Abstract
The association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk has been widely investigated, but remains controversial. We therefore undertook a comprehensive meta-analysis to provide a high-quality evaluation of this association. A literature search was performed among Pubmed, EMBASE and Chinese National Knowledge Infrastructure (CNKI) databases prior to July 31, 2016, and the strength of the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk was assessed based on odds ratio (OR) and 95% confidence interval (95% CI). In total, 12 studies with 50,525 cases and 54,302 controls were included. Pooled risk estimates indicated a significant association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk. Analysis of cases stratified based on ethnicity suggested that the association was significant in both Caucasian and Asian populations. Stratification based on source of controls revealed an association only in population-based studies. These findings indicate the LSP1 rs3817198 T > C polymorphism is associated with increased risk of breast cancer, especially in Caucasian and Asian populations. Large, well-designed studies with different ethnicities are still needed to verify our findings.
Keywords: LSP1, breast cancer, risk, meta-analysis
INTRODUCTION
In 2012, there were approximately 1.7 million newly diagnosed breast cancer patients and 521,900 deaths, accounting for 25% of all new cancer cases and 15% of all cancer-related death in women [1]. Despite the prevalence and severity of breast cancer, the exact mechanism underlying the initiation and progression of breast cancer is still not fully understood. Breast cancer is caused by the interaction of various environmental and genetic risk factors [2, 3]. Environmental variables, such as reproductive factors, hormonal stimulation, high birth weight, obesity, physical inactivity, and alcohol consumption are well-established breast cancer risk factors [4–6]. Moreover, germline mutations in some highly and moderately penetrant genes, including BRCA1, BRCA2, PTEN, TP53, CHEK2, ATM, BRIP1 and PALB2, are associated with high and moderate risk of breast cancer [7, 8]. However, mutations in these genes only explain 25% of breast cancer risk [9]. Single nucleotide polymorphisms (SNPs) in some genes can alter mRNA and protein expression or protein function, and thereby influence cancer susceptibility. A recent genome wide association study (GWAS) has discovered SNPs in 5 lowly penetrant genes as additional susceptibility factors with high frequency, and validated their strong association with breast cancer [10].
One of these genes, lymphocyte-specific protein 1 (LSP1), is located on chromosome 11p15.5. It encodes an F-actin bundling cytoskeletal protein expressed in hematopoietic and endothelial cells [8, 10]. Many polymorphisms in the LSP1 gene have been identified, and one of the most common polymorphisms, the LSP1 s3817198 T > C, has been widely studied for its potential association with breast cancer risk. Several publications have reported a significant association of the LSP1 rs3817198 T > C polymorphism with the risk of breast cancer [11–13]. However, other studies have failed to replicate such an association [14–16]. Chen et al. [17] conducted a meta-analysis in 2010, and concluded that the LSP1 rs3817198 T > C polymorphism was significantly correlated with breast cancer risk. However, only seven studies were available at that time. Since then, some new case-control studies evaluating the association have emerged [13, 16, 18–20]. Therefore, we performed an updated meta-analysis to provide a more comprehensive and accurate assessment of the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk.
