Abstract
Purpose of review
The observed increase in incidence of allergic disease in many regions over the past 3 decades has intensified interest in understanding the epidemiology of severe allergic reactions. We discuss the issues in collecting and interpreting these data, and highlight current deficiencies in the current methods of data gathering.
Recent findings
Anaphylaxis, as measured by hospital admission rates, is not uncommon and has increased in the UK, USA, Canada and Australia over the last 10-20 years. All large datasets are hampered by a large proportion of uncoded “unspecified” causes of anaphylaxis. Fatal anaphylaxis remains a rare event, but appears to be increasing for medication in Australia Canada and the USA. The rate of fatal food anaphylaxis is stable in the UK and USA, but has increased in Australia. The age-distribution for fatal food anaphylaxis is different to other causes, with data suggesting an age-related predisposition to fatal outcomes in teenagers and adults to the fourth decade of life.
Summary
The increasing rates of food and medication allergy (the latter exacerbated by an ageing population) has significant implications for future fatality trends. An improved ability to accurately gather and analyse population level anaphylaxis data in a harmonised fashion is required, so as to ultimately minimise risk and improve management.
Keywords: Allergy, anaphylaxis, epidemiology
Introduction
Anaphylaxis is a term used widely to describe a severe allergic reaction, however there is no single definition agreed worldwide [1]. There has been significant interest in the epidemiology of anaphylaxis, perhaps driven by trends of increasing allergic disease observed in much of the developed world over the past 3 decades. Measurement of population-based patterns and causes of severe allergic reactions might serve several purposes, including: estimating the regional burden of disease, identifying trends in causal/triggering agents, tracking trends over time and in finding associations which may help to predict or prevent severe disease or death. In this review we aim to outline how studying the epidemiology of anaphylaxis might assist in the prevention of anaphylaxis and anaphylaxis-related fatalities at an individual and population-based level.
Understanding Anaphylaxis
Understanding the pathophysiology of anaphylaxis is crucial to predicting risk and interpreting epidemiological associations. However, human data is limited and performing allergen challenges under medical supervision to investigate mechanisms, raises significant ethical and safety concerns. Even where such challenges are performed, those individuals with prior severe reactions may be excluded and the challenges themselves designed (through dose-limitation) to avoid severe reactions, and are less likely to provide a valid picture of the whole population of individuals at risk from severe reactions. Some investigators have sought to capture blood/bio samples for mechanistic studies from individuals presenting to the Emergency Departments during or immediately following anaphylaxis, but these are prone to difficulties in defining the circumstances prior to arrival in the hospital and lack ‘baseline’ samples to allow detailed assessment of inflammatory mediators. Unfortunately, animal models of mediators and mechanisms of anaphylaxis, particularly food-induced anaphylaxis, cannot be extrapolated to humans (Table 1).
Table 1.
Significant differences in the literature with regard to the pathophysiology of anaphylaxis in murine models and the mechanisms which may occur in humans.
| Murine models | Humans | References | |
|---|---|---|---|
| 1:4 to 1:10 | Neutrophil:lymphocyte ratio in peripheral blood | 2:1 to 1:1 | [2] |
| Polymeric IgA (low serum levels) IgD, IgE, IgM IgG1, IgG2a, IgG2b, IgG3 |
Immunoglobulins | Monomeric IgA, 2 serotypes (IgA1, IgA2), IgA1 abundant in IgD, IgE, IgM IgG1, IgG2, IgG3, IgG4 |
[3] |
| Yes | High affinity IgE receptor (FcεRI) on mast cells and basophils | Yes | [4] |
| No | FcεRI receptor on antigen presenting cells | Yes | [4] |
| Yes | IgE-dependent anaphylaxis | Yes | [4] |
| Yes | IgG-dependent anaphylaxis | No evidence for IgG-mediated activation of human mast cells. If present, likely to require very high levels of antigen exposure |
[4,5*] |
| Very high: in murine models of peanut allergy, dose/weight equivalent to a human eating ≅1000 peanuts! | Allergen dose required through oral exposure to cause anaphylaxis | Very low doses (mgs) e.g. for peanut allergy, 10% of individuals react to 1/70 of a peanut | [6,7] |
| + | Sensitivity to histamine | ++++ | [8,9] |
| Yes | Anaphylaxis inhibited by H1-antihistamines |
Little clinical evidence for this. Significant interspecies differences exist in histamine receptor pharmacology. | [10,11,12] |
| Yes | Basophils secrete Platelet Activating Factor (PAF) | Unclear | [13,14] |
Defining Anaphylaxis and severe anaphylaxis: why is it important?
Anaphylaxis is most commonly accepted to be a potentially life threatening systemic IgE-mediated allergic reaction, with a spectrum of severity. To accurately compare anaphylaxis data from different regions and across centres within regions it is useful to have harmonised definitions of anaphylaxis. However ongoing regional variations in definition persist (Table 2) [15–19], which are further confounded by confusion between what constitutes a definition of anaphylaxis as opposed to a description of symptoms, i.e. the clinical presentation of anaphylaxis [20]. Indeed, the NIAID criteria for anaphylaxis (which have gained widespread acceptance) are not a definition per se, but were developed to capture at least 95% of cases [19].
Table 2.
