 100% of IgG can be attained in one-step, as well as higher recovery yields. The structural integrity of IgG was found to be maintained during the extraction process and in presence of ILs, with the exception of the [P4444]Br and [N4444]Br ILs. The tuning ability of ILs was also confirmed by performing extractions of IgG from rabbit serum samples, where the complete extraction in a single-step was maintained and an enhancement of ca. 37% in the IgG purity was obtained by the use of ILs as adjuvants (as compared to the IL-free ABS). Thus, low amounts of ILs in the formulation of ABS are enough to achieve complete extractions in a single-step and to enhance their selectivity for target proteins. Albeit further investigations are still required, ABS composed of ILs as adjuvants can be envisioned as an alternative and more efficient method for the scalable purification of high-cost biopharmaceuticals, such as antibodies.
Supplementary Material
Acknowledgements
This work was developed in the scope of the project CICECO-Aveiro Institute of Materials (Ref. FCT UID/CTM/50011/2013), financed by national funds through the FCT/MEC and co-financed by FEDER under the PT2020 Partnership Agreement. A.M. Ferreira acknowledges FCT for the doctoral grant SFRH/BD/92200/2013. The research leading to reported results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 337753.
References
- Andrew SM, Titus JA. Current Protocols in Cell Biology. John Wiley & Sons, Inc; 2001. Purification of immunoglobulin G. [DOI] [PubMed] [Google Scholar]
- Andrews BA, Nielsen S, Asenjo JA. Partitioning and purification of monoclonal antibodies in aqueous two-phase systems. Bioseparation. 1996;6:303–313. [PubMed] [Google Scholar]
- Azevedo AM, Rosa PA, Ferreira IF, Aires-Barros MR. Optimisation of aqueous two-phase extraction of human antibodies. J Biotechnol. 2007;132:209–217. doi: 10.1016/j.jbiotec.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Azevedo AM, Rosa PA, Ferreira IF, Aires-Barros MR. Integrated process for the purification of antibodies combining aqueous two-phase extraction, hydrophobic interaction chromatography and size-exclusion chromatography. J Chromatogr A. 2008;12:154–161. doi: 10.1016/j.chroma.2008.09.115. [DOI] [PubMed] [Google Scholar]
- Azevedo AM, Gomes AG, Rosa PAJ, Ferreira IF, Pisco AMMO, Aires-Barros MR. Partitioning of human antibodies in polyethylene glycol–sodium citrate aqueous two-phase systems. Sep Purif Technol. 2009;65:14–21. [Google Scholar]
- Azevedo AMP, Rosa AJ, Ferreira IF, Pisco AMMO, de Vries J, Korporaal R, Visser TJ, Aires-Barros MR. Affinity-enhanced purification of human antibodies by aqueous two-phase extraction. Sep Purif Technol. 2009;65:31–39. [Google Scholar]
- Benavides J, Rito-Palomares M. Practical experiences from the development of aqueous two-phase processes for the recovery of high value biological products. J Chem Technol Biotechnol. 2008;83:133–142. [Google Scholar]
- Berggren K, Johansson H-O, Yjerneld F. Effects of salts and the surface hydrophobicity of proteins on partitioning in aqueous two-phase systems containing thermoseparating ethylene oxide-propylene oxide copolymers. J Chromatogr A. 1995;718:67–79. [Google Scholar]
- Bora MM, Borthakur S, Rao PC, Dutta NN. Aqueous two-phase partitioning of cephalosporin antibiotics: effect of solute chemical nature. Sep Purif Technol. 2005;45:153–156. [Google Scholar]
- Borlido L, Azevedo AM, Aires-Barros MR. Extraction of human IgG in thermo-responsive aqueous two-phase systems: Assessment of Structural Stability by Circular Dichroism. Sep Sci Technol. 2010;45:2171–2179. [Google Scholar]