RESULTS
Study characteristics
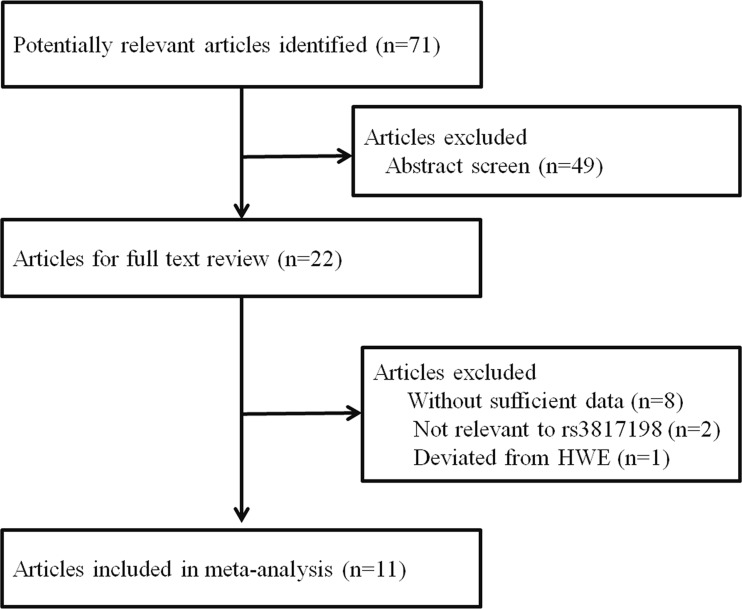
As shown in Figure 1, a total of 71 articles were found from Pubmed, EMBASE, and Chinese National Knowledge Infrastructure (CNKI) databases with the use of specific search terms. Of these, 49 articles were excluded after reviewing the titles and abstracts. The remaining 22 articles were subsequently evaluated for full-text review. Another 11 articles were excluded because they lacked sufficient data, were not relevant to the rs3817198 polymorphism, or not in compliance with Hardy-Weinberg equilibrium (HWE). Finally, 11 articles were eligible for the meta-analysis [11–14, 16, 18–23]. Among them, one article reported the association separately in both Caucasian and African populations, thus we extracted two independent studies from the investigation [11]. In the end, 11 articles with 12 studies, comprising 50,525 cases and 54,302 controls were included in our meta-analysis. As listed in Table 1, 6 studies were conducted in Caucasians, 3 in Asians, 1 in Africans, and 2 in mixed populations. Of the 12 studies, 7 were population-based, 2 were hospital-based, and 3 were nested. The genotype frequency distribution of the LSP1 rs3817198 T > C polymorphism in controls was in compliance with HWE in all studies. Furthermore, 10 articles were considered high quality (quality score ≥ 9), and only 2 were considered low quality (quality score < 9).
Figure 1. Flowchart of articles included in the meta-analysis.
Table 1. Characteristics of included studies in this meta-analysis.
| Surname | Year | Country | Ethnicity | Source of control | N (cases/controls) | MAF | HWE | Score | |
|---|---|---|---|---|---|---|---|---|---|
| Antoniou | 2008 | UK | Caucasian | Nested | 7811 | 6607 | 0.32 | 0.413 | 9 |
| Garcia-Closas | 2008 | USA | Mixed | Nested | 22397 | 26012 | 0.30 | 0.398 | 9 |
| Barnholtz-Sloan | 2010 | USA | African | PB | 742 | 658 | 0.17 | 0.157 | 14 |
| Barnholtz-Sloan | 2010 | USA | Caucasian | PB | 1228 | 1117 | 0.31 | 0.332 | 14 |
| Gorodnova | 2010 | Russia | Caucasian | PB | 140 | 174 | 0.28 | 0.856 | 11 |
| Latif | 2010 | UK | Caucasian | HB | 922 | 366 | 0.33 | 0.938 | 12 |
| Tamimi | 2010 | USA | Caucasian | PB | 680 | 737 | 0.29 | 0.400 | 13 |
| Long | 2010 | China | Asian | PB | 6435 | 3839 | 0.12 | NA | 8 |
| Campa | 2011 | Germany | Mixed | Nested | 8292 | 11558 | 0.30 | 0.779 | 7 |
| Jiang | 2011 | China | Asian | PB | 492 | 510 | 0.14 | 0.078 | 12 |
| Butt | 2012 | Sweden | Caucasian | PB | 689 | 1330 | 0.29 | 0.579 | 9 |
| Sueta | 2012 | Japan | Asian | HB | 697 | 1394 | 0.15 | 0.367 | 11 |
PB, population based; HB, hospital based; MAF, Minor Allele Frequency; HWE, Hardy-Weinberg equilibrium; NA, not available.