Current descriptions of anaphylaxis in the literature
| EAACI [15] | WAO [16] | AAAAI/ACAAI [17] | ASCIA [18] | NIAID [19] |
|---|---|---|---|---|
| A severe life-threatening generalized or systemic hypersensitivity reaction. An acute, potentially fatal, multi-organ system, allergic reaction. |
A serious life-threatening generalized or systemic hypersensitivity reaction. A serious allergic reaction that is rapid in onset and might cause death |
An acute life-threatening systemic reaction with varied mechanisms, clinical presentations, and severity that results from the sudden release of mediators from mast cells and basophils. | Anaphylaxis is a serious, rapid-onset, allergic reaction that may cause death. Severe anaphylaxis is characterised by life-threatening upper airway obstruction, bronchospasm and/or hypotension. |
Anaphylaxis is a serious allergic reaction that involves more than one organ system (for example, skin, respiratory tract, and/or gastrointestinal tract). It can begin very rapidly, and symptoms may be severe or life-threatening. |
In terms of accurately defining anaphylaxis, we are faced with a number of important challenges. Anaphylaxis is typically defined as a generalised reaction, however, at least in the UK and Australia, we have found that coroner’s reports of fatal anaphylaxis (particularly to food) often describe only symptoms localised to the throat and lower airways before collapse and death (Pumphrey P, personal communication). Severe anaphylaxis may, therefore, initially present without significant systemic symptoms. Not all episodes of anaphylaxis necessarily result in a life-threatening situation. Many individuals experiencing anaphylaxis will exhibit symptom resolution without receiving appropriate treatment. For example, in a prospective survey of teenagers attending UK allergy clinics, 245 of 969 had at least one episode of anaphylaxis (according to NIAID criteria) in the previous year; only 41 (17%) had used or been administered epinephrine (adrenaline), despite all being prescribed an auto-injector device. Thus, over 80% of teenagers in this cohort recovered spontaneously from symptoms (including loss of consciousness and difficulty in breathing) despite not receiving epinephrine [21*].
Arguably, our greatest challenge is the inability to predict which patients, including those not previously known to be allergic, are most at risk of fatal reactions [22**]: can the literature assist us in addressing this question? In this regard, it may be helpful to focus on data related to ‘severe’ or life-threatening anaphylaxis. However, what are the determinants of severity? Is it the reaction itself, or the outcome of the reaction (which includes treatment and any homeostatic compensation by the allergic individual)? If an individual has anaphylaxis (with both respiratory and cardiovascular symptoms) but promptly receives a single dose of intramuscular epinephrine with good effect, is this still to be considered a severe reaction?
One approach to narrowing anaphylaxis literature to more severe reactions is to only include cases or reports which have case definitions requiring hospitalization, on the assumption that only severe cases need hospitalisation. However, the decision to admit an individual peri- and post-anaphylaxis may depend not just on severity, but also on local practice, individual healthcare professional and patient preference, which may, in turn, be influenced by national guidelines. Arguably, near-fatal (requiring intensive care) or fatal anaphylaxis may be a more objective criterion of severity, but even here many factors will influence an admission to ICU.
Methodological challenges in gathering and using population-based anaphylaxis data
The use of different definitions of anaphylaxis and severe anaphylaxis are just one challenge in terms of deriving accurate anaphylaxis-related data. Different methodologies are used in the literature: many studies use self-report, which may overestimate the true incidence by at least a factor of 10 [23]. Further confusion may arise from variations in the use of epidemiological terms. Incidence is the number of new cases occurring during a given time period in a defined population: but many studies do not distinguish between those having their first ever episode, and others with previous anaphylaxis who have a further anaphylaxis within the study period (and are therefore, strictly speaking, not new cases). Identification of cases by medical coding systems, such as the ICD-10 system, is a common methodological approach, but is prone to misclassification [23,24]. Retrospective data collection is subject to incomplete reporting and recall bias. Determining causality of anaphylaxis can be challenging, particularly for reactions due to medication/iatrogenic causes where medicolegal concerns may affect reporting. Population-based datasets are hampered by a large proportion of uncoded “unspecified” causes of anaphylaxis [25*–28*]. Using stricter criteria to define anaphylaxis (e.g. admission to intensive care) reduces the number of cases, resulting in the same challenges as those encountered in defining the epidemiology of rare diseases, where very large sample sizes are needed. It also has the likelihood of skewing trigger data towards those where exposure more commonly occurs in the hospital setting (such as parenteral medications and contrasts agents) and avoids severe cases where resolution (either survival or death) occurs prior to admission. Prospective collection of data, for example through case registries, are prone susceptible to similar issues regarding the case definition, and in any event are unlikely to include all cases [22**].
Both anaphylaxis and fatal anaphylaxis commonly occur in the community and not in a medical setting, so the circumstances of reaction and description of symptoms are usually acquired from non-medical witnesses, further complicating “anaphylaxis” categorization. Using data from national death registries and coroner’s reports to attribute cause and risk factors is particularly problematic. In these instances, reports of symptoms are second-hand and usually entered into coroner’s reports by the attending police official. Despite these shortcomings, data registries and admission statistics remain a valuable source of information which can provide insights into at risk populations.
What do we know about the epidemiology of severe and fatal anaphylaxis?
Fatal anaphylaxis is a rare occurrence. Estimates of all-cause fatal anaphylaxis rates across the UK [25*], US [26,27*] and Australia [28*] over the past 2 decades range from 0.064-0.099 deaths, per 100,000 population per annum. These rates are likely underestimates, however the true extent of misdiagnosis (such as acute severe asthma, and coronary infarct) and miscoding is unknown. In a European anaphylaxis registry, only 5.5% of 1155 cases of severe anaphylaxis (laryngeal oedema, bronchospasm, cyanosis, shock) involved cardiorespiratory arrest [29]. In a subsequent paediatric series from the same registry, 1.3% (26/1970) cases involved life-threatening or fatal reactions (5 cases) [30*].