- Chen X, Liu J, Wang J. Ionic liquids in the assay of proteins. Anal Methods. 2010;2:1222–1226. [Google Scholar]
- Cláudio AF, Swift L, Hallett JP, Welton T, Coutinho JA, Freire MG. Extended scale for the hydrogen-bond basicity of ionic liquids. Phys Chem Chem Phys. 2014;16:6593–6601. doi: 10.1039/c3cp55285c. [DOI] [PubMed] [Google Scholar]
- Desai RK, Streefland M, Wijffels RH, Eppink MHM. Extraction and stability of selected proteins in ionic liquid based aqueous two phase systems. Green Chem. 2014;16:2670–2679. [Google Scholar]
- Dreyer S, Kragl U. Ionic liquids for aqueous two-phase extraction and stabilization of enzymes. Biotech Bioeng. 2008;99:1416–1424. doi: 10.1002/bit.21720. [DOI] [PubMed] [Google Scholar]
- Ferreira IF, Azevedo AM, Rosa PA, Aires-Barros MR. Purification of human immunoglobulin G by thermoseparating aqueous two-phase systems. J Chromatogr A. 2008;1195:94–100. doi: 10.1016/j.chroma.2008.04.077. [DOI] [PubMed] [Google Scholar]
- Freire MG, Claudio AF, Araujo JM, Coutinho JA, Marrucho IM, Canongia Lopes JN, Rebelo LP. Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem Soc Rev. 2012;41:4966–4995. doi: 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- Jha I, Venkatesu P. Endeavour to simplify the frustrated concept of protein-ammonium family ionic liquid interactions. Phys Chem Chem Phys. 2015;17:20466–20484. doi: 10.1039/c5cp01735a. [DOI] [PubMed] [Google Scholar]
- Kammoun R, Chouayekh H, Abid H, Naili B, Bejar S. Purification of CBS 819.72 α-amylase by aqueous two-phase systems: Modelling using Response Surface Methodology. Biochem Eng J. 2009;46:306–312. [Google Scholar]
- Kohno Y, Saita S, Murata K, Nakamura N, Ohno H. Extraction of proteins with temperature sensitive and reversible phase change of ionic liquid/water mixture. Polym Chem. 2011;2:862–867. [Google Scholar]
- Kong J, Yu S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim Biophys Sin. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Leenaars M, Hendriksen CFM. Critical Steps in the Production of Polyclonal and Monoclonal Antibodies: Evaluation and Recommendations. Ilar J. 2005;46:269–279. doi: 10.1093/ilar.46.3.269. [DOI] [PubMed] [Google Scholar]
- Marsh KN, Boxall JA, Lichtenthaler R. High pressure phase equilibrium for ethylene + 1-propanol system at 283.65 K. Fluid Phase Equilib. 2010;219:93–98. [Google Scholar]
- Martínez-Aragón M, Burghoff S, Goetheer ELV, de Haan AB. Guidelines for solvent selection for carrier mediated extraction of proteins. Sep Purif Technol. 2009;65:65–72. [Google Scholar]
- Martínez-Aragón M, Goetheer ELV, de Haan AB. Host–guest extraction of immunoglobulin G using calix[6]arenas. Sep Purif Technol. 2009;65:73–78. [Google Scholar]
- Merchuk JC, Andrews BA, Asenjo JA. Aqueous two-phase systems for protein separation: Studies on phase inversion. J Chromatogr B Biomed Sci Appl. 1998;711:285–293. doi: 10.1016/s0378-4347(97)00594-x. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- Passos H, Ferreira AR, Cláudio AFM, Coutinho JAP, Freire MG. Characterization of aqueous biphasic systems composed of ionic liquids and a citrate-based biodegradable salt. Biochem Eng J. 2012;67:68–76. [Google Scholar]
- Pei Y, Wang J, Wu K, Xuan X, Lu X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep Purif Technol. 2009;64:288–295. [Google Scholar]
- Pereira JFB, Lima AS, Freire MG, Coutinho JAP. Ionic liquids as adjuvants for the tailored extraction of biomolecules in aqueous biphasic systems. Green Chem. 2010;12:1661–1669. [Google Scholar]