Meta-analysis results
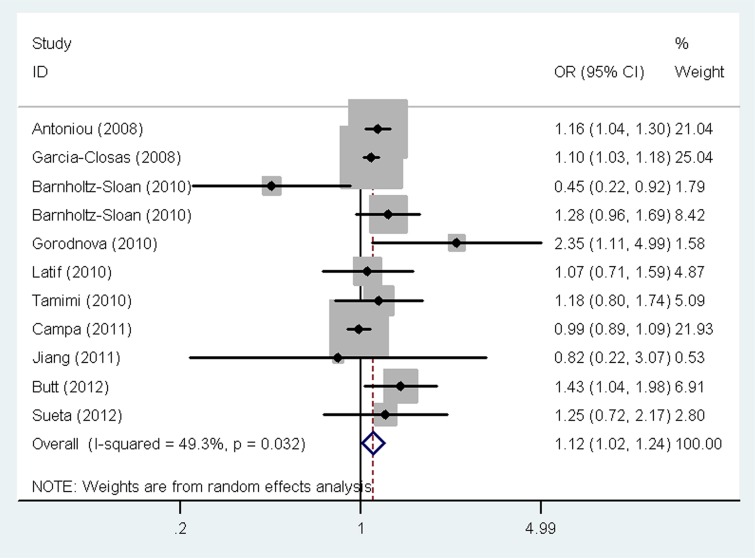
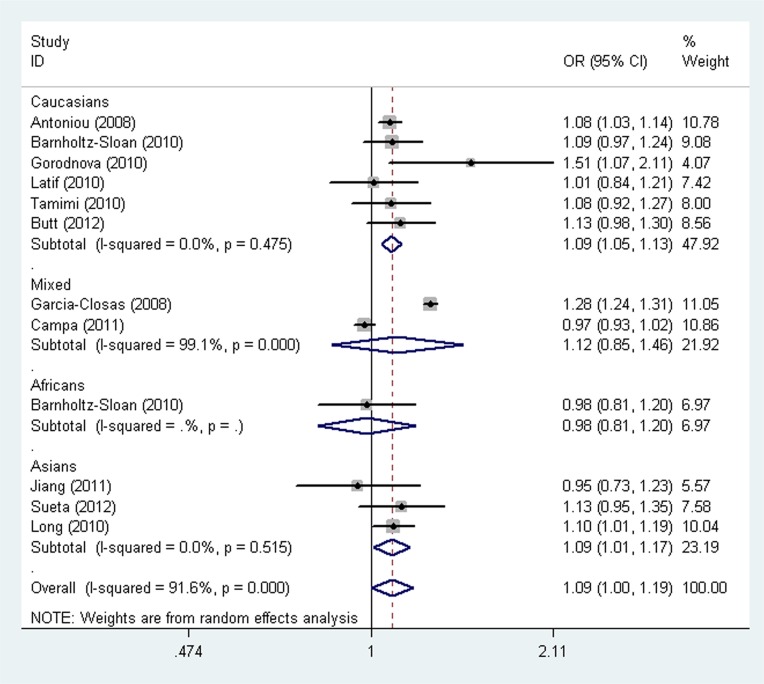
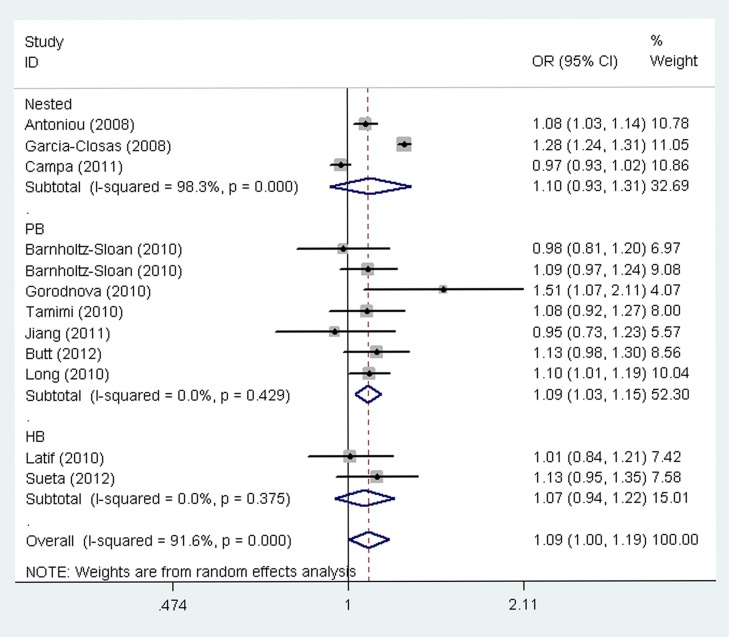
The main results of the meta-analysis for the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk are listed in Table 2. Pooled analysis indicated that there was a significant association between the LSP1 rs3817198 T > C polymorphism and increased breast cancer risk (homozygous model (CC vs. TT): odds ratio (OR) = 1.12, 95% confidence interval (CI) = 1.02–1.24, P = 0.021, Figure 2; as well as comparison of allele frequencies (C vs. T): OR = 1.09, 95% CI = 1.00–1.19, P = 0.039). Stratified analysis by ethnicity revealed an increased risk of breast cancer associated with rs3817198 T > C in Caucasian populations (homozygous model: OR = 1.21, 95% CI = 1.10–1.32, P < 0.001; heterozygous model (TC vs. TT): OR = 1.07, 95% CI = 1.01–1.13, P = 0.017; recessive model (CC vs. TC + TT): OR = 1.16, 95% CI = 1.07–1.27, P = 0.001; dominant model (TC +CC vs. TT): OR = 1.10, 95% CI = 1.04–1.16, P = 0.001; as well as comparison of allele frequencies: OR = 1.09, 95% CI = 1.05–1.13, P < 0.001, Figure 3), and also in Asian populations (comparison of allele frequencies model: OR = 1.09, 95% CI = 1.01–1.17, P = 0.023, Figure 3). Additionally, in the stratified analysis by source of controls, it was noted that the LSP1 rs3817198 variant allele (C) was significantly associated with an increased breast cancer risk in population-based studies (comparison of allele frequencies model: OR = 1.09, 95% CI = 1.03–1.15, P = 0.001, Figure 4).
Table 2. Results of meta-analysis for the association between LSP1 rs1817198 T > C polymorphism and breast cancer risk.
| Variables | N (cases/controls) | Homozygous | Heterozygous | Recessive | Dominant | Allele | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC vs. TT | TC vs. TT | CC vs. (TC + TT) | (TC +CC) vs. TT | C vs. T | ||||||||||||