Rates of hospital admissions for anaphylaxis are reported to have increased in the UK [25*], US [26], Canada [31] and Australia [28*,32] over the past 2 decades, however an increase in all-cause fatality rates has only been observed to date in Australia, not the UK [25*], Canada (Ontario State) [33] or USA [27*]. There was a 7-fold increase in UK-hospitalizations for (all-cause) anaphylaxis from 1992-2012 (using government hospital datasets), but no significant increase in fatalities over the same time period (0.047 cases per 100,000 per annum) [25*]; a similar pattern has been reported in the USA, with a modest annual increase in all-cause hospitalizations between 1999-2009 of 2.2% but not fatalities [27*] (Table 3). In contrast, an analysis of government hospital and death registry datasets in Australia found an increase in both hospitalizations and all-cause fatalities: admissions increased almost 4-fold (from 5.0 to 19.2 per 100,000 population) between 1997-2013, with a near doubling of fatalities (0.05 to 0.09 cases per 100,000) over the same time period [28*]. In both USA and Australia, an increase in fatalities due to medication related anaphylaxis has been reported [27*,28*]. This may, in part, reflect the significant changes in prescription and intra-operative/intervention practice, such that at-risk individuals are being exposed to more medications and contrast agents.
Table 3.
Rates of hospitalization and fatalities (per 100,000 population, per annum) in UK, USA and Australia.
| UK [25*] | USA [26,27*] | Australia [28*,32] | |||||
|---|---|---|---|---|---|---|---|
| 1992 | 1998 | 2012 | 1999-2001 | 2008/10 | 1997 | 2013 | |
| All cause anaphylaxis - hospitalizations - fatalities |
1.0 0.036 |
3.7 0.043 |
7.0 0.054 |
2.1 [26] 0.076 [26] |
2.5 [26] 0.071 [26] |
5.0 0.054 |
19.2 0.099 |
| Anaphylaxis: Food - hospitalizations - fatalities |
- 0.010 |
1.2 0.012 |
2.4 0.012 |
0.61 [26] 0.003 [26] 0.005 [27] |
0.83 [26] 0.004 [26] 0.005 [27] |
1.6 0 |
8.9 0.009 |
| Anaphylaxis: Venom - hospitalizations - fatalities |
- 0.008 |
0.09 0.008 |
0.46 0.005 |
- 0.009 [26] 0.013 [27] |
- 0.009 [26] 0.010 [27] |
- 0.027 |
- 0.013 |
| Anaphylaxis: medication - hospitalizations - fatalities |
- 0.018 |
0.78 0.026 |
1.4 0.028 |
- 0.013 [26] 0.027 [27] |
- 0.013 [26] 0.051 [27] |
1.4^ 0.005 |
4.4 0.013 |
data for 1999
Despite a worldwide focus on increasing food allergy prevalence, the most frequent cause of reported anaphylaxis-related deaths across Europe, UK, North America and Australia is medication [25–31]. Although anaphylaxis to food is relatively common, fatal food-triggered anaphylaxis is rare, with a reported incidence of 1.35 to 2.71 per million person-years [34]. The community focus on food-allergy deaths may, perhaps, be explained by the fact that in children and young adults, food is the commonest cause of severe anaphylaxis and these deaths appear, at least superficially, to be the most preventable.
Food-anaphylaxis: populations and individuals at risk
Children and young adults are proportionately overrepresented in food-related anaphylaxis admission and fatalities. In the recent UK, US and Australian reports, young people under 19 years accounted for 4.8-15.8% of total fatalities, but only 0-3.4% of those due to medication deaths and 0-1.6% due to insect stings [25*,27*,28*]. The trend is similar for admissions [25*,26,28*]. Australia has a higher rate of reported hospitalization due to food-anaphylaxis than elsewhere. However, the rate of fatal food anaphylaxis is similar to the UK (Table 3), and the rates for both regions almost double that reported for the USA. It is interesting to speculate on the possible reasons for this. Accurate reporting of admissions data may be responsible for some differences. Food allergy appears higher per se in Australian children [35], and could account for the differences in admission rates, while perhaps lack of health insurance is a disincentive for hospitalization (rather than observation in Emergency only) in the USA. The differences in fatality rates – if not due to incorrect attribution of causality or miscoding – could be related to the differences in the management of anaphylaxis between regions, or possibly some underlying environmental protective factor. This reinforces the need to establish accurate national fatality registries for anaphylaxis-related deaths.
An emerging trend are the differences in risk for fatal anaphylaxis by racial/ethnic origin: African-American heritage was a significant risk factor for fatal anaphylaxis due to food more than other causes in the USA [27*], and has also been associated with increased asthma mortality [36]. Whether this is due to inequalities to healthcare or some predisposing factor is unclear. Within the UK Fatal Anaphylaxis Registry, there is an excess of deaths in male children due to cow’s milk allergy in families of African, Middle-East or Far-East descent [20]. In a retrospective analysis, Buka et al found a higher rate of severe anaphylaxis in British children from families of South Asian descent, an observation that was not due to confounding by socioeconomic deprivation [37]. Differences in the prevalence of peanut allergy have been observed amongst infants where parents are born in Asia versus Australia [38], although whether this is associated with a more severe phenotype is unclear.
