Abstract
Background
Unnecessary and non-first-choice antibiotic prescribing is a significant problem in primary care. It is often argued that irrational prescribing is higher during out-of-hours (OOH) consultations.
Aim
To obtain insight into the quantity and quality of OOH antibiotic prescribing for commonly presented infectious diseases.
Design and setting
Two two-way comparisons of 1) nationally dispensed antibiotics during office hours and OOH care, using data from the Dutch Foundation of Pharmaceutical Statistics, and 2) regional prescribing quality data from 45 primary care practices from Utrecht and its vicinity, and two large OOH services in Utrecht and Woerden.
Method
From the national data, yearly dispensed antibiotics were analysed per prescriber type, with respect to time (office hours or OOH) of prescription, types of antibiotics, and patients’ age group. Regional prescribing rates, choice of antibiotic, and appropriateness of prescribing were compared for otitis media, sinusitis, tonsillitis, bronchitis, cystitis, and impetigo. Appropriateness was assessed by comparing all relevant information from medical files with the guideline recommendations.
Results
Only 6% of GP-prescribed antibiotics were prescribed OOH. OOH, cystitis and acute otitis media presented most often. First-choice prescribing was comparable for the two settings, whereas prescribing rates were higher OOH, with comparatively more amoxicillin(/clavulanate). The appropriateness evaluation, however, revealed that overprescribing was comparable, or even lower than, for daily practice.
Conclusion
The suggestion that OOH antibiotic prescribing quality is worse than in daily practice does not seem founded. The higher OOH prescribing rates can be explained by a different population of presenting patients. The appropriateness of prescribing rather than prescribing rates, therefore, should be used to determine quality.
Keywords: antibiotics, drug prescribing, infectious disease, out-of-hours service, prescribing quality, primary care
INTRODUCTION
To improve the quality of antibiotic prescribing, GPs have been encouraged to follow evidence-based guidelines in their decision whether or not to prescribe, and regarding the choice of antibiotic. Still, there is considerable overprescribing for respiratory tract infections (RTIs)1,2 and increasing prescribing for urinary tract infection.3,4 The consequences of irrational antibiotic use are the development of antimicrobial resistance, medicalisation of patients, unnecessary exposure to adverse drug events, and costs.5,6
When irrational antibiotic prescribing (overprescribing and non-first-choice prescribing) is discussed with GPs, they often argue that irrational prescribing is higher for out-of-hours (OOH) consultations.7 In OOH services, GPs are generally not familiar to the patients and GPs often do not have access to the patient’s complete medical history. In addition, patients might exert more pressure for medical treatment, and GPs face a high workload and have less time and no personal relationship with the patient for communicating (non-) treatment considerations and advising alternative approaches.8,9 These aspects could result in more irrational antibiotic prescribing. Higher antibiotic prescribing rates could, however, be justified during OOH, as patients are more likely to present with severe illness, often with fever,10 or have higher risk of complications.11,12 In the Netherlands, about 26% of patients consulting during OOH present with an infection: 4.5% with cystitis, 4.3% with acute upper RTI, and 2.8% with acute otitis media (AOM).13
Very little research has been performed on OOH antibiotic prescribing quantity and quality. One Norwegian study described frequencies and characteristics,9 and another found that in a Norwegian setting GPs prescribed antibiotics for AOM and tonsillitis in a similar way in-hours and OOH.14 A recent study from the UK showed that antibiotic prescribing at OOH services rose, which could be explained by a partial displacement of antibiotic prescribing from in-hours to OOH primary care.15 In Belgium, an antibiotic prescribing quality comparison was done using prescribing and second-choice rates. This revealed an overall suboptimal quality, with similar outcomes for both settings.16 In the Netherlands, office hours antibiotic prescribing quality is comparatively good. This offers better opportunities to detect quality differences between the two settings. With a lower prescribing quality OOH, consideration of measures to improve rational antibiotic use in this setting is warranted.
This study aimed at obtaining a detailed insight into quantity and quality differences in office hours and OOH antibiotic prescribing. For this purpose, two two-way comparisons were made:
dispensed antibiotic quantities and types at a national level; and
the quality of antibiotic prescribing for commonly occurring RTIs, ear, urinary tract, and skin infections (prescribing rate, choice of antibiotic, and medical appropriateness by guideline benchmarking) at a regional level.
How this fits in
It is often argued that irrational antibiotic prescribing is higher in out-of-hours (OOH) consultations than during office hours. The present study shows that only 6% of antibiotics are prescribed OOH, and that first-choice prescribing is similar to office hours practice. Although OOH antibiotic prescribing rates are higher, the appropriateness of prescribing is comparable, or even better than, in office hours practice. Therefore, focus for intervention can remain on daily practice.