| OR (95% CI) | Phet | I2 (%) | OR (95% CI) | Phet | I2 (%) | OR (95% CI) | Phet | I2 (%) | OR (95% CI) | Phet | I2 (%) | OR (95% CI) | Phet | I2 (%) | ||
| All | 12(50,525/54302) | 1.12 (1.02-1.24) | 0.032 | 49.3 | 1.12 (0.90-1.39) | <0.001 | 97.6 | 1.07 (0.91-1.25) | <0.001 | 82.1 | 1.13 (0.94-1.36) | <0.001 | 97.1 | 1.09 (1.00-1.19) | <0.001 | 91.6 |
| Ethnicity | ||||||||||||||||
| Caucasian | 6(11470/10331) | 1.21 (1.10-1.32) | 0.408 | 1.4 | 1.07 (1.01-1.13) | 0.812 | 0.0 | 1.16 (1.07-1.27) | 0.436 | 0.0 | 1.10 (1.04-1.16) | 0.686 | 0.0 | 1.09 (1.05-1.13) | 0.475 | 0.0 |
| Asian | 3(7624/5743) | 1.17 (0.70-1.95) | 0.563 | 0.0 | 1.07 (0.90-1.26) | 0.324 | 0.0 | 1.14 (0.69-1.89) | 0.606 | 0.0 | 1.07 (0.89-1.28) | 0.277 | 15.4 | 1.09 (1.01-1.17) | 0.515 | 0.0 |
| African | 1(742/658) | 0.45 (0.22-0.92) | - | - | 1.16 (0.92-1.47) | - | - | 0.43 (0.22-0.88) | - | - | 1.08 (0.86-1.35) | - | - | 0.98 (0.81-1.20) | - | - |
| Mixed | 2(30689/37570) | 1.05 (0.94-1.17) | 0.082 | 67.0 | 1.29 (0.70-2.41) | <0.001 | 99.7 | 0.90 (0.72-1.13) | <0.001 | 93.1 | 1.26 (0.73-2.17) | <0.001 | 99.6 | 1.12 (0,85-1.46) | <0.001 | 99.1 |
| Source of control | ||||||||||||||||
| Nested | 3(38500/44177) | 1.08 (0.99-1.18) | 0.076 | 61.2 | 1.22 (0.79-1.88) | <0.001 | 99.5 | 0.97 (0.79-1.19) | <0.001 | 93.8 | 1.20 (0.83-1.74) | <0.001 | 99.4 | 1.10 (0.93-1.31) | <0.001 | 98.3 |
| PB | 7(10406/8365) | 1.19 (0.88-1.63) | 0.036 | 57.9 | 1.06 (0.97-1.17) | 0.763 | 0.0 | 1.16 (0.86-1.56) | 0.040 | 57.1 | 1.09 (0.99-1.19) | 0.644 | 0.0 | 1.09 (1.03-1.15) | 0.429 | 0.0 |
| HB | 2(1619/1760) | 1.13 (0.81-1.56) | 0.651 | 0.0 | 1.07 (0.91-1.25) | 0.332 | 0.0 | 1.12 (0.82-1.54) | 0.756 | 0.0 | 1.08 (0.92-1.26) | 0.345 | 0.0 | 1.07 (0.94-1.22) | 0.375 | 0.0 |
PB, population based; HB, hospital based.