- Porto TS, Medeiros e Silva GM, Porto CS, Cavalcanti MTH, Neto BB, LimaFilho JL, Converti A, Porto ALF, Pessoa A. Liquid–liquid extraction of proteases from fermented broth by PEG/citrate aqueous two-phase system. Chem Eng Process Process Intensif. 2008;47:716–721. [Google Scholar]
- Quental MV, Caban M, Pereira MM, Stepnowski P, Coutinho JA, Freire MG. Enhanced extraction of proteins using cholinium-based ionic liquids as phase-forming components of aqueous biphasic systems. Biotechnol J. 2015;10:1457–1466. doi: 10.1002/biot.201500003. [DOI] [PubMed] [Google Scholar]
- Raghavarao KSMS, Ranganathan TV, Srinivas ND, Barhate RS. Aqueous two phase extraction—an environmentally benign technique. Clean Technol Environ Policy. 2003;5:136–141. [Google Scholar]
- Rito-Palomares M, Dale C, Lyddiatt A. Generic application of an aqueous two-phase process for protein recovery from animal blood. Process Biochem. 2000;35:665–673. [Google Scholar]
- Rosa PAJA, Azevedo M, Aires-Barros MR. Application of central composite design to the optimisation of aqueous two-phase extraction of human antibodies. J Chromatogr A. 2007;1141:50–60. doi: 10.1016/j.chroma.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Rosa PA, Azevedo AM, Ferreira IF, Sommerfeld S, Backer W, Aires-Barros MR. Downstream processing of antibodies: Single-stage versus multi-stage aqueous two-phase extraction. J Chromatogr A. 2009;11:8741–8749. doi: 10.1016/j.chroma.2009.02.024. [DOI] [PubMed] [Google Scholar]
- Rosa PA, Azevedo AM, Sommerfeld S, Mutter M, Backer W, Aires-Barros MR. Continuous purification of antibodies from cell culture supernatant with aqueous two-phase systems: from concept to process. Biotechnol J. 2013;8:352–362. doi: 10.1002/biot.201200031. [DOI] [PubMed] [Google Scholar]
- Schuster M, Nechansky A, Kircheis R. Cancer immunotherapy. Biotechnol J. 2006;1:138–147. doi: 10.1002/biot.200500044. [DOI] [PubMed] [Google Scholar]
- Shahriari S, Neves CMSS, Freire MG, Coutinho JAP. Role of the Hofmeister Series in the Formation of Ionic-Liquid-Based Aqueous Biphasic Systems. J Phys Chem B. 2012;116:7252–7258. doi: 10.1021/jp300874u. [DOI] [PubMed] [Google Scholar]
- Silva MFF, Fernandes-Platzgummer A, Aires-Barros MR, Azevedo AM. Integrated purification of monoclonal antibodies directly from cell culture medium with aqueous two-phase systems. Sep Purif Technol. 2014;132:330–335. [Google Scholar]
- Soares RRG, Azevedo AM, Van Alstine JM, Aires-Barros MR. Partitioning in aqueous two-phase systems: Analysis of strengths, weaknesses, opportunities and threats. Biotechnol J. 2015;10:1158–1169. doi: 10.1002/biot.201400532. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lomakin A, Latypov RF, Laubach JP, Hideshima T, Richardson PG, Munshi NC, Anderson KC, Benedek GB. Phase transitions in human IgG solutions. J Chem Phys. 2013;139:121904. doi: 10.1063/1.4811345. (1-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Danielson ND. Fluorescence and Circular Dichroism Spectroscopy of Cytochrome c in Alkylammonium Formate Ionic Liquids. Biomacromolecules. 2011;12:290–297. doi: 10.1021/bm1008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiqiang A. Antibody therapeutics—a mini review. Trends in Bio/Pharmaceutical Industry. 2008;2:24–29. [Google Scholar]
- Zijlstra GM, Michielsen MJ, de Gooijer CD, van der Pol LA, Tramper J. Separation of hybridoma cells from their IgG product using aqueous two-phase systems. Bioseparation. 1996;6:201–210. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.