METHOD
National antibiotic dispensing data
Dutch pharmaceutical dispensing data were collected from the Dutch Foundation of Pharmaceutical Statistics (SFK). The SFK gathers all data on dispensed drugs from over 95% of Dutch community pharmacies, serving about 15.3 million people.17 From this national database, all oral antibiotics (J01) were retrieved for 2012.18 Information was available on type of antibiotic, the prescriber (GP, specialist, or others: dentist, nursing home doctor, public health practitioner), the time of prescription (office hours or OOH), and the patient’s age (<18 years, or ≥18 years of age).
Prescriptions from OOH services are registered differently from those made by GPs in their own practice. All prescriptions from the comparatively few practices with extended opening hours after 5 pm therefore account for office hours prescribing.
Office hours: presentation and antibiotic management data
Antibiotic prescribing data for each new diagnosis of AOM (ICPC code: H71), acute or chronic sinusitis (R75), acute tonsillitis (R76), acute bronchitis or bronchiolitis (R78), impetigo (S84), and cystitis (U71) were retrieved from the Julius General Practitioners’ Network (JHN) database for 2012. This database contains anonymous routine care data during office hours from digital patient records of 45 general practices in Utrecht and its vicinity. These practices have 266 417 registered patients. This network, data extraction, and analyses of prescribing data were previously described in more detail.3 Diagnoses are coded according to the International Classification of Primary Care (ICPC)19 and antibiotic prescriptions according to the Anatomical Therapeutic Classification (ATC) system of the World Health Organization.18
Out-of-hours: presentation and antibiotic management data
Antibiotic prescribing data for H71, R75, R76, R78, S84, and U71 during OOH in 2012 were retrieved from ‘Primair’ OOH services in Utrecht and Woerden, with a coverage area of approximately 220 000 persons. This area does not fully cover the JHN practices, but does cover the city of Utrecht and some surrounding rural villages. ‘Primair’ provides primary care for (sub)acute medical problems during OOH and operates daily between 5 pm and 8 am, and on weekends from Friday 5 pm to Monday 8 am.20 Dutch OOH care is provided by normal GPs in rotation. The initial telephone contact is handled by trained nurses, using the Dutch Triage System, to determine the urgency level. Urgency is classified as shown in Box 1. Based on this, the nurse can provide advice, or schedule a GP consultation at the centre or at home.21
Box 1. Dutch Triage System to determine the urgency level.
| U0 | Needing resuscitation |
| U1 | Life-threatening/ambulance |
| U2 | Patient needs to be seen within an hour |
| U3 | Patient needs to be seen within a couple of hours |
| U4 | Routine care without time pressure |
| U5 | Advice |
Per ICPC code, all anonymised consultations and home visits were evaluated for whether an antibiotic was prescribed, and, if so, which one (registered in generic names). For U71 only, (telephone) consultations with the nurse were also included. When a patient recognises cystitis complaints from a previous episode and/or when the nurse tests a positive urine sample, they can ask the GP to authorise a prescription. A patient can only receive an antibiotic without a GP consultation with uncomplicated urinary tract infection. This management is also common practice during office hours involving the practice assistant.
Analyses
From the national data, yearly dispensed antibiotics were analysed per prescriber type, with respect to the time of prescription, the types of antibiotics prescribed, and the patients’ age group, and presented as total numbers and percentages.
From the JHN and Primair data, the following were determined:
Prescribing rates. For office hours, this was the percentage of consultations for a new diagnosis in which antibiotics were prescribed (excluding return or follow-up visits); for OOH, this was the percentage of consultations in which antibiotics were prescribed. It was felt this was justified as usually there are no return, or follow-up, visits at these centres. Patients who were already on antibiotic treatment for the indication of consultation were excluded from the analysis.
The percentages of prescribed first-choice antibiotics (according to the guidelines of the Dutch College of General Practitioners [NHG], Appendix 1)22 and which non-first-choice antibiotics.
SPSS 20.0 and Excel 2010 were used for the analyses.
Appropriateness of antibiotic prescribing
For office hours prescribing for RTIs, a thorough analysis of the appropriateness of the prescribing decision had been performed previously.1 For OOH practice, this was done by specific comparison of the first 100 patient files of each ICPC code of interest with the recommendations of the NHG guidelines for AOM, rhinosinusitis, acute sore throat, acute cough, urinary tract infections, and bacterial skin infections (Appendix 1).22 To this aim, age, sex, urgency level, as well as fever, medical history (including relevant comorbidity), history taking of the current illness episode, illness duration, physical examination, results from diagnostic tests, when available, and all other free text were used. Overprescription was defined as the percentage of antibiotic prescriptions that were not indicated by the guidelines. Underprescription was defined as the percentage of non-prescriptions in which an antibiotic was actually indicated.