Figure 2. Forest plot of the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk under a homozygous model.
Figure 3. Forest plot of the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk stratified by ethnicity under an allele contrast model.
Figure 4. Funnel plot analysis for publication bias by source of control under an allele contrast model.
Heterogeneity and sensitivity analyses
There were significant heterogeneities detected while evaluating the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk under all five genetic models (homozygous model: P = 0.032; heterozygous model: P < 0.001; recessive model: P < 0.001; dominant model: P < 0.001; comparison of allele frequencies: P < 0.001). Thus, the random-effects model was applied to calculate pooled ORs and 95% CIs. The leave-one-out sensitivity analysis found that no single study had qualitatively altered the pooled ORs, suggesting that our meta-analysis were relatively robust.
Publication bias
Egger's test was used to assess publication bias in this meta-analysis. No publication bias was found for any of the five models (homozygous model: P = 0.637; heterozygous model: P = 0.156; recessive model: P = 0.191; dominant model: P = 0.194; comparison of allele frequencies: P = 0.268).
DISCUSSION
The LSP1 gene encodes an F-actin bundling protein, which is expressed in lymphocytes, neutrophils, and endothelial cells. LSP1 protein regulates neutrophil motility, adhesion to fibrinogen matrix proteins, and transendothelial migration [24, 25]. Polymorphisms in the LSP1 gene may lead to alterations in the expression and function of the protein as well as the regulation of downstream signaling pathways, thereby modulating breast cancer susceptibility [7, 8, 26]. The LSP1 rs3817198 T > C polymorphism has been widely studied for its potential association with the risk of breast cancer; however, the findings were inconclusive. This updated meta-analysis was performed to draw a more precise conclusion about the association, with the addition of recently published studies. In the current meta-analysis, a total of 50,525 cases and 54,302 controls were retrieved to assess the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk. We found that an increased risk of breast cancer was observed for the LSP1 rs3817198 T > C polymorphism under both a homozygous model and a comparison of allele frequencies model. Further stratified analysis showed that this association was notable in Caucasian populations, Asian populations, and in population-based studies. Our results suggest that the LSP1 rs3817198 T > C polymorphism is a risk factor for breast cancer.
Previously, only one meta-analysis (in 2011) investigated the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk [17]. The previous meta-analysis included only 7 studies with 33,920 cases and 35,671 controls and found a significant association between the LSP1 rs3817198 T > C polymorphism and breast cancer under homozygous model and comparison of allele frequencies model. A number of new studies [13, 16, 18–20] comprising 16,605 cases and 18,631 controls were also included in our current meta-analysis. As a result, the statistical power of our meta-analysis was greatly increased. Consistent with the previous meta-analysis, we observed an increased risk of breast cancer associated with the LSP1 rs3817198 T > C polymorphism under both a homozygous model and a comparison of allele frequencies model. Stratification analysis in the previous meta-analysis also indicated that the LSP1 rs3817198 T > C polymorphism was significantly associated with breast cancer in Caucasians under homozygous and recessive models and in mixed ethnicities under a homozygous model [17]. However, our meta-analysis showed that the LSP1 rs3817198 T > C polymorphism was significantly associated with breast cancer in Caucasians under all five genetic models and we failed to replicate the association for mixed ethnicities. These discrepancies between the two meta-analyses may be accredited to the differences in the sample size and the classification of ethnicities. Chen et al. did not include studies conducted among Asians, possibly leading to bias in their results. Our meta-analysis included 3 studies performed among Asian populations [18–20]. We also first found an increased risk of breast cancer with the LSP1 rs3817198 T > C polymorphism in an Asian population, although there was a stronger association in the Caucasian population. Moreover, a significantly elevated risk of breast cancer in nested case-control studies was observed by Chen et al. [17] and was not replicated in our meta-analysis. Instead, we found that the polymorphism increased the risk of breast cancer by at least 9% in population-based studies, which may be attributed to our relatively large sample size.
