Abstract
Plasma D-dimer level is clinically useful for diagnosing patients with suspected deep vein thrombosis (DVT). However, the cut-off value for the D-dimer level remains controversial and undetermined with regard to total hip arthroplasty (THA). The objective of this study was to estimate the efficacy of an age- and D-dimer-based index for diagnosing DVTs in asymptomatic cases before THA. We enrolled 224 patients with no symptoms associated with DVT before THA. All the patients underwent ultrasonography, and the plasma D-dimer level was recorded about 1 month preoperatively. The optimal cut-off value was calculated using multiple logistic regression and receiver operating curve analyses. DVTs were detected in 13 patients (5.8%) using ultrasonography. Multiple logistic regression analysis demonstrated that age (odds ratio [OR]: 1.13; p = 0.007) and D-dimer value (OR: 1.74; p = 0.003) were related to the existence of preoperative DVT. A DVT index (0.12 × age + 0.45 × the D-dimer value) of 8.15 was the most reasonable cut-off value according to the receiver operating curve analysis. This value caused 100% sensitivity and 70.1% specificity, with an area under the curve (AUC) of 0.905 (range, 0.836–0.975). For age and D-dimer value, the AUCs were 0.828 (0.749–0.907) and 0.716 (0.522–0.910), respectively. This study demonstrated that age and D-dimer index can be useful in screening patients for DVTs before THA. This DVT index is also easy to calculate and may be clinically significant.
Introduction
Pulmonary thromboembolism (PTE) following deep vein thrombosis (DVT) is one of the general complications that can be related to significant mortality after orthopedic operation, particularly total hip arthroplasty (THA) [1–4]. Therefore, in addition to appropriate triage, earlier and more reliable diagnoses of PTE and DVT are necessary for the affected patients to improve their outcomes [5–8]. Søresen et al. [9] described that the relative risks for patients with DVT vary from 1.60 for myocardial infarction to 2.19 for stroke in the first year after the thrombotic event and are also increased during the subsequent 20 years of follow-up. Søgaard et al. [10] argued that patients with DVT or PTE are at increased risk of dying, particularly within the first year after diagnosis, and during the entire 30 years of follow-up, with DVT or PE as an important cause of death. Therefore, we believe that DVT is not only a postoperative complication after THA, but also an important factor that influences prognosis. Although many studies on the occurrence of DVT postoperatively have been conducted [1–8], few reports on the presence of DVT preoperatively are available. A number of physicians believe that almost all patients, before undergoing THA, are not complicated by DVT; however, screening of DVT before THA may be clinically valuable.
If a patient who is undergoing THA has a DVT, physicians should consider anticoagulation therapies, such as warfarization, inferior vena cava filter placement, and thrombolytic therapy, or the procedure may need to be postponed. Thus, the presence of preoperative DVT should not be ignored, but its clinical symptoms are unreliable. Venography and ultrasonography are considered the most suitable methods for DVT diagnosis despite their several limitations. Venography is invasive, expensive, and not easily repeatable, whereas ultrasonography requires experienced technicians, equipment, and labor force [8]. Since these procedures are time consuming and/or costly, repeated assessments of DVT are almost impossible at institutions where many orthopedic operations are conducted annually. Given this reason, we routinely perform ultrasonography before and after THA in our institutions to screen DVT.
Therefore, plasma markers are commonly used to diagnose DVTs. However, debate exists about the level of plasma D-dimer, a fibrin degradation product of the crosslinked fibrin mesh. Nevertheless, D-dimer level is considered a useful diagnostic marker for patients with suspected DVT [11,12].
Schouten et al. [13] argued that the application of age-adjusted cut-off values to the D-dimer value (0.1 × age [years] × true D-dimer value [μg/mL]) considerably elevates specificity without altering sensitivity in patients aged >50 years with suspected PTE. Therefore, the cut-off value of the D-dimer is still controversial and undetermined. The purpose of this study was to evaluate the relation of the existence of DVT before THA and the use of plasma D-dimer value, age, sex, body height, body weight, and body mass index for the screening of DVTs.