RESULTS
National antibiotic dispensing data
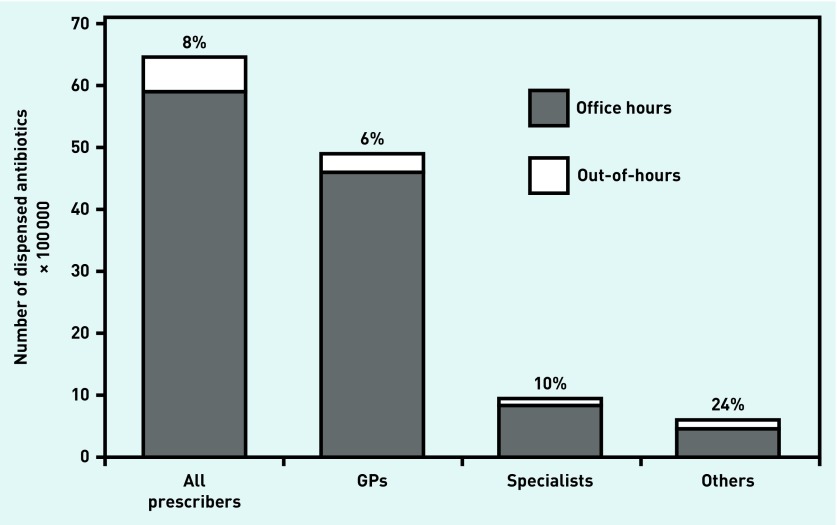
In 2012, 6 434 640 antibiotic courses were dispensed by community pharmacies, of which 8.4% were prescribed OOH. Of all antibiotics, 76% were prescribed by GPs, 15% by specialists, and 9% by dentists, nursing home practitioners, and public health practitioners. This last group prescribed comparatively more during OOH (in absolute numbers); however, GPs prescribed most OOH dispensed antibiotics (Figure 1). When analysed per age group, 13.1% of antibiotics for patients <18 years of age were prescribed during OOH contacts, compared with 7.8% of antibiotics for patients aged ≥18 years. This higher percentage of antibiotics prescribed for children during OOH was apparent for all types of prescribers (data not shown).
Figure 1.
Dispensed antibiotics per prescriber, split for office hours and out-of-hours prescribing. These national data were derived from community pharmacies via the SFK. Absolute numbers of dispensed antibiotics are shown, in total and per prescriber; others refer to dentists, nursing home practitioners, and public health practitioners. SFK = Dutch Foundation of Pharmaceutical Statistics.
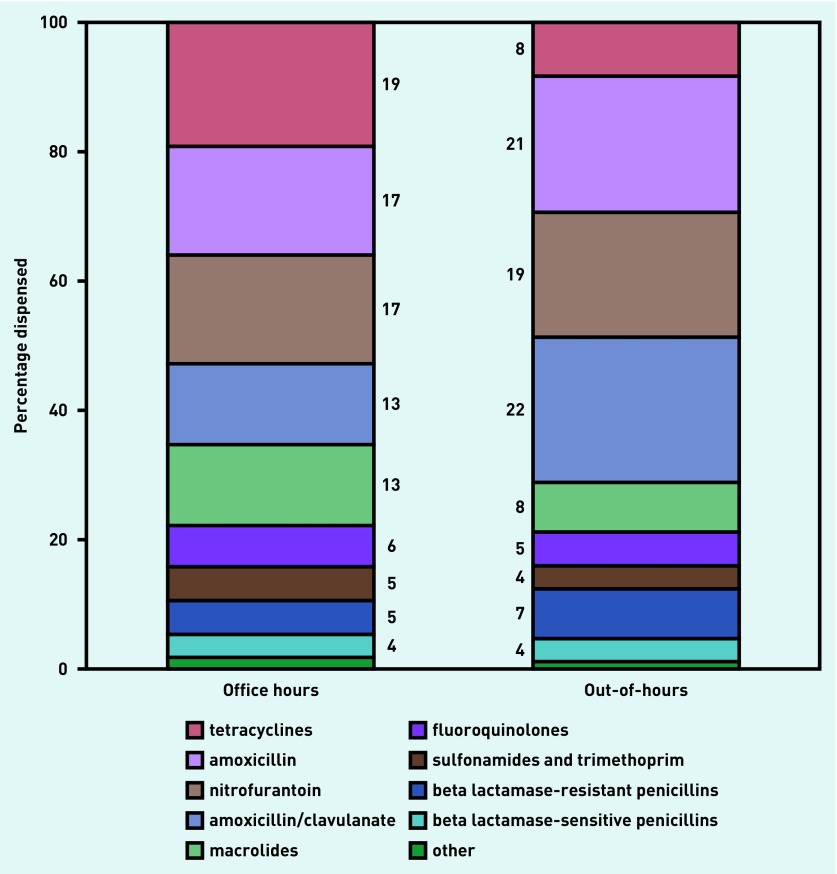
Tetracyclines, amoxicillin, nitrofurantoin, amoxicillin/clavulanate, and macrolides were most often prescribed by GPs during office hours. Out-of-hours, comparatively more amoxicillin, nitrofurantoin, and amoxicillin/clavulanate were prescribed, and comparatively fewer tetracyclines and macrolides (Figure 2).
Figure 2.