Several limitations to our meta-analysis should be noted. First, in the stratification analysis by ethnicity, the numbers of studies among Asian and Africans were relatively small. Therefore, the statistical power might be not sufficient to assess the relationship. Second, the source of controls was not uniformly defined. Some studies adopted population-based controls or hospital-based controls, while other studies had nested controls. Third, our meta-analysis results were based on unadjusted risk estimates. We did not have sufficient data to conduct a more precise analysis with adjustment for age, obesity, smoking, drinking, menopausal status, environmental factors and lifestyle. Nonetheless, our meta-analysis provides a more comprehensive assessment of the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk, and is based on a relatively large sample size. Our results indicate that the LSP1 rs3817198 T > C polymorphism increases susceptibility to breast cancer, especially in Caucasian and Asian populations.
MATERIALS AND METHODS
Identification of eligible relevant studies
To retrieve all eligible articles that assessed the association between the LSP1 rs3817198 T > C polymorphism and breast cancer risk, we performed a literature search using Pubmed, EMBASE, and Chinese National Knowledge Infrastructure (CNKI) databases prior to July 31, 2016. The search terms that used were as follows: “Lymphocyte-specific protein 1 or LSP1”, “variant or polymorphism” and “cancer or tumor or carcinoma”. Reference lists of relevant studies and review articles were also screened manually for additional eligible articles. Only articles written in English and Chinese were retrieved for further screening.
Inclusion and exclusion criteria
Eligible studies had to satisfy all of the following criteria: (a) case-control studies in human populations; (b) investigation of the association of the LSP1 rs3817198 T > C polymorphism with breast cancer risk; (c) sufficient information for estimating the ORs and 95% CIs; and (d) genotype frequency distributions in the control group in compliance with HWE. The exclusion criteria were: (a) not a case-control study; (b) abstracts, reviews, or comments; (c) lacking sufficient data; (d) replicating data. If several studies shared the same or overlapping subjects, only the most recent study or the study with the largest number of participants or most complete data was selected.
Data extraction
Two authors independently reviewed and extracted the information from the studies and applied the inclusion and exclusion criteria. If any discrepancy was encountered, a consensus was finally reached by consultation and discussion with a third author. The following data were extracted from each eligible study: the surname of the first author, year of publication, country of origin, ethnicity, source of control, number of cases and controls, allele or genotype frequencies of the LSP1 rs3817198 for cases and controls, evidence of HWE, and quality score (high quality articles with score ≥ 9, low quality articles with score < 9) [27, 28].
Statistical analysis
The strength of the association between the LSP1 rs3817198 T > C polymorphism and the risk of breast cancer was assessed by ORs and corresponding 95% CIs under five different genetic models. The models were as follows: homozygous model (CC vs. TT), heterozygous model (TC vs. TT), recessive model (CC vs. TC + TT) and dominant model (TC +CC vs. TT), as well as comparison of allele frequencies (C vs. T). We use the Q-statistic to evaluate between-study heterogeneity. For the Q test, a P value greater than 0.10 indicated a lack of heterogeneity. In the case of no heterogeneity, the fixed-effects model (Mantel-Haenszel method) was applied [29]. Otherwise, the random-effects model (the DerSimonian and Laird method) was selected [30]. In addition, the I2 test was also used to quantify the heterogeneity among studies [31]. We also conducted sensitivity analysis to assess the stability of our meta-analysis. In order to do so, we consecutively omitted one study at a time and recalculated OR and 95% CI. Funnel plots and Egger's linear regression test was used to check for publication bias [32]. All statistical analyses were conducted with STATA Software (version 11.0; Stata Corporation, College Station, TX).