Materials and methods
This retrospective study was approved by the ethics committee of our institution (Niigata University Medical and Dental Hospital, Niigata, Japan), and written informed consent was obtained from all the participants. Two hundred and thirty-eight patients underwent primary THA at Niigata University Medical and Dental Hospital from January 1, 2010 to December 31, 2014. Patients with a previous history of thromboembolism and those who received anticoagulation or antiplatelet agents for pre-existing cardiac or cerebrovascular diseases were excluded. Subsequently, 224 patients were enrolled. The preoperative diagnosis was osteoarthritis of the hip in 198 patients and osteonecrosis of the femoral head in 26.
The age, sex, body height, body weight, body mass index (weight in kilograms divided by the square of the height in meters), and plasma D-dimer level of the patients were recorded about 1 month preoperatively. All the patients underwent ultrasonography around the same time that the plasma D-dimer level was measured. Plasma D-dimer levels were collected for routine health purposes for this study. Skilled radiologists who were blinded to the D-dimer value performed all the examinations. Ultrasonography was conducted with compression and color Doppler imaging in B-mode for the common femoral, superficial, popliteal, and calf veins bilaterally. DVT was diagnosed by a loss of compressibility of the vein, the existence of intraluminal echogenicity, and the absence of venous flow. Plasma D-dimer levels were measured using latex agglutination turbidimetry via the CA8000 (Sysmex Co., Kobe, Japan). The normal range is <0.5 μg/mL.
Fisher's exact test was performed to compare the qualitative data such as the number of males or females and the presence or absence of DVT. Unpaired Student’s t-test was conducted to analyze the quantitative data such as age, body height, body weight, body mass index, and D-dimer value. We used multiple logistic regression analysis to determine which of the following factors contributed to the presence of DVT: age, sex, body height, body weight, body mass index, and D-dimer value. Receiver operating characteristic (ROC) curves were also applied to determine the cut-off value with regard to the statistically significant factor(s). The area under the curve (AUC) was also calculated from ROC curves, and the cut-off value was determined by the Youden index [14,15]. SPSS (version 21; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses, and a p value <0.05 was considered statistically significant.
Results
None of the 224 patients showed symptoms suggestive of DVT such as calf pain, tenderness, and swelling. Of these patients, 13 (5.8%) had DVTs with distal type detected by ultrasonography. The patients’ clinical features are presented in Table 1. Patients with and without DVT demonstrated that age (p < 0.001) and D-dimer value (p = 0.048) were significantly different (Table 1).
Table 1. Patients’ characteristics.
| Total patients (n = 224) | Patients with DVT (n = 13) | Patients without DVT (n = 211) | p value✝ | |
|---|---|---|---|---|
| Age (years) | 58.8 ± 10.6 | 69.1 ± 5.3 | 58.2 ± 10.5 | <0.001 |
| Sex (male/female) | 46/178 | 1/12 | 45/166 | 0.476* |
| Side (right/left) | 117/107 | 5/8 | 112/99 | 0.395* |
| Body height (cm) | 155.5 ± 9.2 | 155.6 ± 9.2 | 153.0 ± 9.0 | 0.397 |
| Body weight (kg) | 56.9 ± 12.3 | 52.7 ± 8.2 | 57.1 ± 12.5 | 0.138 |
| Body mass index (kg/m2) | 23.6 ± 4.2 | 22.7 ± 3.7 | 23.6 ± 4.2 | 0.432 |
| Preoperative D-dimer (μg/mL) | 1.49 ± 1.33 | 2.86 ± 2.93 | 1.39 ± 1.20 | 0.048 |
| White blood cell count(/μL) | 5947 ± 1446 | 6117 ± 1219 | 5858 ± 1580 | 0.674 |
| Hemoglobin (g/dL) | 13.0 ± 1.4 | 12.6 ± 1.4 | 13.0 ± 1.6 | 0.308 |
| Hematocrit (%) | 40.3 ± 3.9 | 39.7 ± 3.9 | 40.8 ± 4.0 | 0.511 |
| Platelet count (×104/μL) | 23.7 ± 6.8 | 22.3 ± 4.6 | 23.8 ± 6.9 | 0.479 |
| APTT (s) | 30.7 ± 4.0 | 29.9 ± 3.2 | 30.7 ± 4.0 | 0.518 |
| PT (%) | 106.3 ± 15.0 | 102.7 ± 8.5 | 106.4 ± 15.5 | 0.452 |
| PT–INR | 0.98 ± 0.07 | 0.99 ± 0.04 | 0.98 ± 0.08 | 0.758 |
Data are presented as mean ± standard deviation.