Types of antibiotics prescribed by GPs during office and out-of-hours contacts. These national data were obtained from the SFK. Of all GP-prescribed antibiotics (J01 at the ATC4 or 5 level), their relative contribution as a percentage was determined for office hours and out-of-hours prescribing. SFK = Dutch Foundation of Pharmaceutical Statistics.
Office hours and out-of-hours primary care: presentation of six infectious diseases
Table 1 shows the mean patient ages and numbers of episodes for office hours and contacts for OOH care for AOM, sinusitis, tonsillitis, acute bronchitis, cystitis, and impetigo. During office hours, as well as OOH, cystitis and AOM were most often presented. Comparatively more patients with AOM and tonsillitis presented during OOH. More younger patients presented at OOH, which was most apparent for acute bronchitis and AOM. Most OOH contacts fell in urgency levels 3 and 4. Therefore, a considerable number of contacts concerning sinusitis, cystitis, and impetigo were handled by the triage nurses over the phone. These patients were not invited for a consultation because their symptoms were not severe enough, or could await an office hours consultation with their own GP; in these cases the nurse reassures and provides advice.
Table 1.
Disease presentation of six infectious diseases in office hours and out-of-hours primary carea
| Disease | Urgency level | Office hours (JHN 2012) | Out-of-hours, (Primair 2012) | |
|---|---|---|---|---|
| Acute otitis media | Age, years (mean) | 10 | 7.7 | |
| Contacts, n (T:C) | 9271 | 85:773 | ||
| 2 | 10 | |||
| 3 | 479 | |||
| 4 | 308 | |||
| 5 | 61 | |||
|
| ||||
| Sinusitis | Age, years (mean) | 44.7 | 39 | |
| Contacts, n (T:C) | 7141 | 39:103 | ||
| 2 | 7 | |||
| 3 | 52 | |||
| 4 | 62 | |||
| 5 | 21 | |||
|
| ||||
| Acute tonsillitis | Age, years (mean) | 23.1 | 22.4 | |
| Contacts, n (T:C) | 3575 | 5:246 | ||
| 2 | 14 | |||
| 3 | 139 | |||
| 4 | 94 | |||
| 5 | 4 | |||
|
| ||||
| Acute bronchitis | Age, years (mean) | 45.3 | 28.4 | |
| Contacts, n (T:C) | 5159 | 6:187 | ||
| 2 | 16 | |||
| 3 | 138 | |||
| 4 | 38 | |||
| 5 | 1 | |||
|
| ||||
| Cystitis | Age, years (mean) | 50.8 | 46.8 | |
| Contacts, n (T:C) | 20 158 | 594:702 | ||
| 2 | 22 | |||
| 3 | 460 | |||
| 4 | 577 | |||
| 5 | 237 | |||
|
| ||||
| Impetigo | Age, years (mean) | 17.9 | 11.1 | |
| Contacts, n (T:C) | 3104 | 57:152 | ||
| 2 | 2 | |||
| 3 | 40 | |||
| 4 | 128 | |||
| 5 | 39 | |||
Disease episodes are shown for office hours care. For out-of-hours care, all contacts with the facility, telephone (T), and GP consultations (C) are shown, together with the urgency levels (Box 1) as determined by the triage nurse. Absolute numbers are shown.
Office hours and out-of-hours care: antibiotic management of six infectious diseases
Prescribing rates and types of antibiotics prescribed for the selected indications in office hours and OOH care are shown in Table 2. In OOH care, slightly higher prescribing rates were found for AOM, bronchitis, and impetigo. Larger rate differences were found for sinusitis and tonsillitis. For cystitis, prescribing rates differed the most, 64% in office hours and 94% in OOH practice. With respect to the choice of antibiotic, 80% or more prescriptions for AOM, bronchitis, and sinusitis were the recommended first-choice antibiotics, in both types of care. For tonsillitis, however, slightly fewer first-choice antibiotics were prescribed OOH, with nearly two times more amoxicillin/clavulanate. For impetigo, two-thirds of OOH prescriptions were the first-choice flucloxacillin; however, more amoxicillin/clavulanate was prescribed as well. For cystitis, similar antibiotics were prescribed.
Table 2.