Another emerging trend in fatal anaphylaxis to food has been a change in the triggering allergen: traditionally, peanut has been thought to cause most deaths, however other allergens are now seen to be as likely to cause severe reactions. In the UK, cow’s milk is now the commonest cause of fatal anaphylaxis in children [25*], while in Australia, seafood is now the most common food allergen implicated in fatal food-induced anaphylaxis, although peanuts were still the most common cause of death in children [28*].
A history of asthma is common in cases of fatal food anaphylaxis [25*], more so than for other causes of anaphylaxis [27*,28*]. However, in the UK registry, many cases of fatal food anaphylaxis did not have a history of increased symptoms or reliever use (implying a change in asthma control) prior to the fatal event. Around 50% of children [39,40] and up to 25% of adults [29,41] with food allergy have asthma, yet the vast majority will never have a fatal allergic reaction: thus, the utility of asthma as a predictor for fatal reactions is poor, although this does not negate the value of improving asthma control as a important strategy in risk management.
Venom
It is noteworthy that in North America and UK, the rates of severe anaphylaxis due to venom appear to be falling [25–27,33]. Rates of venom fatalities in Australia have been relatively stable since 1997, with no significant decrease, but importantly have not increased in line with food and medication-related fatalities [28*]. The reason(s) for this are unknown, but may be related to a falling population (“colony collapse”) for bees that has occurred over the last decade, something also now affecting the wasp population in the UK [42]. Species-specific data relating to the proportion of venom-anaphylaxis are lacking, and in any event it can be very difficult to identify the responsible insect for most cases of insect-related anaphylaxis. The Central European Registry reports wasp to be the most common trigger for venom-induced anaphylaxis, presumably this is on the basis of investigations performed as a work-up towards desensitisation [43]; however in Australia Honey Bee, not wasp, accounts for the majority of both venom immunotherapy prescriptions [44] and fatalites due to insect anaphylaxis [28*]. Curiously, venom is the most common cause of anaphylaxis in adults in the European Registry [29], despite the fact that the national datasets report anaphylaxis to medications are more common. This may be explained by the fact that many allergic reactions due to medications are not referred to allergy clinics, where healthcare professionals would then submit these data to the registry. In contrast, venom anaphylaxis is a common referral condition, due to the widespread availability of desensitisation protocols which can significantly reduce the adverse impact of the diagnosis on health-related quality of life [45,46].
In the USA [27*] and Australia [28*] fatal anaphylaxis to insect venom is significantly more common in middle aged adult men. In the USA it is further associated with being white and in Australia, a rural setting, and upright posture during anaphylaxis (e.g. being driven seated in a car to a health care facility) were identified as apparent significant risk factors for death from insect allergy [28*]. Upright posture, is likely to be a risk factor for other anaphylaxis related fatalities [20], excluding contrast and anaesthetic agent deaths (hence many anaphylaxis management plans specify keeping patients supine during treatment and observation). Its prominence in Australian insect fatalities are likely related to stings in remote (often rural/farm settings), where transportation to medical assistance is often not by ambulance in the first instance.
Drug / Iatrogenic causes
Defining the epidemiology of severe anaphylaxis for this group of triggers is particularly challenging, due to under-recognition, limited reporting and possible concerns as to medicolegal consequences. The majority of anaphylactic reactions due to iatrogenic causes occur in older age groups [24,26,28*,47], often in patients with co-morbidities such as coronary artery disease, obesity and chronic obstructive airways disease [28*]. Whether this is due to reverse causality is unknown: in a large series of anaphylaxis presenting to Emergency departments, cardiovascular disease (and use of anti-hypertensives) was significantly associated with age but conferred no additional predictive value for severe reactions on logistic regression analysis [48*]. A similar age-distribution is reported for Korea [49] and the UK: hospital admissions for drug-induced anaphylaxis increased from 0.78 to 1.4 per 100,000 population per annum over the period 1992-2012, predominantly in the 60+ age group. The mean age of fatal cases due to drugs/iatrogenic causes was 58 years (95% CI, 56-61 years), with fatalities rare in those under 40 [25*]. In the USA fatal drug-induced anaphylaxis was significantly more common in African Americans [27*].
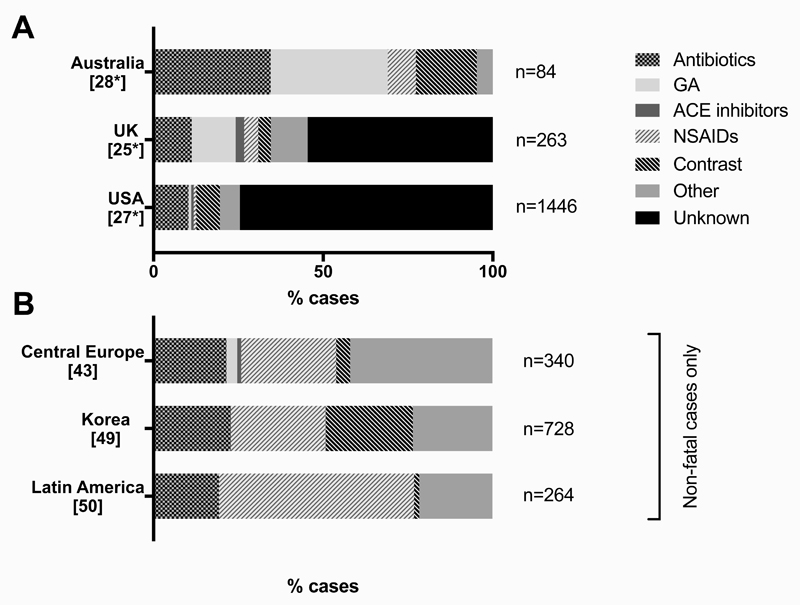
The literature indicates that antibiotics (particularly beta-lactams) and muscle-relaxants are the primary identified causes of medication-related fatal anaphylaxis (Figure 1). Non-steroidal anti-inflammatory agents (NSAIDs) appear to be common triggers for all-cause and severe anaphylaxis, but less so for fatal anaphylaxis. This may be due to a different route of exposure, with NSAIDs commonly taken via the oral route. In a cohort from Korea, drug-induced anaphylaxis was independently associated with more severe anaphylaxis than other causes [49], a finding seen elsewhere [51]. This is often assumed to be due to a parenteral route of exposure, however in a prospective Australian cohort of 315 cases of anaphylaxis presenting to the Emergency Department, both injected and oral drugs (predominantly antibiotics) were strongly predictive for hypotensive reactions [48*].