DVT, deep vein thrombosis; APTT, activated partial thromboplastin time; PT, prothrombin time; PT–INR, prothrombin time–international normalized ratio.
*, Fisher’s exact test.
✝, comparison between patients with DVT and without DVT.
According to multiple logistic regression analysis, when age, sex, body height, body weight, body mass index, and D-dimer value were independent variables, age and D-dimer value were associated with preoperative DVT (Table 2). Multiple logistic regression analysis indicated that age and D-dimer value were considered significant risk factors (coefficient regressions = 0.12 and 0.45, respectively; Table 3).
Table 2. Multiple logistic regression analysis for preoperative deep vein thrombosis.
| Independent variable | Odds ratio | 95% CI | p value |
|---|---|---|---|
| Age (year) | 1.13 | 1.034–1.238 | 0.007 |
| Sex (female) | 5.51 | 0.262–11.559 | 0.272 |
| Side (right) | 0.90 | 0.902–1.231 | 0.507 |
| Bogy height (cm) | 1.06 | 0.867–1.303 | 0.345 |
| Body weight (kg) | 0.95 | 0.743–1.201 | 0.642 |
| Body mass index (kg/m2) | 1.15 | 0.635–2.062 | 0.653 |
| D-dimer (μg/mL) | 1.74 | 1.209–2.504 | 0.003 |
CI, confidence interval.
Table 3. Multiple logistic regression analysis for age and D-dimer value.
| Independent variable | Coefficient regression | Odds ratio | 95% CI | p value |
|---|---|---|---|---|
| Age (years) | 0.12 | 1.12 | 1.046–1.208 | 0.002 |
| D-dimer (μg/mL) | 0.45 | 1.56 | 1.172–2.081 | 0.002 |
CI, confidence interval.
ROC curves were created for age, D-dimer value, and the value that was calculated by the following formula: DVT index = 0.12 × age (years) + 0.45 × the D-dimer value (μg/mL) (Figs 1–3). For the screening of DVTs, a DVT index of 8.15 was the most suitable cut-off value, as determined by the Youden index [14,15]. This value yielded 100% sensitivity and 70.1% specificity (Table 4). The cut-off value for age was 63.5, which caused a sensitivity of 88.9% and a specificity of 74.1%; and that of the D-dimer value was 1.85, which yielded a sensitivity of 60.0% and a specificity of 82.4% (Table 4).
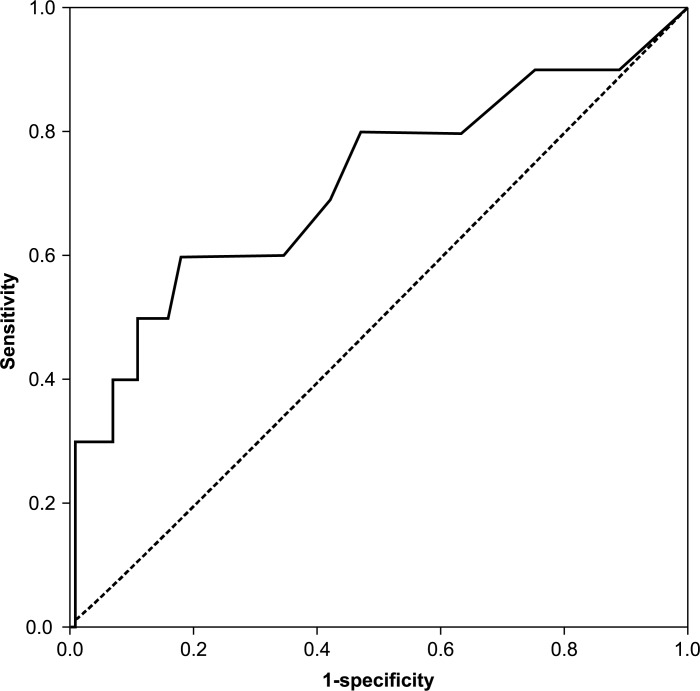
Fig 1. Receiver operating characteristic curve of the D-dimer value preoperatively.