Antibiotic management in office hours and out-of-hours care
| Indication | Choice of antibiotic | Prescribing,a % | |
|---|---|---|---|
|
| |||
| Office hours (JHN 2012) | Out-of-hours (Primair 2012) | ||
| Acute otitis media | Prescription rate | 47.6 | 60.5 |
| Amoxicillinb | 83.1 | 85.9 | |
| Azithromycin | 7.8 | 5.6 | |
| Amoxicillin/clavulanate | 4.7 | 5.1 | |
| Co-trimoxazole | 2 | 0.4 | |
|
| |||
| Sinusitis | Prescription rate | 48.1 | 70 |
| Doxycyclineb | 59.1 | 48.6 | |
| Amoxicillinb | 22 | 31.9 | |
| Azithromycin | 9.9 | 9.7 | |
| Amoxicillin/clavulanate | 5 | 2.8 | |
| Clarithromycin | 2.4 | 2.8 | |
|
| |||
| Acute tonsillitis | Prescription rate | 56.9 | 78 |
| Pheneticillinb | 71.3 | 59.9 | |
| Amoxicillin | 10 | 11.5 | |
| Azithromycin | 8.9 | 8.3 | |
| Amoxicillin/clavulanate | 6.2 | 11.5 | |
|
| |||
| Acute bronchitis | Prescription rate | 53.1 | 65.8 |
| Doxycyclineb | 44.8 | 32.5 | |
| Amoxicillinb | 37.5 | 56.9 | |
| Azithromycin | 8.7 | 2.4 | |
| Amoxicillin/clavulanate | 5.5 | 5.7 | |
| Clarithromycin | 2.1 | 2.4 | |
|
| |||
| Cystitis | Prescription rate | 64.2 | 93.9 |
| Nitrofurantoinb | 65.5 | 62.5 | |
| Amoxicillin/clavulanateb | 11.4 | 15.6 | |
| Trimethoprim | 8.7 | 6 | |
| Norfloxacin | 5.5 | 2.5 | |
| Ciprofloxacin | 3.5 | 3.9 | |
| Fosfomycin | 2.4 | 2.5 | |
| Co-trimoxazole | 2.3 | 3.2 | |
|
| |||
| Impetigo | Prescription rate | 23.9 | 30.9 |
| Flucloxacillinb | 59.8 | 66 | |
| Amoxicillin | 11.2 | 2.1 | |
| Azithromycin | 9 | 6.4 | |
| Clarithromycin | 6.7 | 2.1 | |
| Amoxicillin/clavulanate | 5.8 | 21.3 | |
| Pheneticillin | 1.5 | 2.1 | |
Antibiotics with prescribed percentages over 2% are shown.
First-choice antibiotics according to the Dutch prescribing guidelines (Appendix 1).
Out-of-hours care: appropriateness of antibiotic prescribing
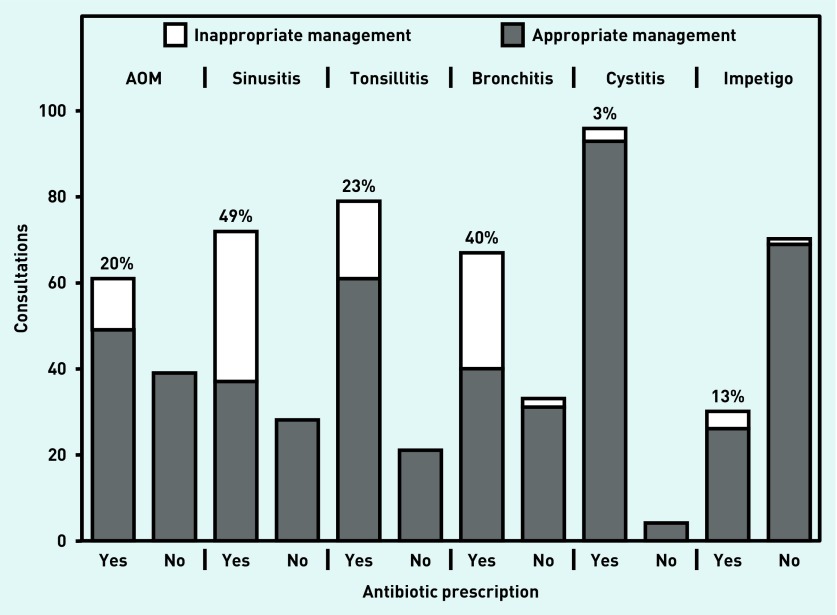
To determine to what extent the higher prescribing rates found OOH corresponds to more irrational prescribing, 100 consultations per indication were analysed for appropriateness of (non-)antibiotic management (Figure 3). Guidelines adherence for the prescribing decision was over 80% for the management of AOM, tonsillitis, cystitis, and impetigo; overprescribing was, respectively, 20%, 23%, 3%, and 13% for these indications. Adherence to guidelines for sinusitis and bronchitis was, respectively, 65% and 71%, with overprescribing of 49% for sinusitis and 40% for bronchitis.
Figure 3.
Appropriateness of (non-)antibiotic management in out-of-hours care. Data are from Primair patient files. Per indication, all available information of 100 consultations were compared with the prescribing guidelines (Appendix 1). The numbers of (in-)appropriate (non-)antibiotic prescriptions are shown, and the percentages of overprescription are given.