Footnotes
CONFLICTS OF INTEREST
None.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Song M, Lee KM, Kang D. Breast cancer prevention based on gene-environment interaction. Mol Carcinog. 2011;50:280–290. doi: 10.1002/mc.20639. [DOI] [PubMed] [Google Scholar]
- 3.Wolff MS, Weston A. Breast cancer risk and environmental exposures. Environ Health Perspect. 1997;105:891–896. doi: 10.1289/ehp.97105s4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Manson JE, Anderson GL, Cauley JA, Aragaki AK, Stefanick ML, Lane DS, Johnson KC, Wactawski-Wende J, Chen C, Qi L, Yasmeen S, Newcomb PA, Prentice RL. Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study. J Natl Cancer Inst. 2013;105:526–535. doi: 10.1093/jnci/djt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung KL. Endocrine therapy for breast cancer: an overview. Breast. 2007;16:327–343. doi: 10.1016/j.breast.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Silva Idos S, De Stavola B, McCormack V. Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med. 2008;5:e193. doi: 10.1371/journal.pmed.0050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ripperger T, Gadzicki D, Meindl A, Schlegelberger B. Breast cancer susceptibility: current knowledge and implications for genetic counselling. Eur J Hum Genet. 2009;17:722–731. doi: 10.1038/ejhg.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanale D, Amodeo V, Corsini LR, Rizzo S, Bazan V, Russo A. Breast cancer genome-wide association studies: there is strength in numbers. Oncogene. 2012;31:2121–2128. doi: 10.1038/onc.2011.408. [DOI] [PubMed] [Google Scholar]
- 9.Thompson D, Easton D. The genetic epidemiology of breast cancer genes. J Mammary Gland Biol Neoplasia. 2004;9:221–236. doi: 10.1023/B:JOMG.0000048770.90334.3b. [DOI] [PubMed] [Google Scholar]
- 10.Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R, Wareham N, Ahmed S, Healey CS, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnholtz-Sloan JS, Shetty PB, Guan X, Nyante SJ, Luo J, Brennan DJ, Millikan RC. FGFR2 and other loci identified in genome-wide association studies are associated with breast cancer in African-American and younger women. Carcinogenesis. 2010;31:1417–1423. doi: 10.1093/carcin/bgq128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorodnova TV, Kuligina E, Yanus GA, Katanugina AS, Abysheva SN, Togo AV, Imyanitov EN. Distribution of FGFR2, TNRC9, MAP3K1, LSP1, and 8q24 alleles in genetically enriched breast cancer patients versus elderly tumor-free women. Cancer Genet Cytogenet. 2010;199:69–72. doi: 10.1016/j.cancergencyto.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Butt S, Harlid S, Borgquist S, Ivarsson M, Landberg G, Dillner J, Carlson J, Manjer J. Genetic predisposition, parity, age at first childbirth and risk for breast cancer. BMC Res Notes. 2012;5:414. doi: 10.1186/1756-0500-5-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latif A, Hadfield KD, Roberts SA, Shenton A, Lalloo F, Black GC, Howell A, Evans DG, Newman WG. Breast cancer susceptibility variants alter risks in familial disease. J Med Genet. 2010;47:126–131. doi: 10.1136/jmg.2009.067256. [DOI] [PubMed] [Google Scholar]
- 15.Travis RC, Reeves GK, Green J, Bull D, Tipper SJ, Baker K, Beral V, Peto R, Bell J, Zelenika D, Lathrop M. Gene-environment interactions in 7610 women with breast cancer: prospective evidence from the Million Women Study. Lancet. 2010;375:2143–2151. doi: 10.1016/S0140-6736(10)60636-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campa D, Kaaks R, Le Marchand L, Haiman CA, Travis RC, Berg CD, Buring JE, Chanock SJ, Diver WR, Dostal L, Fournier A, Hankinson SE, Henderson BE, et al. Interactions between genetic variants and breast cancer risk factors in the breast and prostate cancer cohort consortium. J Natl Cancer Inst. 2011;103:1252–1263. doi: 10.1093/jnci/djr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen MB, Li C, Shen WX, Guo YJ, Shen W, Lu PH. Association of a LSP1 gene rs3817198T > C polymorphism with breast cancer risk: evidence from 33,920 cases and 35,671 controls. Mol Biol Rep. 2011;38:4687–4695. doi: 10.1007/s11033-010-0603-3. [DOI] [PubMed] [Google Scholar]
