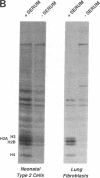
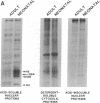
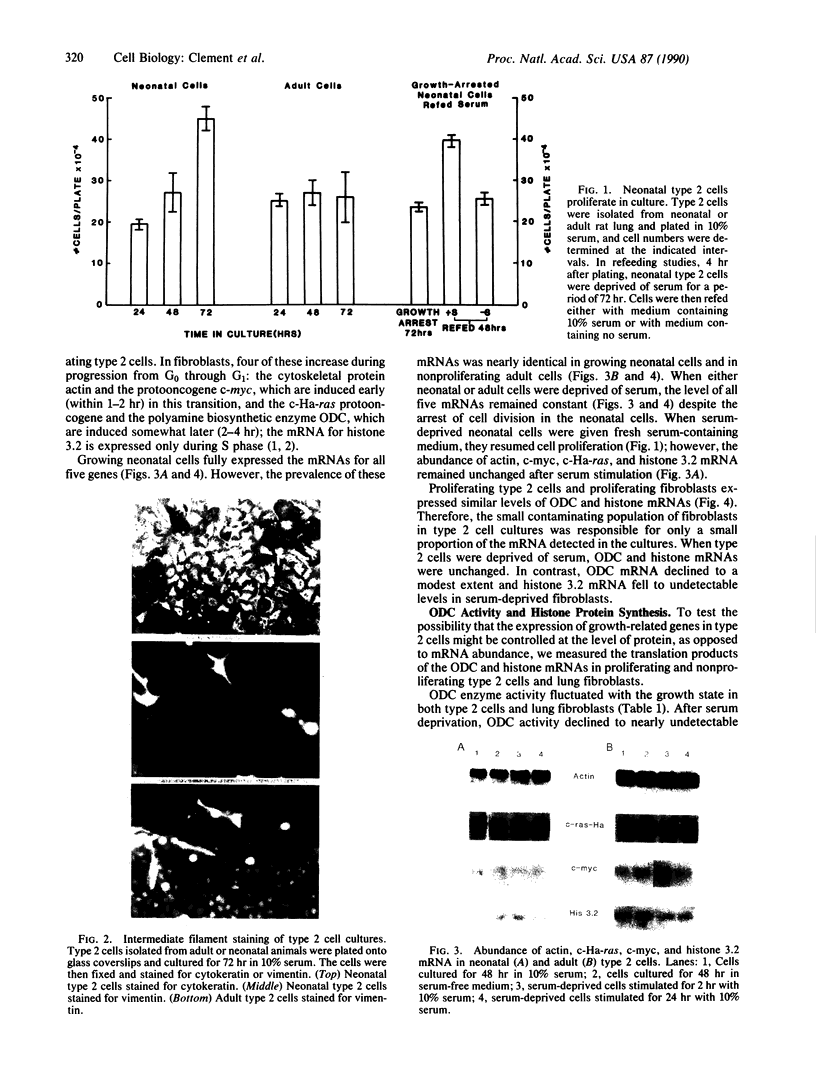
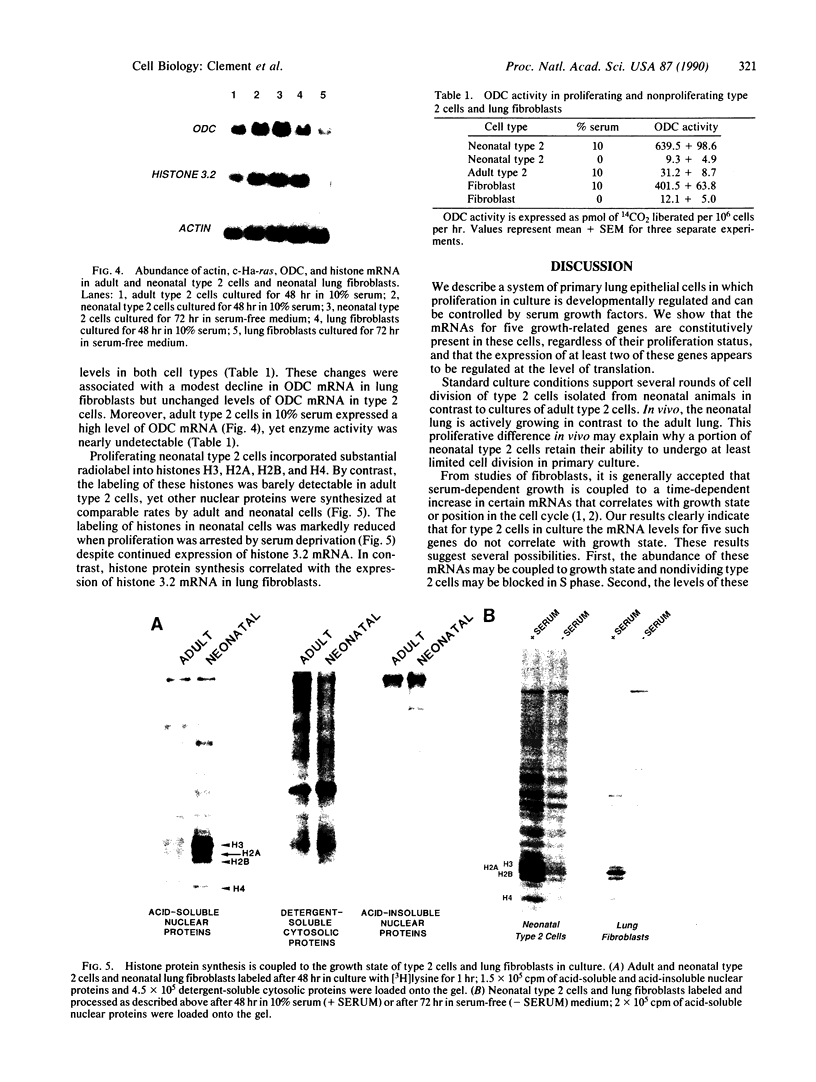
Abstract
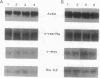
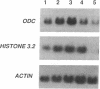
We describe the control of proliferation and growth-related gene expression in primary cultures of epithelial cells derived from rat lung. Type 2 epithelial cells line the gas-exchange surface of the alveoli where they produce and secrete surfactant. When isolated from adult animals, type 2 cells do not proliferate in culture, although they have a limited ability to do so in vivo. We show that type 2 cells isolated from neonatal rats proliferate in culture and that growth can be reversibly arrested by withdrawing serum from the medium. We studied the expression of five genes whose mRNA levels fluctuate with the state of proliferation in several cell systems: the c-myc and c-Ha-ras protooncogenes and the genes encoding actin, ornithine decarboxylase (L-ornithine carboxy-lyase, EC 4.1.1.17), and histone 3.2. All five mRNAs were constitutively expressed at identical levels in proliferating and nonproliferating (serum deprived) neonatal cells and in adult cells. Thus, at the level of mRNA abundance, the expression of these five genes was uncoupled from the growth state of the cells. By contrast, synthesis of the replication-dependent histones and the activity of ornithine decarboxylase were detectable only in proliferating neonatal cells and not in serum-deprived neonatal cells or in adult cells. The results suggest that, in type 2 cells, growth factors might regulate the translation, rather than the mRNA abundance, of at least some growth-related genes and that the ability to respond to this translational control may be developmentally regulated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Brody J. S., Burki R., Kaplan N. Deoxyribonucleic acid synthesis in lung cells during compensatory lung growth after pneumonectomy. Am Rev Respir Dis. 1978 Feb;117(2):307–316. doi: 10.1164/arrd.1978.117.2.307. [DOI] [PubMed] [Google Scholar]
- Chen K., Heller J., Canellakis E. S. Studies on the regulation of ornithine decarboxylase activity by the microtubules: the effect of colchicine and vinblastine. Biochem Biophys Res Commun. 1976 Jan 26;68(2):401–408. doi: 10.1016/0006-291x(76)91159-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Greenspan S. Similarities in ornithine decarboxylase regulation in intact and enucleated 3T3 cells. Exp Cell Res. 1979 Feb;118(2):253–260. doi: 10.1016/0014-4827(79)90150-2. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Edwards D. R., Parfett C. L. Gene expression during the mammalian cell cycle. Biochim Biophys Acta. 1986 Oct 28;865(2):83–125. doi: 10.1016/0304-419x(86)90024-7. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Gonzalez R., Williams M. C. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis. 1986 Jul;134(1):141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Gilman M. Z., Maruyama M., Weinberg R. A. c-myc and c-fos expression in differentiating mouse primary keratinocytes. EMBO J. 1986 Nov;5(11):2853–2857. doi: 10.1002/j.1460-2075.1986.tb04579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppi V. E., Jr, Coffino P. G1 and S phase mammalian cells synthesize histones at equivalent rates. Cell. 1980 Aug;21(1):195–204. doi: 10.1016/0092-8674(80)90127-0. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Thompson C. B., Eisenman R. N. c-myc oncogene protein synthesis is independent of the cell cycle in human and avian cells. 1985 Mar 28-Apr 3Nature. 314(6009):366–369. doi: 10.1038/314366a0. [DOI] [PubMed] [Google Scholar]
- Hirvonen A. Ornithine decarboxylase activity and the accumulation of its mRNA during early stages of liver regeneration. Biochim Biophys Acta. 1989 Jan 23;1007(1):120–123. doi: 10.1016/0167-4781(89)90140-1. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Calabretta B., Baserga R. Expression of cell-cycle-dependent genes in phytohemagglutinin-stimulated human lymphocytes. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5375–5379. doi: 10.1073/pnas.82.16.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Translational regulation of mammalian ornithine decarboxylase by polyamines. J Biol Chem. 1985 Dec 15;260(29):15390–15393. [PubMed] [Google Scholar]
- Kauffman S. L., Burri P. H., Weibel E. R. The postnatal growth of the rat lung. II. Autoradiography. Anat Rec. 1974 Sep;180(1):63–76. doi: 10.1002/ar.1091800108. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Skelly H., Botteri F., van der Putten H., Barber J. R., Verma I. M., Leffert H. L. Proto-oncogene expression in regenerating liver is simulated in cultures of primary adult rat hepatocytes. J Biol Chem. 1986 Jun 15;261(17):7929–7933. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., McCormick-Shannon K., Robinson P. C., Mason R. J. Stimulation of DNA synthesis in cultured rat alveolar type II cells. Exp Lung Res. 1985;8(1):53–66. doi: 10.3109/01902148509069679. [DOI] [PubMed] [Google Scholar]