DISCUSSION
Summary
Of all GP-prescribed antibiotics, only 6% were prescribed OOH. Comparatively more amoxicillin and amoxicillin/clavulanate were prescribed; amoxicillin, as children more often present at OOH,23 and higher amoxicillin/clavulanate use was specifically seen for tonsillitis, cystitis, and impetigo (Table 2). Overall, OOH first-choice prescribing was comparable with office hours prescribing. And, although prescribing rates, especially for sinusitis, tonsillitis, and cystitis, were higher for OOH, overprescribing was comparable, or even lower than had been determined in a previous study for daily practice.1
Oral antibiotics have a limited effectiveness in treatment of RTI and ear infections. With risk factors, for a severe infection, or pneumonia, the benefits of antibiotic treatment offset the risk of harm. A confirmed urinary tract infection is treated effectively with antibiotics, although there is evidence that a wait-and-see policy can be appropriate for uncomplicated cystitis.4 Topical antibiotics are effective for impetigo; oral antibiotics are indicated only for extensive disease with general illness and insufficient effect of topical treatment.
For various reasons, like time pressure, no personal relationship with the patient, and the organisation of emergency care, it was suspected that OOH antibiotic prescribing quality was lower. Indeed, prescribing rates for all indications were higher OOH. When interpreting this, however, one should be aware that the patient population presenting at OOH differs from daily practice. First, a different triage system is used: the OOH system aims to select patients with urgent problems that cannot wait until the next day for evaluation by the patient’s own GP. As a result, more severe illness will be presented in OOH, with a higher likelihood of requiring antibiotics.24,25 Second, comparatively more young children23 and patients aged >75 years present at OOH; patients in these age categories could belong to risk groups. Third, the threshold to contact the OOH service is higher than contacting own GP during office hours. Therefore, higher prescription rates do not necessarily implicate more irrational prescribing.
A previous Dutch study showed office hours overprescribing for AOM, sinusitis, tonsillitis, and bronchitis of, respectively, 4%, 50%, 53%, and 69%.1 OOH, the present study found lower overprescribing for sinusitis, tonsillitis, and bronchitis. Only AOM overprescribing was higher OOH. According to the Dutch AOM guideline, antibiotic treatment can be considered for patients with non-reducing complaints for 3 days. When disease duration was not specifically mentioned in the patient file and no other antibiotic prescribing criterion was present, the present study classified prescribing as inappropriate. This missing information might have resulted in an overestimation. Alternatively, there might really be more irrational prescribing for children with AOM. GPs might ‘give in’ to antibiotic treatment more easily as they lack time for communicating their non-treatment considerations with the concerned parent.
For cystitis, the daytime prescribing rate was around 60%, in agreement with a previous study.3 This seems low as, according to the guideline, antibiotic treatment is indicated for confirmed cystitis. Patients might choose a wait-and-see policy, or the diagnosis and/or prescription might have been inappropriately registered. GPs and practice assistants are both involved in the management of females with suspected urinary tract infection, which might decrease proper coding. The higher OOH prescribing rate is not alarming, as there was hardly any overprescribing. This indicates that GPs and nurses followed the guideline and only prescribed antibiotics after urine testing. Overprescribing for impetigo seems low, but could not be compared with daytime data, as these were not available.
Strengths and limitations
To the authors’ knowledge this is the first study evaluating OOH antibiotic prescribing quantity and quality, as well as the appropriateness of prescribing for the most often presented infectious diseases in primary care. The main strength of this study is that for the main infections all office hours were analysed and OOH contacts from 1 complete year were analysed. Data were retrieved from the same region in the Netherlands. This is important for a valid quality comparison, as antibiotic use varies throughout the country.26 As routine care data were used, GPs were not aware of the analysis of their prescribing behaviour. Second, the appropriateness of prescribing, using guideline benchmarking, has not been studied before for OOH care. By benchmarking all available patient and disease characteristics to the guidelines, it has been shown that higher prescribing rates do not go hand-in-hand with more irrational prescribing.
The following limitations must be acknowledged. First, in determining of overprescribing during office hours, a registration form was used to capture all relevant patient and disease characteristics.1 For OOH overprescribing, information from the patient files was used. This harbours the risk of missing relevant information, as GPs might not have registered all relevant items from the guideline. It was noted, however, that GPs tended to file extensive information during OOH, with the aim of informing the patient’s own GP. When specific information was not provided, it was regarded as absent; this might have overestimated overprescribing in this setting. Second, the analysis of OOH overprescribing was done 1–2 years later than the office hours registration. In this period, however, antibiotic use for RTIs was very stable in the Netherlands,26,27 and guideline recommendations for antibiotic treatment did not change. Third, the data were retrieved by selecting for ICPC codes. There is no reason to assume that ICPC coding was done differently in the two settings, as in the Netherlands OOH services are done by GPs.
Comparison with existing literature
Another Dutch study showed that adherence to antibiotic prescribing guidelines was 69% in OOH care; overtreatment was mostly seen for sinusitis and sore throat.25 Prescribing rates and types of antibiotics were not presented, and the data from that study were not compared with daytime practice. In the present study, using all free text from patient records, higher OOH guideline adherence was found. In two other low-prescribing countries, Denmark and Norway, high small -spectrum prescribing during OOH9 and comparable prescribing between the two settings were found as well.14 In Belgium, a high prescribing country, prescribing quality indicator outcomes for seven acute infections also revealed similar outcomes for the two settings.16 Although medical appropriateness was not determined in these studies, the results do point towards a similar quality in office hours and OOH care.