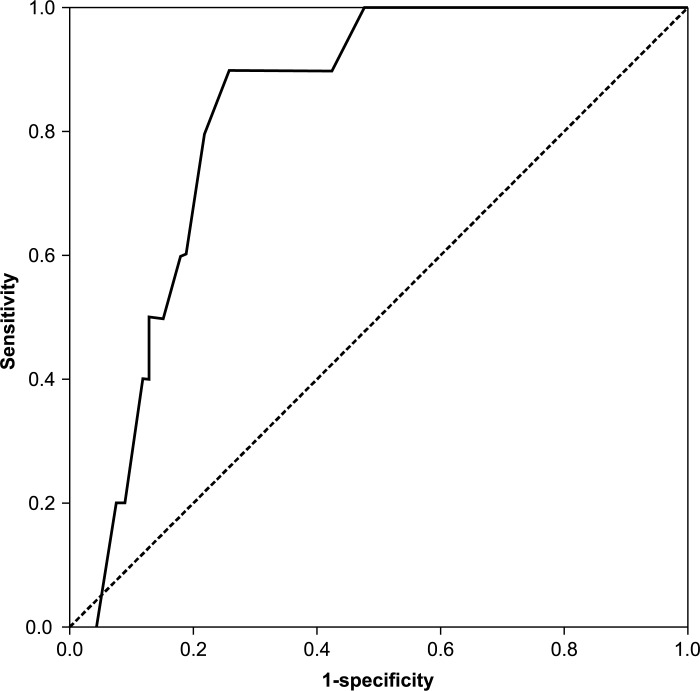
Fig 3. Receiver operating characteristic curve of the deep vein thrombosis index.
Table 4. Area under the curve and cut-off value.
| Independent variable | Cut-off value | Sensitivity* | Specificity* | AUC | 95% CI | p value |
|---|---|---|---|---|---|---|
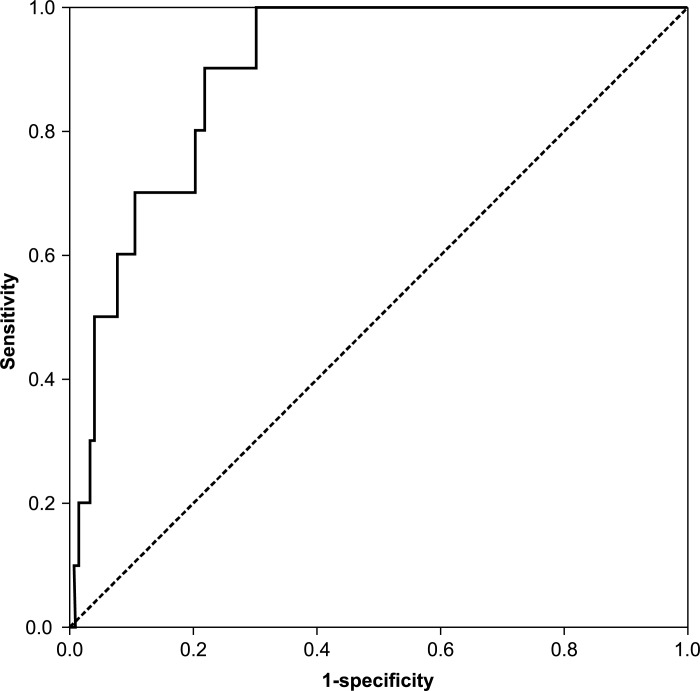
| Age | 63.5 years | 0.889 | 0.741 | 0.828 | 0.749–0.907 | <0.001 |
| D-dimer | 1.85 μg/mL | 0.600 | 0.824 | 0.716 | 0.522–0.910 | 0.021 |
| DVT index✝ | 8.15 | 1.000 | 0.701 | 0.905 | 0.836–0.975 | <0.001 |
AUC, area under the curve; CI, confidence interval; DVT, deep vein thrombosis.