- Maksvytis H. J., Niles R. M., Simanovsky L., Minassian I. A., Richardson L. L., Hamosh M., Hamosh P., Brody J. S. In vitro characteristics of the lipid-filled interstitial cell associated with postnatal lung growth: evidence for fibroblast heterogeneity. J Cell Physiol. 1984 Feb;118(2):113–123. doi: 10.1002/jcp.1041180203. [DOI] [PubMed] [Google Scholar]
- McCaffrey P., Ran W., Campisi J., Rosner M. R. Two independent growth factor-generated signals regulate c-fos and c-myc mRNA levels in Swiss 3T3 cells. J Biol Chem. 1987 Feb 5;262(4):1442–1445. [PubMed] [Google Scholar]
- McConlogue L., Dana S. L., Coffino P. Multiple mechanisms are responsible for altered expression of ornithine decarboxylase in overproducing variant cells. Mol Cell Biol. 1986 Aug;6(8):2865–2871. doi: 10.1128/mcb.6.8.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepveu A., Levine R. A., Campisi J., Greenberg M. E., Ziff E. B., Marcu K. B. Alternative modes of c-myc regulation in growth factor-stimulated and differentiating cells. Oncogene. 1987;1(3):243–250. [PubMed] [Google Scholar]
- Paine R., Ben-Ze'ev A., Farmer S. R., Brody J. S. The pattern of cytokeratin synthesis is a marker of type 2 cell differentiation in adult and maturing fetal lung alveolar cells. Dev Biol. 1988 Oct;129(2):505–515. doi: 10.1016/0012-1606(88)90396-x. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Persson L., Holm I., Heby O. Translational regulation of ornithine decarboxylase by polyamines. FEBS Lett. 1986 Sep 15;205(2):175–178. doi: 10.1016/0014-5793(86)80892-4. [DOI] [PubMed] [Google Scholar]
- Ran W., Dean M., Levine R. A., Campisi J. Activation of proto-oncogene expression by growth regulatory signals. Curr Top Microbiol Immunol. 1986;132:313–319. doi: 10.1007/978-3-642-71562-4_46. [DOI] [PubMed] [Google Scholar]
- Smith L. J., Brody J. S. Influence of methylprednisolone on mouse alveolar type 2 cell response to acute lung injury. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):459–464. doi: 10.1164/arrd.1981.123.4.459. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday J., Carson L., Lawson E. E. Saturated phosphatidylcholine in amniotic fluid and prediction of the respiratory-distress syndrome. N Engl J Med. 1979 Nov 8;301(19):1013–1018. doi: 10.1056/NEJM197911083011901. [DOI] [PubMed] [Google Scholar]
- Van Golde L. M., Batenburg J. J., Robertson B. The pulmonary surfactant system: biochemical aspects and functional significance. Physiol Rev. 1988 Apr;68(2):374–455. doi: 10.1152/physrev.1988.68.2.374. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]