In Norway, an intervention in an OOH service using peer academic detailing did not affect prescribing rates, but did reduce macrolide and lincosamide prescribing.7
Implications for practice
It seems that the OOH triage, which specifically aims to select urgent cases and to prevent complications, results in a patient population with more severe disease. The low threshold to consult a GP during the daytime is valued in Dutch primary care, but also results in patients consulting with mild, self-limiting disease. OOH triage might be an inspirational model to adapt daytime triage to reassure patients with self-limiting disease on the phone, and to stimulate self-management. This could help to decrease antibiotic overprescribing.
In conclusion, the quality of antibiotic prescribing during OOH is comparable, or even slightly better than, in daily practice. Given the numbers of dispensed antibiotics during OOH (6%), the generally correct choice of antibiotic, and the non-excessive rates of irrational prescribing, Dutch antibiotic-related problems cannot be attributed to management at OOH services.
Acknowledgments
The authors thank HAP Primair, specifically Iddo de Ruiter, for sharing the anonymised electronic out-of-hours patient files, and Monique Lunstroo for helping with the data and files retrieval. The Dutch Working Party on Antibiotic Policy (SWAB), specifically Marco Lourens, is thanked for initiating the retrieval of national antibiotic dispensing data, and the Dutch Foundation of Pharmaceutical Statistics, specifically Jan Dirk Kroon, for extracting the required data. Thanks also to the GPs who participate in the ‘Julius General Practitioners’ Network’ for sharing their anonymised medical record data.
Appendix 1. Recommendations for antibiotic prescribing according to the guidelines of the Dutch College of GPs (NHG)a
| Guideline | Antibiotic | Definition of patient- and disease characteristics | First-choice antibiotic |
|---|---|---|---|
| Acute otitis media (2006) | Indicated |
|
Amoxicillin |
|
| |||
| May be considered |
|
||
| Acute sore throat (2007) | Indicated |
|
Pheneticillin, phenoxymethyl penicillin |
| Rhinosinusitis (2005) | May be considered |
|
Adults: doxycycline or amoxicillin Children: amoxicillin |
| Acute cough (2011) | Indicated | Signs and symptoms indicative for pneumonia:
|
Adults: doxycycline |
|
|
|||
Risk for complicated course:
|
Children: amoxicillin | ||
| Bacterial skin infections (2007) | Indicated |
|
Flucloxacillin |
| Urinary tract infections (2005) | Indicated |
|
Uncomplicated or without tissue invasion: nitrofurantoin |
|
| |||
| Complicated with tissue invasion: amoxicillin/clavulanate | |||
Non-antibiotic management, with reassurance and, if possible, symptomatic treatment advice, is advised for all other patients. In the Netherlands, all GPs use the NHG guidelines and no other local primary care guidelines are in place. AOM = acute otitis media. COPD = chronic obstructive pulmonary disease. NHG = Dutch College of General Practitioners.
Funding
This work was carried out as part of the authors’ routine work.
Ethical approval
This study was approved by the medical ethics committee of the University Medical Center Utrecht, the Netherlands: METC-protocol number 14-313/C.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Dekker AR, Verheij TJ, van der Velden AW. Inappropriate antibiotic prescription for respiratory tract indications: most prominent in adult patients. Fam Pract. 2015;32(4):401–407. doi: 10.1093/fampra/cmv019. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother. 2014;69(1):234–240. doi: 10.1093/jac/dkt301. [DOI] [PubMed] [Google Scholar]
- 3.Van den Broek d’Obrenan J, Verheij TJ, Numans ME, van der Velden AW. Antibiotic use in Dutch primary care: relation between diagnosis, consultation and treatment. J Antimicrob Chemother. 2014;69(6):1701–1707. doi: 10.1093/jac/dku005. [DOI] [PubMed] [Google Scholar]
- 4.Willems CS, van den Broek D’Obrenan J, Numans ME, et al. Cystitis: antibiotic prescribing, consultation, attitudes and opinions. Fam Pract. 2014;31(2):149–155. doi: 10.1093/fampra/cmt077. [DOI] [PubMed] [Google Scholar]
- 5.Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 6.Smith R, Coast J. The true cost of antimicrobial resistance. BMJ. 2013;346:f1493. doi: 10.1136/bmj.f1493. [DOI] [PubMed] [Google Scholar]
- 7.Dyrkorn R, Gjelstad S, Espnes KA, Lindbæk M. Peer academic detailing on use of antibiotics in acute respiratory infections. A controlled study in an urban Norwegian out-of-hours service. Scand J Prim Health Care. 2016;34(2):180–185. doi: 10.3109/02813432.2016.1163035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulscher ME, van der Meer JW, Grol RP. Antibiotic use: how to improve it? Int J Med Microbiol. 2010;300(6):351–356. doi: 10.1016/j.ijmm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Huibers L, Moth G, Christensen MB, Vedsted P. Antibiotic prescribing patterns in out-of-hours primary care: a population-based descriptive study. Scand J Prim Health Care. 2014;32(4):200–207. doi: 10.3109/02813432.2014.972067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huibers L, Moth G, Bondevik GT, et al. Diagnostic scope in out-of-hours primary care services in eight European countries: an observational study. BMC Fam Pract. 2011;12:30. doi: 10.1186/1471-2296-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elshout G, Kool M, Van der Wouden JC, et al. Antibiotic prescription in febrile children: a cohort study during out-of-hours primary care. J Am Board Fam Med. 2012;25(6):810–818. doi: 10.3122/jabfm.2012.06.110310. [DOI] [PubMed] [Google Scholar]
- 12.Giesen P, Willekens M, Mokkink H, et al. Out-of-hours primary care: development of indicators for prescribing and referring. Int J Qual Health Care. 2007;19(5):289–295. doi: 10.1093/intqhc/mzm027. [DOI] [PubMed] [Google Scholar]
- 13.NIVEL, Netherlands institute for health services research Medical consumption at out-of-hours services: top-5 ICPC-codes. http://www.nivel.nl/NZR/top-5-icpc-codes (accessed 27 Jan 2017).