Figure 1. Triggering agents for medication-induced anaphylaxis from series of fatal reactions (A) and anaphylaxis registries (non-fatal reactions) (B) [from data in 50].
It is possible that many medication-related anaphylaxis hospitalizations and fatalities are coded as “unspecified cause” in registries (from all regions), resulting in significant underreporting of death and anaphylaxis from medications occurs. This again highlights the need for a coordinated approach to the collection of high quality accurate data, to inform prescribing practice and identify risk factors, so as to mitigate against serious allergic reactions and deaths.
Contrast agents are an important cause of anaphylaxis. Palmiere and Bonetti reviewed 34 fatalities due to contrast reported in the literature and a further 8 cases in their own hospital network [52]. Post-mortem findings were non-specific but consistent with an IgE-mediated mechanism. Of note, the vast majority of cases had their fatal event following a first exposure to the contrast agent: thus, the mechanism of primary sensitisation remains unknown.
Mast Cell Tryptase as a biomarker for anaphylaxis
There is currently no reliable sensitive and specific biomarker for anaphylaxis or for risk of anaphylaxis.. Anaphylaxis has been assumed to be largely mast cell or basophil mediated, usually involving an IgE-mediated mechanism, and although an elevated mast cell tryptase (MCT) is the most well known and used biomarker of recent anaphylaxis, it has poor sensitivity. Moreover, the value of MCT in attributing anaphylaxis as the cause of death as is hindered by lack of specificity in elevation of MCT post mortem [53]. Individuals with a raised baseline mast cell tryptase (MCT) are known to be at increased risk for severe reactions to venom [54,55]; in a retrospective analysis in patients attending an allergy clinic in Spain, Fellinger et al. reported a similar finding for other causes of anaphylaxis, although less that two thirds of cases were due to insect stings [56]. Whether a raised baseline MCT is an independent risk factor for anaphylaxis due to drugs is therefore unclear. Although Sahiner et al has proposed that baseline MCT is of value in predicting severity of reactions in a small cohort of food-allergic children, the significant overlap between baseline MCT levels limits the conclusions which can be made [57].
Learning from epidemiology?
It is noteworthy that in series of fatal anaphylaxis from the UK [25*], USA [27*] and Australia [28*], the age-distribution varies significantly according to the eliciting agent. This raises important questions about differences in the pathogenesis of anaphylaxis between different triggers. The symptoms of anaphylaxis also vary according to trigger in both fatal [20], severe (with hypoxemia and/or hypotension) [48*] and non-fatal reactions [58]. While cardiovascular compromise is common in severe reactions to drugs and insect venom [48*,58], this is uncommon in food-triggered reactions, where life-threatening manifestations are generally due to laryngopharyngeal and/or respiratory compromise; where cardiovascular arrest occurs, this is generally secondary to respiratory arrest [20]. Children with food-related anaphylaxis tend to present with respiratory and not cardiovascular symptoms [59]; this may give rise to delays in the use of epinephrine in those presenting with anaphylaxis [60].
On one level, the differences in symptoms with differing triggers might be predicted given the routes of exposure: parenteral allergens (e.g. venom, non-oral medication) result in rapidly systemic exposure, and therefore might be expected to cause cardiovascular involvement more than food allergens which need to be absorbed across the oro-gastrointestinal mucosa. However, this explanation may be too simplistic. Food allergens such as peanut can be rapidly absorbed through the buccal mucosa resulting in plasma levels sufficient to trigger a systemic effector cell response [61]. Furthermore, there is some evidence to suggest that severe allergic reactions to oral medication (such as antibiotics) frequently cause cardiovascular manifestations – in common with parentally-administered medication in terms of symptoms elicited – rather than respiratory involvement seen with food-triggered anaphylaxis, despite the fact that gastrointestinal absorption is still required [48*]. The epidemiological data therefore raise the possibility that food-induced anaphylaxis might not have the same pathophysiologic basis as anaphylaxis caused by other triggers (Table 4). In this context, it is interesting to note that MCT is often not increased in severe/fatal food-induced anaphylaxis [62,63] and when observed, the increase is generally more modest than that seen in severe anaphylaxis due to other, non-food triggers [48*,63]. Evaluating this prospectively through mechanistic assessments might lead to important advances in our understanding of determinants of severity in food-allergic individuals.