*, Value at the cut-off value.
✝, DVT index = 0.12 × age (years) + 0.45 × D-dimer value (μg/mL).
Fig 2. Receiver operating characteristic curve of age on the day of surgery.
With regard to the AUC, the DVT index was 0.905, and the AUCs were 0.828 and 0.716 for age and the D-dimer value, respectively. Therefore, the DVT index was the most accurate diagnostic test among the three tests.
Discussion
Detecting DVTs preoperatively is necessary because approximately 90% of symptomatic PTE cases originate from a DVT in the lower limb [16]. If patients who are undergoing THA have a DVT, the DVT may be enlarged and/or expanded, which may subsequently cause PTE, a generally critical postoperative complication following arthroplasty. Therefore, venography or ultrasonography of the lower extremities must be performed in all patients before THA despite the limitations of these diagnostic tools [17].
In this study, the DVT index was calculated using the previously described formula, which showed 100% sensitivity and 70.1% specificity with an AUC of 0.905. This index was calculated using two coefficients of significant risk factors determined with multiple logistic regression. However, only age and D-dimer value demonstrated sensitivities of 88.9% and 60.0%; specificities of 74.1% and 82.4%; and AUCs of 0.831 and 0.712, respectively.
In previous reports, age-adjusted cut-off values by Schouten et al. [13] for the D-dimer value (0.1 × age [years]) [μg/mL] substantially increase specificity without modifying the sensitivity in patients suspected for DVT; a high sensitivity (>97%) was reported, but the specificity was <60%. If this Schouten’s index applied to the participants of our study, high specificity was observed (0.899), while sensitivity was 0.077. Moreover, Signorelli et al. [18] identified the risk of DVT by using routine blood tests, such as erythrocyte sedimentation rate, antithrombin III, C-reactive protein, D-dimer, and N-terminal pro-brain natriuretic peptide, in 295 consecutive medical patients. They reported that the DVT risk stratification system is moderately prognostic (AUC: 0.838; 95% confidence interval: 0.771–0.904; p < 0.001), whereas its sensitivity and specificity were 100% and 20%, respectively. In the present study, the DVT index was easy to calculate using only age and preoperative D-dimer value, and the sensitivity was 100%. The DVT index of all patients with a DVT was >8.15; therefore, patients with a DVT will never be missed, even in the absence of any clinical signs or symptoms associated with it.
Our DVT index may have a great potential benefit because DVT screening was performed with high accuracy in many patients. For example, in the current study, 150 of 224 patients had a DVT index <8.15; thus, ultrasonography or venography was not required for DVT screening.
The current study has several limitations. First, only a few patients were enrolled. Second, D-dimer concentrations at our institution are often measured using latex agglutination turbidimetry; conversely, in North America and Europe, they are measured using enzyme-linked immunoassay (ELISA). Latex agglutination turbidimetry is not recommended over the ELISA method, which may lead to lower sensitivity and specificity in this study. Finally, we did not examine the plasma levels of blood natriuretic peptide (BNP), a useful marker to detect increased pulmonary pressure. This marker is considered crucial for identifying patients at high risk for venous thromboembolism. However, given that BNP measurement is covered by health insurance in Japan for heart or renal failure only, we could not routinely examine BNP. Therefore, BNP evaluation was not included in this study.
In conclusion, this study showed that the DVT index is the most accurate screening method for the presence of DVTs before THA. This index was also easy to calculate and may be clinically useful. Nevertheless, further investigations are required to verify our findings and ensure more precise diagnoses.