- 14.Fagan M. Is otitis and tonsillitis handled in the same way within normal working hours and out-of-hours? [In Norwegian] Tidsskr Nor Laegeforen. 2008;128(20):2340–2342. [PubMed] [Google Scholar]
- 15.Hayward GN, Fisher RF, Spence GT, Lasserson DS. Increase in antibiotic prescriptions in out-of-hours primary care in contrast to in-hours primary care prescriptions: service evaluation in a population of 600 000 patients. J Antimicrob Chemother. 2016;71(9):2612–2619. doi: 10.1093/jac/dkw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adriaenssens N, Bartholomeeusen S, Ryckebosch P, Coenen S. Quality of antibiotic prescription during office hours and out-of-hours in Flemish primary care, using European quality indicators. Eur J Gen Pract. 2014;20(2):114–120. doi: 10.3109/13814788.2013.828200. [DOI] [PubMed] [Google Scholar]
- 17.Stichting Farmaceutische Kengetallen Foundation for pharmaceutical statistics. http://www.sfk.nl/english (accessed 27 Jan 2017).
- 18.World Health Organization Collaborating Centre for Drug Statistics Methodology Structure and principles. http://www.whocc.no/atc/structure_and_principles (accessed 27 Jan 2017).
- 19.Hofmans-Okkes IM, Lamberts H. The International Classification of Primary Care (ICPC): new applications in research and computer-based patient records in family practice. Fam Pract. 1996;13(3):294–302. doi: 10.1093/fampra/13.3.294. [DOI] [PubMed] [Google Scholar]
- 20.Den Boer-Wolters D, Knol MJ, Smulders K, de Wit NJ. Frequent attendance of primary care out-of-hours services in the Netherlands: characteristics of patients and presented morbidity. Fam Pract. 2010;27(2):129–134. doi: 10.1093/fampra/cmp103. [DOI] [PubMed] [Google Scholar]
- 21.Huibers L, Moth G, Andersen M, et al. Consumption in out-of-hours health care: Danes double Dutch? Scand J Prim Health Care. 2014;32(1):4450. doi: 10.3109/02813432.2014.898974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nederlands Huisartsen Genootschap Richtlijnen en praktijk. [Guidelines and practice]. https://www.nhg.org/richtlijnen-praktijk (accessed 27 Jan 2017).
- 23.De Bont EG, Lepot JM, Hendrix DA, et al. Workload and management of childhood fever at general practice out-of-hours care: an observational study. BMJ Open. 2015;5:e007365. doi: 10.1136/bmjopen-2014-007365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giesen P, Franssen E, Mokkink H, et al. Patients either contacting a general practice cooperative or accident and emergency department out of hours: a comparison. Emerg Med J. 2006;23(9):731–734. doi: 10.1136/emj.2005.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willekens M, Giesen P, Plat E, et al. Quality of after-hours primary care in The Netherlands: adherence to national guidelines. BMJ Qual Saf. 2011;20(3):223–227. doi: 10.1136/bmjqs.2010.043091. [DOI] [PubMed] [Google Scholar]
- 26.The Dutch Working Party on Antibiotic Policy (SWAB) Report on regional antibiotic use. [In Dutch]. http://www2.sfk.nl/producten/swab/regionaal/index_html (accessed 27 Jan 2017).
- 27.The Dutch Working Party on Antibiotic Policy (SWAB) Antibiotic use in the Netherlands. [In Dutch]. http://www2.sfk.nl/producten/swab/landelijk (accessed 27 Jan 2017).