Table 4. Differences in the epidemiology and pathophysiology of anaphylaxis due to food versus non-food causes.
| Food | Medication / iatrogenic causes | Venom sting | |
|---|---|---|---|
| Age distribution: anaphylaxis (all severity) | Most common in preschool children, less common in older adults | Predominantly older ages | All ages |
| Age distribution: fatal anaphylaxis | Young adults into 4th decade of life. Rare in younger children. | Unusual until 5th decade of life. | 4th to 6th decade |
| Symptoms | Respiratory | Cardiovascular (respiratory less common) | Cardiovascular (respiratory less common) |
| Asthma/atopy | Common | Uncommon | Uncommon |
| Onset | Less rapid | Rapid | Rapid |
| Site of Antigen presentation | Usually orogastric route | Usually parenteral route | Parenteral |
| Triggering threshold dose | ++ interperson variability (up to 4 log) | Poor data for medications | Less variability for insect stings |
| Mechanism | No or relatively modest increases in MCT generally observed | Increased MCT often seen | Increased MCT often seen |
| Sex | M=F | M=F | M>>F |
| Ethnic distribution | ? higher risk in persons of Asian decent ? more common in male children of African American decent |
More common in persons of African American decent | More common in Caucasians |
Conclusion and future perspectives
Anaphylaxis is not uncommon and appears to be increasing in North American, UK, and Australia. Fatal anaphylaxis remains a rare occurrence, but the fear of such a reaction, particularly to food triggers, is widespread. Lack of universal definitions, underreporting, miscoding, misdiagnosis and lack of robust national data collecting systems hinders the interpretation of current data. Our current lack of knowledge about the mechanisms of anaphylaxis further impairs our capacity to understand why different allergic triggers are associated with different presentations, risk factors and age/sex susceptibilities.
Given the worldwide trends for increasing rates of anaphylaxis to food and medication, there are significant implications for future fatality trends with an ageing population accumulating comorbidities such as cardiovascular disease and diabetes. An improved ability to accurately gather and analyse population-level anaphylaxis and fatal anaphylaxis data in a harmonised fashion is required so as to ultimately improved risk assessment and management.
Key Points.
Anaphylaxis is increasing but fatal anaphylaxis remains rare
Medications appear to be the most common cause of anaphylaxis admissions and fatalities in the UK, US and Australia
Triggers such as medications, foods and insect venoms are associated with specific demographic patterns and clinical presentations
Large data sets are hindered by lack of robust utilised coding systems, with underreporting, miscoding and many cases of “unspecified” triggers in admissions and fatality registers
Acknowledgements
Financial Support and Sponsorship
PJT is in receipt of a Clinician Scientist award funded by the UK Medical Research Council (reference MR/K010468/1), and is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or the Department of Health.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Simons FE, Ardusso LR, Bilò MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014;7:9. doi: 10.1186/1939-4551-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doeing DC, Borowicz JL, Crockett ET. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin Pathol. 2003;3:3. doi: 10.1186/1472-6890-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 4.Finkelman FD, Rothenberg ME, Brandt EB, et al. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–457. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- 5.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol. 2016;137:1674–80. doi: 10.1016/j.jaci.2016.02.015. [A recent review assessing the relevance of IgG-dependent anaphylaxis observed in murine models to human anaphylaxis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smit JJ, Willemsen K, Hassing I, et al. Contribution of Classic and Alternative Effector Pathways in Peanut-Induced Anaphylactic Responses. PLoS ONE. 2011;6(12):e28917. doi: 10.1371/journal.pone.0028917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen KJ, Remington BC, Baumert JL, Crevel RW, Houben GF, Brooke-Taylor S, Kruizinga AG, Taylor SL. Allergen reference doses for precautionary labeling (VITAL 2.0): clinical implications. J Allergy Clin Immunol. 2014;133:156–164. doi: 10.1016/j.jaci.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Bergman RK, Munoz JJ. Increased histamine sensitivity in mice after administration of endotoxins. Infect Immun. 1977;15:72–77. doi: 10.1128/iai.15.1.72-77.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaliner M, Shelhamer JH, Ottesen EA. Effects of infused histamine: correlation of plasma histamine levels and symptoms. J Allergy Clin Immunol. 1982;69:283–289. doi: 10.1016/s0091-6749(82)80005-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang M, Han J, Domenico J, et al. Combined Blockade of the Histamine H1 and H4 Receptor Suppresses Peanut-Induced Intestinal Anaphylaxis by Regulating Dendritic Cell Function. Allergy. 2016 doi: 10.1111/all.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheikh A, Ten Broek V, Brown SG, Simons FE. H1-antihistamines for the treatment of anaphylaxis: Cochrane systematic review. Allergy. 2007;62:830–837. doi: 10.1111/j.1398-9995.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu C, Wilson SJ, Kuei C, Lovenberg TW. Comparison of human, mouse, rat, and guinea pig histamine H4 receptors reveals substantial pharmacological species variation. J Pharmacol Exp Ther. 2001;299:121–130. [PubMed] [Google Scholar]
- 13.Tsujimura Y, Obata K, Mukai K, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Betz SJ, Lotner GZ, Henson PM. Generation and release of platelet-activating factor (PAF) from enriched preparations of rabbit basophils; failure of human basophils to release PAF. J Immunol. 1980;125:2749–2755. [PubMed] [Google Scholar]
- 15.Panesar SS, Javad S, de Silva D, et al. on behalf of the EAACI Food Allergy and Anaphylaxis Group The epidemiology of anaphylaxis in Europe: a systematic review. Allergy. 2013;68:1353–1361. doi: 10.1111/all.12272. [DOI] [PubMed] [Google Scholar]