Data Availability
All relevant data are contained within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Sasaki S, Miyakoshi N, Matsuura H, Saitoh H, Kudoh D, Shimada Y. Prospective randomized controlled trial on the effect of fondaparinux sodium for prevention of venous thromboembolism after hip fracture surgery. J Orthop Sci. 2009;14: 491–496. 10.1007/s00776-009-1365-4 [DOI] [PubMed] [Google Scholar]
- 2.Yoshitaka T, Abe N, Minagawa H, Date H, Sakoma Y, Nishida K. Disease-specific screening for deep venous thrombosis and pulmonary thromboembolism using plasma D-dimer values after total knee arthroplasty. Mod Rheumatol. 2008;18: 359–365. 10.1007/s10165-008-0068-6 [DOI] [PubMed] [Google Scholar]
- 3.Sudo A, Wada H, Nobori T, Yamada N, Ito M, Niimi R, et al. Cut-off values of D-dimer and soluble fibrin for prediction of deep vein thrombosis after orthopaedic surgery. Int J Hematol. 2009;89: 572–576. 10.1007/s12185-009-0323-4 [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Hasegawa M, Niimi R, Sudo A. Incidence and time course of asymptomatic deep vein thrombosis with fondaparinux in patients undergoing total joint arthroplasty. Thromb Res. 2010;126: e323–e326. 10.1016/j.thromres.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 5.Cosmi B, Palareti G. Update on the predictive value of D-dimer in patients with idiopathic venous thromboembolism. Thromb Res. 2010;125: S62–S65. 10.1016/S0049-3848(10)70016-5 [DOI] [PubMed] [Google Scholar]
- 6.Bounameaux H, Cirafici P, de Moerloose P, Schneider PA, Slosman D, Reber G, et al. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet. 1991;337: 196–200. [DOI] [PubMed] [Google Scholar]
- 7.Kearon C, Ginsberg JS, Douketis J, Turpie AG, Bates SM, Lee AY, et al. An evaluation of D-dimer in the diagnosis of pulmonary embolism: a randomized trial. Ann Intern Med. 2006;144: 812–821. [DOI] [PubMed] [Google Scholar]
- 8.Kamikura Y, Wada H, Nobori T, Matsumoto T, Shiku H, Ishikura K, et al. Elevated plasma levels of fibrin degradation products by granulocyte-derived elastase in patients with deep vein thrombosis. Thromb Res. 2005;115: 53–57. 10.1016/j.thromres.2004.07.019 [DOI] [PubMed] [Google Scholar]
- 9.Sørensen HT, Horváth-Puhó E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370: 1773–1779. 10.1016/S0140-6736(07)61745-0 [DOI] [PubMed] [Google Scholar]
- 10.Søgaard KK, Schmidt M, Pedersen L, Horváth-Puhó E, Sørensen HT. 30-year mortality after venous thromboembolism: a population-based cohort study. Circulation. 2014;130: 829–836. 10.1161/CIRCULATIONAHA.114.009107 [DOI] [PubMed] [Google Scholar]
- 11.Gaffney PJ, Edgell T, Creighton-Kempsford LJ, Wheeler S, Tarelli E. Fibrin degradation product (FnDP) assays: analysis of standardization issues and target antigens in plasma. Br J Haematol. 1995;90: 187–194. [DOI] [PubMed] [Google Scholar]
- 12.Lip GYH, Lowe GDO. Fibrin D-dimer: a useful clinical marker of thrombogenesis? Clin Sci. 1995;89: 205–214. [DOI] [PubMed] [Google Scholar]
- 13.Schouten HJ, Geersing GJ, Koek HL, Zuithoff NPA, Janssen KJM, Douma RA, et al. Diagnostic accuracy of conventional or age-adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346: f2492 10.1136/bmj.f2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins NJ, Schisterman EF. The inconsistency of ‘optimal’ cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163: 670–675. 10.1093/aje/kwj063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47: 458–472. [DOI] [PubMed] [Google Scholar]
- 16.Fuji T, Fujita S, Ochi T. Fondaparinux prevents venous thromboembolism after joint replacement surgery in Japanese patients. Int Orthop. 2008;32: 443–451. 10.1007/s00264-007-0360-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niimi R, Hasegawa M, Shi DQ, Sudo A. The influence of fondaparinux on the diagnosis of postoperative deep vein thrombosis by soluble fibrin and D-dimer. Thrombosis Res. 2012;130: 759–764. [DOI] [PubMed] [Google Scholar]
- 18.Signorelli SS, Valerio F, Davide C, Conti GO, Maria F, Ignazio M, et al. Evaluating the potential of routine blood tests to identify the risk of deep vein thrombosis: a 1-year monocenter cohort study. Angiology. 2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are contained within the paper.