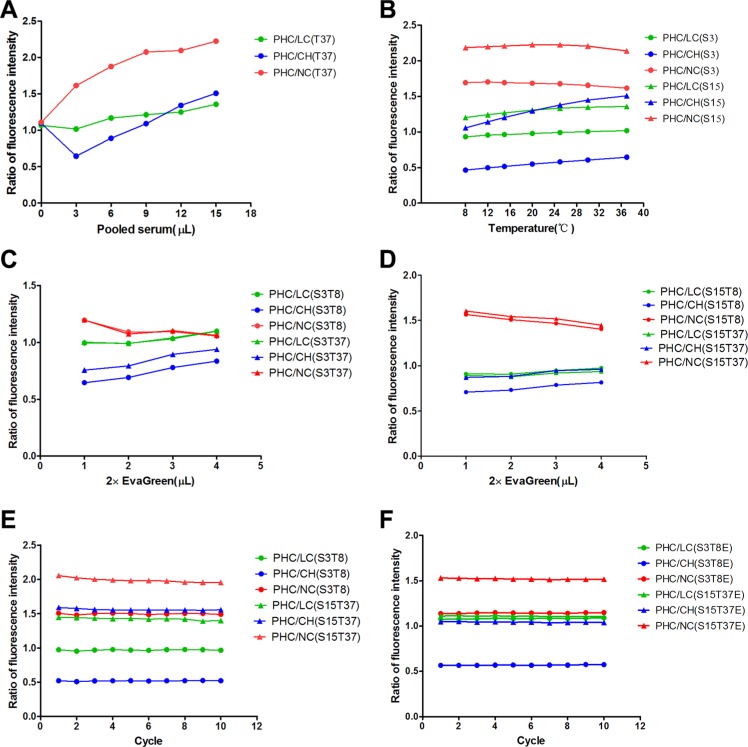
Figure 1. Optimum conditions for the fluorescence intensity measurements of pooled serum samples.
(A) The fluorescence intensity ratios of PHC to LC, CH and NC correspond to pooled serum volumes at 37°C (T37). (B) The fluorescence intensity ratios of PHC to LC, CH and NC correspond to detection temperatures for 3 μL (S3) and 15 μL (S15) of pooled serum. (C, D) The fluorescence intensity ratios of PHC to LC, CH and NC correspond to EvaGreen volumes for 3 μL (S3) and 15 μL (S15) of pooled serum at 8°C (T8) and 37°C (T37). (E, F) The fluorescence intensity ratios of PHC to LC, CH and NC correspond to the cycle numbers (incubation time) for 3 μL of pooled serum at 8°C (S3T8) and 15 μL of pooled serum at 37°C (S15T37) in the absence of EvaGreen and for 3 μL of pooled serum at 8°C (S3T8E) and 15 μL of pooled serum at 37°C (S15T37E) in the presence of EvaGreen. PHC: primary hepatic carcinoma; LC: liver cirrhosis; CH: chronic hepatitis; NC: normal control.