- 16.Simons FER, Ardusso LRF, Bilò MB, et al. World Allergy Organization Guidelines for the Assessment and Management of Anaphylaxis. The World Allergy Organization Journal. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieberman P, Nicklas RA, Oppenheimer J, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477–480. doi: 10.1016/j.jaci.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Brown SG, Mullins RJ, Gold MS. Anaphylaxis: diagnosis and management. Med J Aust. 2006;185:283–289. doi: 10.5694/j.1326-5377.2006.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 19.Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report – Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117:391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 20.Pumphrey RSH. An epidemiological approach to reducing the risk of fatal anaphylaxis. In Anaphylaxis and hypersensitivity reactions. In: Castells MC, editor. Anaphylaxis and Hypersensitivity Reactions. New York, Dordrecht, Heidelberg, London: Springer Science Ltd, Humana Press; 2011. pp. 13–31. Asthma and COPD. [Google Scholar]
- 21.Noimark L, Wales J, Du Toit G, et al. The use of adrenaline autoinjectors by children and teenagers. Clin Exp Allergy. 2012;42:284–292. doi: 10.1111/j.1365-2222.2011.03912.x. [A survey which reported most children and young adults experiencing anaphylaxis recover from the event despite not using appropriate rescue medication.] [DOI] [PubMed] [Google Scholar]
- 22.Turner PJ, Baumert JL, Beyer K, et al. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy. 2016 doi: 10.1111/all.12924. [A state-of-the-art review assessing the evidence-base for factors which healthcare professionals often use to try and risk-stratify food-allergic individuals. The risks involved in mis-interpreting the literature are discussed, with reference to current international efforts to reduce risk of severe food-allergic reactions from unintended allergen exposure.] [DOI] [PubMed] [Google Scholar]
- 23.Umasunthar T, Leonardi-Bee J, Turner PJ, et al. Incidence of food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2015;45:1621–1636. doi: 10.1111/cea.12477. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee M, Wyatt JC, Simpson CR, Sheikh A. Usage of allergy codes in primary care electronic health records: a national evaluation in Scotland. Allergy. 2016 doi: 10.1111/all.12928. [DOI] [PubMed] [Google Scholar]
- 25.Turner PJ, Gowland MH, Sharma V, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol. 2015;135:956–963.e1. doi: 10.1016/j.jaci.2014.10.021. [A report of time-trends in anaphylaxis (hospitalizations and fatal cases) over 20 years, using national health datasets and a large prospective fatal anaphylaxis registry.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L, Danoff TM, Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:1075–1083. doi: 10.1016/j.jaci.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jerschow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014;134:1318–1328.e7. doi: 10.1016/j.jaci.2014.08.018. [Time-trends in fatal anaphylaxis in the USA, using national databases.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullins RJ, Wainstein BK, Barnes EH, et al. Increases in anaphylaxis fatalities in Australia 1997 to 2013. Clin Exp Allergy. 2016 doi: 10.1111/cea.12748. [An analysis of fatal anaphylaxis datasets in Australia, incorporating case verification] [DOI] [PubMed] [Google Scholar]
- 29.Worm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA) Allergy. 2014;69:1397–1404. doi: 10.1111/all.12475. [DOI] [PubMed] [Google Scholar]
- 30.Grabenhenrich LB, Dölle S, Moneret-Vautrin A, et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137:1128–1137.e1. doi: 10.1016/j.jaci.2015.11.015. [A series of 1970 cases of paediatric anaphylaxis from 70 different centres in 10 European countries.] [DOI] [PubMed] [Google Scholar]
- 31.Canadian Institute for Health Information. Anaphylaxis and Allergy in the Emergency Department. [Accessed 5 June 2016];2015 Available at: https://www.cihi.ca/sites/default/files/document/anaphylaxis_infosheet_enweb.pdf.
- 32.Mullins RJ, Dear KB, Tang ML. Time trends in Australian hospital anaphylaxis admissions in 1998-1999 to 2011-2012. J Allergy Clin Immunol. 2015;136:367–375. doi: 10.1016/j.jaci.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Xu YS, Kastner M, Harada L, et al. Anaphylaxis-related deaths in Ontario: a retrospective review of cases from 1986 to 2011. Allergy Asthma Clin Immunol. 2014;10:38. doi: 10.1186/1710-1492-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umasunthar T, Leonardi-Bee J, Hodes M, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43:1333–1341. doi: 10.1111/cea.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prescott SL, Pawankar R, Allen KJ, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6(1):21. doi: 10.1186/1939-4551-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;94:1–8. [PubMed] [Google Scholar]
- 37.Buka RJ, Crossman RJ, Melchior CL, et al. Anaphylaxis and ethnicity: higher incidence in British South Asians. Allergy. 2015;70:1580–1587. doi: 10.1111/all.12702. [DOI] [PubMed] [Google Scholar]
- 38.Koplin JJ, Peters RL, Ponsonby AL, et al. Increased risk of peanut allergy in infants of Asian-born parents compared to those of Australian-born parents. Allergy. 2014;69:1639–1647. doi: 10.1111/all.12487. [DOI] [PubMed] [Google Scholar]
- 39.Clark AT, Ewan PW. Good prognosis, clinical features, and circumstances of peanut and tree nut reactions in children treated by a specialist allergy center. J Allergy Clin Immunol. 2008;122:286–289. doi: 10.1016/j.jaci.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Rudders SA, Banerji A, Vassallo MF, et al. Trends in pediatric emergency department visits for food-induced anaphylaxis. J Allergy Clin Immunol. 2010;126:385–388. doi: 10.1016/j.jaci.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Asai Y, Yanishevsky Y, Clarke A, et al. Rate, triggers, severity and management of anaphylaxis in adults treated in a Canadian emergency department. Int Arch Allergy Immunol. 2014;164:246–252. doi: 10.1159/000365631. [DOI] [PubMed] [Google Scholar]
- 42.Who, what, why: Where are all the wasps? [Accessed 28 May 2016]; http://www.bbc.co.uk/news/magazine-23356578.