- 18.Long J, Shu XO, Cai Q, Gao YT, Zheng Y, Li G, Li C, Gu K, Wen W, Xiang YB, Lu W, Zheng W. Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol Biomarkers Prev. 2010;19:2357–2365. doi: 10.1158/1055-9965.EPI-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y, Han J, Liu J, Zhang G, Wang L, Liu F, Zhang X, Zhao Y, Pang D. Risk of genome-wide association study newly identified genetic variants for breast cancer in Chinese women of Heilongjiang Province. Breast Cancer Res Treat. 2011;128:251–257. doi: 10.1007/s10549-010-1327-8. [DOI] [PubMed] [Google Scholar]
- 20.Sueta A, Ito H, Kawase T, Hirose K, Hosono S, Yatabe Y, Tajima K, Tanaka H, Iwata H, Iwase H, Matsuo K. A genetic risk predictor for breast cancer using a combination of low-penetrance polymorphisms in a Japanese population. Breast Cancer Res Treat. 2012;132:711–721. doi: 10.1007/s10549-011-1904-5. [DOI] [PubMed] [Google Scholar]
- 21.Tamimi RM, Lagiou P, Czene K, Liu J, Ekbom A, Hsieh CC, Adami HO, Trichopoulos D, Hall P. Birth weight, breast cancer susceptibility loci, and breast cancer risk. Cancer Causes Control. 2010;21:689–696. doi: 10.1007/s10552-009-9496-7. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Closas M, Hall P, Nevanlinna H, Pooley K, Morrison J, Richesson DA, Bojesen SE, Nordestgaard BG, Axelsson CK, Arias JI, Milne RL, Ribas G, Gonzalez-Neira A, et al. Heterogeneity of breast cancer associations with five susceptibility loci by clinical and pathological characteristics. PLoS Genet. 2008;4:e1000054. doi: 10.1371/journal.pgen.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antoniou AC, Spurdle AB, Sinilnikova OM, Healey S, Pooley KA, Schmutzler RK, Versmold B, Engel C, Meindl A, Arnold N, Hofmann W, Sutter C, Niederacher D, et al. Common breast cancer-predisposition alleles are associated with breast cancer risk in BRCA1 and BRCA2 mutation carriers. Am J Hum Genet. 2008;82:937–948. doi: 10.1016/j.ajhg.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanigan F, O'Connor D, Martin F, Gallagher WM. Molecular links between mammary gland development and breast cancer. Cell Mol Life Sci. 2007;64:3159–3184. doi: 10.1007/s00018-007-7386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vachon CM, Scott CG, Fasching PA, Hall P, Tamimi RM, Li J, Stone J, Apicella C, Odefrey F, Gierach GL, Jud SM, Heusinger K, Beckmann MW, et al. Common breast cancer susceptibility variants in LSP1 and RAD51L1 are associated with mammographic density measures that predict breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:1156–1166. doi: 10.1158/1055-9965.EPI-12-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher O, Dudbridge F. Candidate gene-environment interactions in breast cancer. BMC Med. 2014;12:195. doi: 10.1186/s12916-014-0195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He J, Liao XY, Zhu JH, Xue WQ, Shen GP, Huang SY, Chen W, Jia WH. Association of MTHFR C677T, A1298C polymorphisms with non-Hodgkin lymphoma susceptibility: evidence from a meta-analysis. Sci Rep. 2014;4:6159. doi: 10.1038/srep06159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue WQ, He YQ, Zhu JH, Ma JQ, He J, Jia WH. Association of BRCA2 N372H polymorphism with cancer susceptibility: a comprehensive review and meta-analysis. Sci Rep. 2014;4:6791. doi: 10.1038/srep06791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]