- 43.Worm M, Eckermann O, Dölle S, et al. Triggers and treatment of anaphylaxis: an analysis of 4000 cases from Germany, Austria and Switzerland. Dtsch Arztebl Int. 2014;111:367–75. doi: 10.3238/arztebl.2014.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Department of Human Services AG. Pharmaceutical Benefits Schedule Item Reports. [Accessed 5 June 2016]; Available at http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp.
- 45.Boyle RJ, Elremeli M, Hockenhull J, et al. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012 Oct 17;10 doi: 10.1002/14651858.CD008838.pub2. CD008838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paolocci G, Folletti I, Torén K, et al. Hymenoptera venom allergy: work disability and occupational impact of venom immunotherapy. BMJ Open. 2014;4:e005593. doi: 10.1136/bmjopen-2014-005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clark S, Wei W, Rudders SA, Camargo CA., Jr Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J Allergy Clin Immunol. 2014;134:1125–1130. doi: 10.1016/j.jaci.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 48.Brown SG, Stone SF, Fatovich DM, et al. Anaphylaxis: clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013;132:1141–1149.e5. doi: 10.1016/j.jaci.2013.06.015. [A unique observational study describing the symptoms, pattern of severity and correlates with biomarkers in 315 patients with anaphylaxis presenting to Emergency Departments in Australia] [DOI] [PubMed] [Google Scholar]
- 49.Ye Y-M, Kim MK, Kang H-R, et al. Predictors of the Severity and Serious Outcomes of Anaphylaxis in Korean Adults: A Multicenter Retrospective Case Study. Allergy, Asthma & Immunology Research. 2015;7:22–29. doi: 10.4168/aair.2015.7.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jares EJ, Baena-Cagnani CE, Sánchez-Borges M, et al. Drug-Induced Anaphylaxis in Latin American Countries. J Allergy Clin Immunol Pract. 2015;3:780–788. doi: 10.1016/j.jaip.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Nassiri M, Babina M, Dölle S, et al. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol. 2015;135:491–499. doi: 10.1016/j.jaci.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Palmiere C, Reggiani Bonetti L. Risk factors in fatal cases of anaphylaxis due to contrast media: a forensic evaluation. Int Arch Allergy Immunol. 2014;164:280–288. doi: 10.1159/000366204. [DOI] [PubMed] [Google Scholar]
- 53.McLean-Tooke A, Goulding M, Bundell C, et al. Postmortem serum tryptase levels in anaphylactic and non-anaphylactic deaths. J Clin Pathol. 2014;67:134–138. doi: 10.1136/jclinpath-2013-201769. [DOI] [PubMed] [Google Scholar]
- 54.Ruëff F, Przybilla B, Biló MB, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase: a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J Allergy Clin Immunol. 2009;124:1047–1054. doi: 10.1016/j.jaci.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 55.Borer-Reinhold M, Haeberli G, Bitzenhofer M, et al. An increase in serum tryptase even below 11.4 ng/mL may indicate a mast cell-mediated hypersensitivity reaction: a prospective study in Hymenoptera venom allergic patients. Clin Exp Allergy. 2011;41:1777–1783. doi: 10.1111/j.1365-2222.2011.03848.x. [DOI] [PubMed] [Google Scholar]
- 56.Fellinger C, Hemmer W, Wöhrl S, et al. Clinical characteristics and risk profile of patients with elevated baseline serum tryptase. Allergol Immunopathol (Madr) 2014;42:544–552. doi: 10.1016/j.aller.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Sahiner UM, Yavuz ST, Buyuktiryaki B, et al. Serum basal tryptase may be a good marker for predicting the risk of anaphylaxis in children with food allergy. Allergy. 2014;69:265–268. doi: 10.1111/all.12317. [DOI] [PubMed] [Google Scholar]
- 58.Worm M, Edenharter G, Ruëff F, et al. Symptom profile and risk factors of anaphylaxis in Central Europe. Allergy. 2012;67:691–698. doi: 10.1111/j.1398-9995.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 59.de Silva IL, Mehr SS, Tey D, Tang ML. Paediatric anaphylaxis: a 5 year retrospective review. Allergy. 2008;63:1071–1076. doi: 10.1111/j.1398-9995.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 60.O'Leary FM, Hokin B, Enright K, Campbell DE. Treatment of a simulated child with anaphylaxis: an in situ two-arm study. J Paediatr Child Health. 2013 Jul;49(7):541–7. doi: 10.1111/jpc.12276. [DOI] [PubMed] [Google Scholar]
- 61.Dirks CG, Pedersen MH, Platzer MH, et al. Does absorption across the buccal mucosa explain early onset of food-induced allergic systemic reactions? J Allergy Clin Immunol. 2005;115:1321–1323. doi: 10.1016/j.jaci.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 62.Lin RY, Schwartz LB, Curry A, et al. Histamine and tryptase levels in patients with acute allergic reactions: An emergency department-based study. J Allergy Clin Immunol. 2000;106:65–71. doi: 10.1067/mai.2000.107600. [DOI] [PubMed] [Google Scholar]
- 63.Sala-Cunill A, Cardona V, Labrador-Horrillo M, et al. Usefulness and limitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int Arch Allergy Immunol. 2013;160:192–199. doi: 10.1159/000339749. [DOI] [PubMed] [Google Scholar]